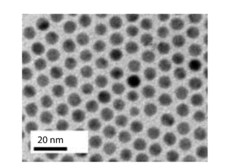
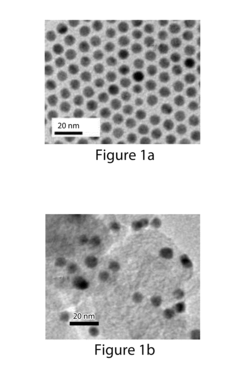
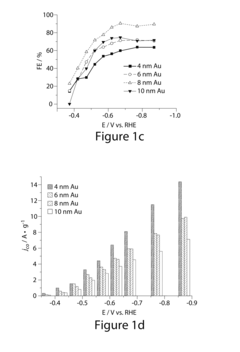
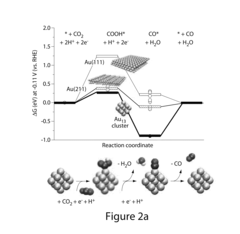
Analysis of Electrocatalytic CO2 reduction for electrode kinetics and adsorption performance
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Electrocatalysis Background and Objectives
Electrocatalytic CO2 reduction has emerged as a promising approach to address the dual challenges of climate change and sustainable energy production. The evolution of this technology can be traced back to the early 1980s when pioneering work by Hori and colleagues demonstrated the feasibility of converting CO2 to various hydrocarbons and alcohols using copper electrodes. Since then, the field has witnessed significant advancements in catalyst design, reaction mechanisms understanding, and process optimization.
The technological trajectory has been characterized by three distinct phases: initial discovery (1980s-1990s), mechanistic understanding (2000s-2010s), and rational catalyst design (2010s-present). Recent years have seen a paradigm shift from trial-and-error approaches to theory-guided catalyst development, leveraging computational methods and in-situ characterization techniques to elucidate reaction pathways and intermediate species.
Current research trends focus on enhancing catalytic selectivity, activity, and stability - the three pillars of effective CO2 reduction catalysts. Particular attention is being paid to understanding electrode kinetics, which governs reaction rates and energy efficiency, and adsorption performance, which determines product selectivity and conversion efficiency. These parameters are critical for scaling laboratory discoveries to industrial applications.
The primary technical objectives in this field include developing catalysts with high Faradaic efficiency toward specific value-added products, achieving industrially relevant current densities (>200 mA/cm²), maintaining catalyst stability over thousands of hours, and reducing the overpotential required to drive the reaction. Additionally, there is growing interest in integrating CO2 reduction systems with renewable energy sources to create carbon-neutral or carbon-negative production cycles.
From a fundamental science perspective, researchers aim to establish clear structure-property relationships that connect catalyst atomic structure to adsorption energetics and reaction kinetics. This includes understanding how surface facets, defects, grain boundaries, and local coordination environments influence CO2 activation and subsequent C-C coupling reactions.
Looking forward, the field is moving toward multifunctional catalyst designs that can orchestrate complex reaction cascades, tandem catalytic systems that combine electrochemical and thermal catalysis, and integrated devices that simultaneously capture and convert CO2. The ultimate goal is to develop economically viable technologies that can be deployed at scale to make meaningful contributions to carbon mitigation efforts while producing valuable chemical feedstocks and fuels.
The technological trajectory has been characterized by three distinct phases: initial discovery (1980s-1990s), mechanistic understanding (2000s-2010s), and rational catalyst design (2010s-present). Recent years have seen a paradigm shift from trial-and-error approaches to theory-guided catalyst development, leveraging computational methods and in-situ characterization techniques to elucidate reaction pathways and intermediate species.
Current research trends focus on enhancing catalytic selectivity, activity, and stability - the three pillars of effective CO2 reduction catalysts. Particular attention is being paid to understanding electrode kinetics, which governs reaction rates and energy efficiency, and adsorption performance, which determines product selectivity and conversion efficiency. These parameters are critical for scaling laboratory discoveries to industrial applications.
The primary technical objectives in this field include developing catalysts with high Faradaic efficiency toward specific value-added products, achieving industrially relevant current densities (>200 mA/cm²), maintaining catalyst stability over thousands of hours, and reducing the overpotential required to drive the reaction. Additionally, there is growing interest in integrating CO2 reduction systems with renewable energy sources to create carbon-neutral or carbon-negative production cycles.
From a fundamental science perspective, researchers aim to establish clear structure-property relationships that connect catalyst atomic structure to adsorption energetics and reaction kinetics. This includes understanding how surface facets, defects, grain boundaries, and local coordination environments influence CO2 activation and subsequent C-C coupling reactions.
Looking forward, the field is moving toward multifunctional catalyst designs that can orchestrate complex reaction cascades, tandem catalytic systems that combine electrochemical and thermal catalysis, and integrated devices that simultaneously capture and convert CO2. The ultimate goal is to develop economically viable technologies that can be deployed at scale to make meaningful contributions to carbon mitigation efforts while producing valuable chemical feedstocks and fuels.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market size for CO2 conversion was valued at approximately $1.8 billion in 2022 and is projected to reach $4.9 billion by 2030, growing at a CAGR of 13.2% during the forecast period. This growth trajectory is supported by substantial investments in research and development, particularly in electrocatalytic CO2 reduction technologies.
The demand for efficient CO2 conversion solutions spans multiple industries, with energy, chemicals, and manufacturing sectors showing the strongest interest. In the energy sector, the integration of CO2 conversion with renewable energy sources presents a promising pathway for energy storage and carbon-neutral fuel production. The chemicals industry views CO2 as a potential feedstock for producing value-added chemicals, reducing dependence on fossil resources.
Regional analysis reveals that North America and Europe currently lead the market, accounting for approximately 60% of the global market share. However, Asia-Pacific is emerging as the fastest-growing region, with China and Japan making significant investments in CO2 utilization technologies. This regional shift is expected to reshape the competitive landscape over the next decade.
Market segmentation by technology type shows that electrochemical reduction methods, particularly those focusing on electrode kinetics and adsorption performance optimization, represent about 35% of the current market. This segment is expected to grow at a higher rate than thermal or biological conversion methods due to its potential for higher selectivity and energy efficiency.
End-product markets for converted CO2 are diversifying rapidly. While methanol and formic acid remain dominant products, accounting for approximately 45% of the market, emerging applications in synthetic fuels and polymers are gaining traction. The market for CO2-derived fuels alone is projected to grow at 16.8% annually through 2030.
Key market drivers include increasingly stringent carbon emission regulations, carbon pricing mechanisms, and corporate sustainability commitments. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for CO2 utilization. Additionally, consumer preference for green products is pushing industries to adopt carbon-neutral or carbon-negative production processes.
Market barriers include high capital costs for implementation, energy intensity of conversion processes, and competition from established fossil-based production routes. The economic viability of many CO2 conversion technologies remains challenging without policy support or carbon pricing mechanisms.
The demand for efficient CO2 conversion solutions spans multiple industries, with energy, chemicals, and manufacturing sectors showing the strongest interest. In the energy sector, the integration of CO2 conversion with renewable energy sources presents a promising pathway for energy storage and carbon-neutral fuel production. The chemicals industry views CO2 as a potential feedstock for producing value-added chemicals, reducing dependence on fossil resources.
Regional analysis reveals that North America and Europe currently lead the market, accounting for approximately 60% of the global market share. However, Asia-Pacific is emerging as the fastest-growing region, with China and Japan making significant investments in CO2 utilization technologies. This regional shift is expected to reshape the competitive landscape over the next decade.
Market segmentation by technology type shows that electrochemical reduction methods, particularly those focusing on electrode kinetics and adsorption performance optimization, represent about 35% of the current market. This segment is expected to grow at a higher rate than thermal or biological conversion methods due to its potential for higher selectivity and energy efficiency.
End-product markets for converted CO2 are diversifying rapidly. While methanol and formic acid remain dominant products, accounting for approximately 45% of the market, emerging applications in synthetic fuels and polymers are gaining traction. The market for CO2-derived fuels alone is projected to grow at 16.8% annually through 2030.
Key market drivers include increasingly stringent carbon emission regulations, carbon pricing mechanisms, and corporate sustainability commitments. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for CO2 utilization. Additionally, consumer preference for green products is pushing industries to adopt carbon-neutral or carbon-negative production processes.
Market barriers include high capital costs for implementation, energy intensity of conversion processes, and competition from established fossil-based production routes. The economic viability of many CO2 conversion technologies remains challenging without policy support or carbon pricing mechanisms.
Current Challenges in Electrode Kinetics and Adsorption
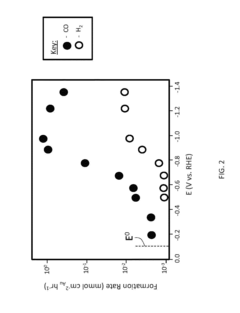
Despite significant advancements in electrocatalytic CO2 reduction (ECR) technology, several critical challenges persist in electrode kinetics and adsorption performance that impede commercial viability. The sluggish kinetics of CO2 reduction represents one of the most formidable obstacles, requiring substantial overpotentials that diminish energy efficiency. This kinetic limitation stems from the initial electron transfer to form the CO2•- intermediate, which constitutes the rate-determining step in many catalytic systems.
Competitive side reactions, particularly hydrogen evolution, further complicate the process by consuming electrons that could otherwise be utilized for CO2 conversion. This parasitic reaction becomes especially problematic in aqueous electrolytes where proton availability facilitates hydrogen formation at potentials similar to those required for CO2 reduction.
Mass transport limitations present another significant challenge, as the low solubility of CO2 in aqueous solutions (approximately 33 mM at ambient conditions) restricts reactant availability at the electrode surface. This limitation creates concentration gradients that hinder reaction rates and contribute to poor current densities, especially at industrially relevant scales.
The adsorption behavior of intermediates on catalyst surfaces remains insufficiently understood, with competing theories about optimal binding strengths for different reaction pathways. The Sabatier principle suggests an optimal intermediate binding energy, but achieving this balance across multiple reaction steps with different intermediates presents a complex optimization problem that current catalyst designs struggle to solve.
Catalyst stability under reaction conditions represents another critical challenge, as many promising materials suffer from degradation through mechanisms including poisoning, restructuring, and leaching. Metal catalysts often experience surface reconstruction during operation, altering their catalytic properties over time and complicating long-term performance predictions.
The complex interplay between electrode surface structure and electrolyte composition further influences reaction pathways and product selectivity. Local pH gradients near the electrode surface can dramatically alter reaction conditions compared to bulk electrolyte properties, creating microenvironments that are difficult to characterize and control.
Advanced in-situ and operando characterization techniques are needed to better understand these dynamic processes occurring at the electrode-electrolyte interface. Current analytical limitations make it challenging to observe intermediate species and surface transformations in real-time, hampering rational catalyst design efforts.
Addressing these interconnected challenges requires interdisciplinary approaches combining computational modeling, advanced materials synthesis, and sophisticated electrochemical characterization to develop next-generation catalysts with improved kinetics and adsorption properties for efficient CO2 electroreduction.
Competitive side reactions, particularly hydrogen evolution, further complicate the process by consuming electrons that could otherwise be utilized for CO2 conversion. This parasitic reaction becomes especially problematic in aqueous electrolytes where proton availability facilitates hydrogen formation at potentials similar to those required for CO2 reduction.
Mass transport limitations present another significant challenge, as the low solubility of CO2 in aqueous solutions (approximately 33 mM at ambient conditions) restricts reactant availability at the electrode surface. This limitation creates concentration gradients that hinder reaction rates and contribute to poor current densities, especially at industrially relevant scales.
The adsorption behavior of intermediates on catalyst surfaces remains insufficiently understood, with competing theories about optimal binding strengths for different reaction pathways. The Sabatier principle suggests an optimal intermediate binding energy, but achieving this balance across multiple reaction steps with different intermediates presents a complex optimization problem that current catalyst designs struggle to solve.
Catalyst stability under reaction conditions represents another critical challenge, as many promising materials suffer from degradation through mechanisms including poisoning, restructuring, and leaching. Metal catalysts often experience surface reconstruction during operation, altering their catalytic properties over time and complicating long-term performance predictions.
The complex interplay between electrode surface structure and electrolyte composition further influences reaction pathways and product selectivity. Local pH gradients near the electrode surface can dramatically alter reaction conditions compared to bulk electrolyte properties, creating microenvironments that are difficult to characterize and control.
Advanced in-situ and operando characterization techniques are needed to better understand these dynamic processes occurring at the electrode-electrolyte interface. Current analytical limitations make it challenging to observe intermediate species and surface transformations in real-time, hampering rational catalyst design efforts.
Addressing these interconnected challenges requires interdisciplinary approaches combining computational modeling, advanced materials synthesis, and sophisticated electrochemical characterization to develop next-generation catalysts with improved kinetics and adsorption properties for efficient CO2 electroreduction.
State-of-the-Art Electrode Materials and Designs
01 Metal-based catalysts for CO2 electroreduction
Various metal-based catalysts can be employed for electrocatalytic CO2 reduction with enhanced electrode kinetics. These include transition metals, metal alloys, and metal oxides that offer different selectivity and activity for CO2 conversion products. The catalysts can be designed with specific surface structures to optimize adsorption of CO2 and reaction intermediates, thereby improving the overall efficiency of the electrochemical reduction process.- Metal-based catalysts for CO2 electroreduction: Various metal-based catalysts can be employed for electrocatalytic CO2 reduction with enhanced kinetics and selectivity. These include transition metals, metal alloys, and metal oxides that provide active sites for CO2 adsorption and conversion. The catalyst composition and structure significantly influence the reaction pathway, product distribution, and energy efficiency. Optimizing the metal catalyst's surface properties can improve electrode kinetics by lowering activation barriers and enhancing electron transfer rates during the CO2 reduction process.
- Carbon-based materials for enhanced adsorption performance: Carbon-based materials such as graphene, carbon nanotubes, and porous carbon structures serve as excellent supports or catalysts for CO2 electroreduction. These materials offer high surface area, tunable porosity, and abundant active sites for CO2 adsorption. The carbon materials can be functionalized or doped with heteroatoms to further enhance their adsorption capabilities and catalytic activity. Their electrical conductivity also facilitates electron transfer during the electrochemical process, improving overall electrode kinetics and reaction efficiency.
- Electrode structure design and interface engineering: The design of electrode structures plays a crucial role in CO2 electroreduction performance. Hierarchical porous structures, 3D architectures, and nanostructured surfaces can significantly enhance mass transport, increase active surface area, and improve CO2 adsorption. Interface engineering between catalyst layers and electrolytes optimizes the local reaction environment, affecting proton and electron transfer kinetics. Controlling the electrode-electrolyte interface properties helps manage the local pH, CO2 concentration, and intermediate species stability, leading to improved reaction pathways and product selectivity.
- Electrolyte composition and reaction conditions optimization: The composition of the electrolyte solution significantly impacts CO2 electroreduction kinetics and adsorption performance. Factors such as pH, ionic strength, buffer capacity, and specific ion effects influence CO2 solubility, mass transport, and the stability of reaction intermediates. Optimizing reaction conditions including temperature, pressure, potential, and current density can enhance electrode performance. Advanced electrolyte systems incorporating ionic liquids or specialized additives can improve CO2 adsorption at the electrode surface and facilitate more efficient conversion pathways.
- In-situ characterization and mechanistic studies: Advanced in-situ and operando characterization techniques provide crucial insights into electrode kinetics and adsorption mechanisms during CO2 electroreduction. Spectroscopic methods, electrochemical impedance spectroscopy, and computational modeling help identify reaction intermediates, rate-determining steps, and adsorption energetics. Understanding the fundamental mechanisms allows for rational catalyst design and process optimization. These studies reveal the dynamic changes at the electrode surface during operation and correlate structural features with catalytic performance, guiding the development of more efficient electrocatalytic systems.
02 Carbon-based materials as CO2 reduction electrodes
Carbon-based materials such as graphene, carbon nanotubes, and doped carbon structures serve as effective electrodes for CO2 reduction. These materials offer high surface area, excellent electrical conductivity, and tunable surface properties that enhance CO2 adsorption and electron transfer kinetics. Functionalization of carbon surfaces with specific groups can further improve the selectivity and efficiency of the electrocatalytic CO2 reduction process.Expand Specific Solutions03 Nanostructured electrodes for improved kinetics
Nanostructured electrodes with controlled morphology and composition demonstrate enhanced kinetics for CO2 electroreduction. These structures provide increased active sites, improved mass transport, and optimized CO2 adsorption properties. Various fabrication techniques can be employed to create nanostructures with specific features such as high porosity, hierarchical structures, and defect sites that contribute to superior catalytic performance and product selectivity.Expand Specific Solutions04 Electrode surface modification for enhanced adsorption
Surface modification strategies can significantly improve CO2 adsorption performance on electrodes. These include atomic layer deposition, plasma treatment, chemical functionalization, and the introduction of specific binding sites. Modified surfaces can stabilize reaction intermediates, lower activation barriers, and enhance the local concentration of CO2 near the electrode surface, resulting in improved reaction rates and product selectivity.Expand Specific Solutions05 Electrolyte engineering for optimized CO2 reduction
The composition and properties of the electrolyte play a crucial role in electrode kinetics and CO2 adsorption. Factors such as pH, ionic strength, buffer capacity, and specific ion effects can significantly influence the local environment at the electrode-electrolyte interface. Tailored electrolytes can enhance CO2 solubility, stabilize intermediates, suppress competing reactions, and ultimately improve the efficiency and selectivity of the electrocatalytic CO2 reduction process.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalytic CO2 reduction technology landscape is currently in a transitional phase from early research to commercial application, with a global market projected to reach significant scale as carbon neutrality initiatives accelerate. The technology maturity varies across key players, with research institutions like Dalian Institute of Chemical Physics, KAIST, and Brown University leading fundamental research, while industrial entities such as Siemens Energy, TotalEnergies, and Saudi Aramco are advancing practical applications. Major corporations including Toshiba, ENEOS, and Repsol are investing in scalable solutions, focusing on electrode materials optimization and reaction kinetics. The competitive landscape shows a collaborative ecosystem between academic institutions developing novel catalysts and industrial players working on system integration and scale-up challenges for commercial deployment.
Dalian Institute of Chemical Physics of CAS
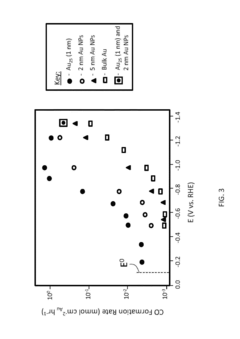
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced copper-based catalysts with precisely controlled morphologies and electronic structures for efficient CO2 electroreduction. Their approach involves creating copper catalysts with specific facets and defects that enhance CO2 adsorption and C-C coupling for higher-value products. DICP researchers have pioneered the use of in-situ/operando characterization techniques including X-ray absorption spectroscopy and ambient pressure XPS to monitor catalyst surface changes during reaction conditions[1]. They've demonstrated catalysts achieving Faradaic efficiencies exceeding 60% for C2+ products at industrially relevant current densities (>200 mA/cm²), with particular focus on ethylene and ethanol production pathways. Their work includes comprehensive kinetic studies correlating adsorption energies of key intermediates with product selectivity across different potential ranges.
Strengths: World-leading expertise in catalyst design with atomic-level precision; exceptional characterization capabilities for mechanistic understanding; strong integration of theoretical and experimental approaches. Weaknesses: Some catalysts show stability limitations under extended operation; scaling relations between different intermediates remain challenging to overcome completely.
Kyushu University
Technical Solution: Kyushu University has developed innovative approaches to electrocatalytic CO2 reduction focusing on metal-organic frameworks (MOFs) and single-atom catalysts. Their research teams have created highly dispersed single-atom catalysts where isolated metal centers (particularly Fe, Co, and Ni) are anchored to nitrogen-doped carbon supports, providing well-defined active sites with tunable electronic properties. These catalysts demonstrate exceptional CO2 adsorption capabilities due to their optimized binding energies. Kyushu researchers have implemented advanced in-situ characterization techniques including X-ray absorption fine structure (XAFS) and operando infrared spectroscopy to monitor catalyst structure and adsorbed intermediates during reaction[5]. Their work has revealed how coordination environment affects the stabilization of key intermediates like *COOH and *CO, enabling rational catalyst design. They've achieved catalysts with CO Faradaic efficiencies exceeding 95% at low overpotentials (<400 mV) while maintaining stability for hundreds of hours of continuous operation.
Strengths: Exceptional atomic-level control over active sites; outstanding stability compared to many competing systems; detailed mechanistic understanding enabling rational design. Weaknesses: Some systems show limited activity for C-C coupling to form multi-carbon products; mass transport limitations in some MOF-based catalysts; challenges in scaling up precise synthetic procedures.
Key Breakthroughs in Catalyst-Substrate Interactions
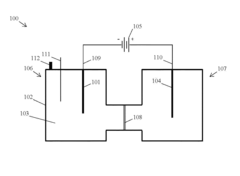
Method for Electrocatalytic Reduction using Au Nanoparticles Tuned or Optimized for Reduction of CO2 to CO
PatentInactiveUS20160230295A1
Innovation
- The use of gold nanoparticles with a diameter of approximately 8 nm, supported on Ketjen carbon, which presents a surface structure with a higher density of CO-converting edge sites and fewer hydrogen-evolving corner sites, facilitating efficient CO2 reduction to carbon monoxide in an alkaline solution.
Efficient electrocatalytic conversion of CO<sub>2 </sub>to CO using ligand-protected Au<sub>25 </sub>clusters
PatentInactiveUS9139920B1
Innovation
- An Au25 electrode with a ligand-protected structure, comprising an icosahedral core of 13 atoms and a shell of six semi-ring structures, is used in an electrochemical cell with a potential of at least −0.1 volts to interact with CO2 and H+ ions, facilitating the reduction of CO2 to CO with high Faradaic efficiency and reduced overpotential.
Techno-Economic Assessment of CO2 Reduction Systems
The techno-economic assessment of CO2 reduction systems requires comprehensive analysis of both technical performance and economic viability. Current electrocatalytic CO2 reduction technologies demonstrate promising potential for carbon utilization but face significant cost barriers to widespread implementation. Capital expenditures for these systems typically range from $500-2,000 per kW of installed capacity, with electrode materials representing 15-30% of this cost depending on catalyst composition and loading.
Operating expenses are dominated by electricity consumption, accounting for 60-75% of total operational costs. With current electricity prices averaging $0.05-0.12 per kWh in industrial settings, the energy cost for producing value-added chemicals through CO2 reduction ranges from $1.20-3.50 per kg of product. This creates a challenging economic landscape when competing with conventional fossil-based production methods that typically cost $0.80-1.50 per kg for similar chemicals.
System efficiency presents another critical economic factor. State-of-the-art CO2 reduction systems achieve Faradaic efficiencies of 60-95% for specific products, but overall energy efficiency often remains below 40%. This efficiency gap significantly impacts the levelized cost of production, which currently stands at $2.00-5.50 per kg of carbon-based products, depending on the target molecule and process configuration.
Sensitivity analysis reveals that a 10% improvement in electrode kinetics could reduce operational costs by 7-12%, while enhanced adsorption performance could improve product selectivity by 15-25%, substantially affecting economic viability. The economic threshold for commercial viability requires either electricity costs below $0.04 per kWh or catalyst systems that maintain stability for over 5,000 operating hours while achieving energy efficiencies above 60%.
Scale-up economics show promising trends, with projected cost reductions of 30-45% when moving from laboratory to industrial scale. However, these projections depend heavily on advances in electrode materials that can simultaneously address kinetics, selectivity, and durability. Current techno-economic models suggest that CO2 reduction systems will reach cost parity with conventional processes by 2028-2032, assuming continued technological improvements and supportive carbon pricing mechanisms in the range of $50-75 per ton of CO2.
Operating expenses are dominated by electricity consumption, accounting for 60-75% of total operational costs. With current electricity prices averaging $0.05-0.12 per kWh in industrial settings, the energy cost for producing value-added chemicals through CO2 reduction ranges from $1.20-3.50 per kg of product. This creates a challenging economic landscape when competing with conventional fossil-based production methods that typically cost $0.80-1.50 per kg for similar chemicals.
System efficiency presents another critical economic factor. State-of-the-art CO2 reduction systems achieve Faradaic efficiencies of 60-95% for specific products, but overall energy efficiency often remains below 40%. This efficiency gap significantly impacts the levelized cost of production, which currently stands at $2.00-5.50 per kg of carbon-based products, depending on the target molecule and process configuration.
Sensitivity analysis reveals that a 10% improvement in electrode kinetics could reduce operational costs by 7-12%, while enhanced adsorption performance could improve product selectivity by 15-25%, substantially affecting economic viability. The economic threshold for commercial viability requires either electricity costs below $0.04 per kWh or catalyst systems that maintain stability for over 5,000 operating hours while achieving energy efficiencies above 60%.
Scale-up economics show promising trends, with projected cost reductions of 30-45% when moving from laboratory to industrial scale. However, these projections depend heavily on advances in electrode materials that can simultaneously address kinetics, selectivity, and durability. Current techno-economic models suggest that CO2 reduction systems will reach cost parity with conventional processes by 2028-2032, assuming continued technological improvements and supportive carbon pricing mechanisms in the range of $50-75 per ton of CO2.
Environmental Impact and Carbon Neutrality Implications
Electrocatalytic CO2 reduction represents a pivotal technology in addressing global climate change challenges. The environmental implications of this technology extend far beyond laboratory settings, offering tangible pathways toward carbon neutrality goals established under international frameworks such as the Paris Agreement. By converting waste CO2 into valuable chemicals and fuels, this process creates a circular carbon economy that significantly reduces net emissions.
The implementation of efficient electrocatalytic CO2 reduction systems could potentially mitigate gigatons of CO2 emissions annually when deployed at industrial scale. Current estimates suggest that optimized systems could achieve carbon reduction efficiencies of 30-45% compared to traditional fossil fuel-based production methods for the same chemical products. This represents a substantial contribution to national and corporate carbon neutrality pledges, particularly in hard-to-abate sectors like chemical manufacturing and heavy industry.
Life cycle assessments of electrocatalytic CO2 reduction technologies reveal complex environmental trade-offs. While the direct process reduces atmospheric CO2, considerations must include the source of electricity powering these systems. Maximum environmental benefits occur when renewable energy sources drive the electrocatalytic process, creating a truly carbon-negative technology pathway. Integration with intermittent renewable energy sources also provides grid stabilization benefits, addressing a critical challenge in renewable energy deployment.
Water usage represents another important environmental consideration, as most electrocatalytic systems require purified water inputs. Advanced catalyst designs that function effectively in seawater or wastewater could significantly enhance the sustainability profile of this technology. Similarly, the environmental footprint of catalyst materials—particularly those utilizing rare earth elements or precious metals—requires careful evaluation against their carbon reduction benefits.
From a policy perspective, electrocatalytic CO2 reduction aligns with emerging carbon pricing mechanisms and regulatory frameworks. As carbon taxes and cap-and-trade systems expand globally, the economic case for these technologies strengthens considerably. The technology also supports sustainable development goals beyond climate action, including clean energy access, responsible consumption, and industrial innovation.
Long-term environmental modeling indicates that widespread adoption of electrocatalytic CO2 reduction could contribute 5-8% of required emissions reductions needed to limit global warming to 1.5°C by 2050. This positions the technology as an essential component of a diversified climate mitigation portfolio, complementing direct emissions reductions and natural carbon sinks.
The implementation of efficient electrocatalytic CO2 reduction systems could potentially mitigate gigatons of CO2 emissions annually when deployed at industrial scale. Current estimates suggest that optimized systems could achieve carbon reduction efficiencies of 30-45% compared to traditional fossil fuel-based production methods for the same chemical products. This represents a substantial contribution to national and corporate carbon neutrality pledges, particularly in hard-to-abate sectors like chemical manufacturing and heavy industry.
Life cycle assessments of electrocatalytic CO2 reduction technologies reveal complex environmental trade-offs. While the direct process reduces atmospheric CO2, considerations must include the source of electricity powering these systems. Maximum environmental benefits occur when renewable energy sources drive the electrocatalytic process, creating a truly carbon-negative technology pathway. Integration with intermittent renewable energy sources also provides grid stabilization benefits, addressing a critical challenge in renewable energy deployment.
Water usage represents another important environmental consideration, as most electrocatalytic systems require purified water inputs. Advanced catalyst designs that function effectively in seawater or wastewater could significantly enhance the sustainability profile of this technology. Similarly, the environmental footprint of catalyst materials—particularly those utilizing rare earth elements or precious metals—requires careful evaluation against their carbon reduction benefits.
From a policy perspective, electrocatalytic CO2 reduction aligns with emerging carbon pricing mechanisms and regulatory frameworks. As carbon taxes and cap-and-trade systems expand globally, the economic case for these technologies strengthens considerably. The technology also supports sustainable development goals beyond climate action, including clean energy access, responsible consumption, and industrial innovation.
Long-term environmental modeling indicates that widespread adoption of electrocatalytic CO2 reduction could contribute 5-8% of required emissions reductions needed to limit global warming to 1.5°C by 2050. This positions the technology as an essential component of a diversified climate mitigation portfolio, complementing direct emissions reductions and natural carbon sinks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!