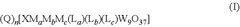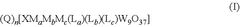Comparative evaluation of Electrocatalytic CO2 reduction thermal and electrochemical properties
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Electrocatalysis Background and Objectives
Electrocatalytic CO2 reduction represents a promising approach to address the global challenge of rising carbon dioxide levels while simultaneously producing valuable chemicals and fuels. The technology has evolved significantly since its initial conceptualization in the 1980s, with major breakthroughs occurring in catalyst design, reaction mechanisms understanding, and system integration over the past decade. The field has witnessed an exponential growth in research publications, particularly since 2015, indicating its increasing importance in the scientific community and industrial sectors.
The thermal and electrochemical properties of catalysts play crucial roles in determining the efficiency, selectivity, and stability of CO2 reduction reactions. Historically, research has focused primarily on noble metal catalysts such as gold, silver, and platinum due to their excellent conductivity and catalytic activity. However, recent trends show a shift toward earth-abundant materials including copper, zinc, nickel-based catalysts, and carbon-supported materials to address cost and sustainability concerns.
Current technological trajectories indicate a convergence of materials science, electrochemistry, and computational modeling to develop next-generation catalysts with precisely controlled thermal and electrochemical properties. The integration of in-situ characterization techniques has enabled deeper insights into reaction mechanisms and catalyst behavior under operating conditions, accelerating the rational design of more efficient systems.
The primary objectives of comparative evaluation in this field include establishing standardized protocols for assessing catalyst performance, identifying structure-property-performance relationships, and developing predictive models for catalyst behavior under various operating conditions. Additionally, there is growing interest in understanding how thermal management affects electrochemical performance, as temperature fluctuations can significantly impact reaction kinetics, selectivity, and catalyst stability.
From an industrial perspective, the goals extend to scaling up laboratory discoveries to commercially viable technologies, reducing energy inputs, enhancing product selectivity, and developing catalysts with prolonged operational lifetimes. The economic viability of electrocatalytic CO2 reduction heavily depends on achieving high Faradaic efficiencies, appropriate current densities, and minimizing degradation mechanisms.
Looking forward, the field aims to achieve multi-carbon product selectivity exceeding 90%, current densities above 200 mA/cm², and catalyst stability beyond 1000 hours of continuous operation. These ambitious targets necessitate comprehensive understanding of the interplay between thermal properties (heat capacity, thermal conductivity, temperature-dependent activity) and electrochemical characteristics (overpotential, exchange current density, Tafel slope) of catalyst materials.
The thermal and electrochemical properties of catalysts play crucial roles in determining the efficiency, selectivity, and stability of CO2 reduction reactions. Historically, research has focused primarily on noble metal catalysts such as gold, silver, and platinum due to their excellent conductivity and catalytic activity. However, recent trends show a shift toward earth-abundant materials including copper, zinc, nickel-based catalysts, and carbon-supported materials to address cost and sustainability concerns.
Current technological trajectories indicate a convergence of materials science, electrochemistry, and computational modeling to develop next-generation catalysts with precisely controlled thermal and electrochemical properties. The integration of in-situ characterization techniques has enabled deeper insights into reaction mechanisms and catalyst behavior under operating conditions, accelerating the rational design of more efficient systems.
The primary objectives of comparative evaluation in this field include establishing standardized protocols for assessing catalyst performance, identifying structure-property-performance relationships, and developing predictive models for catalyst behavior under various operating conditions. Additionally, there is growing interest in understanding how thermal management affects electrochemical performance, as temperature fluctuations can significantly impact reaction kinetics, selectivity, and catalyst stability.
From an industrial perspective, the goals extend to scaling up laboratory discoveries to commercially viable technologies, reducing energy inputs, enhancing product selectivity, and developing catalysts with prolonged operational lifetimes. The economic viability of electrocatalytic CO2 reduction heavily depends on achieving high Faradaic efficiencies, appropriate current densities, and minimizing degradation mechanisms.
Looking forward, the field aims to achieve multi-carbon product selectivity exceeding 90%, current densities above 200 mA/cm², and catalyst stability beyond 1000 hours of continuous operation. These ambitious targets necessitate comprehensive understanding of the interplay between thermal properties (heat capacity, thermal conductivity, temperature-dependent activity) and electrochemical characteristics (overpotential, exchange current density, Tafel slope) of catalyst materials.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.9 billion by 2030, growing at a CAGR of 13.3% during the forecast period. This growth trajectory is supported by substantial investments in research and development, as well as government initiatives promoting carbon capture and utilization technologies.
Electrocatalytic CO2 reduction represents a particularly promising segment within this market, with increasing commercial interest due to its potential for producing value-added chemicals and fuels from waste carbon dioxide. The market for electrocatalytic CO2 reduction specifically is expected to grow from $420 million in 2023 to over $1.2 billion by 2030, reflecting the technology's increasing maturity and commercial viability.
Regional analysis indicates that North America currently leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.7% annually, primarily driven by China's aggressive carbon neutrality targets and substantial investments in green technologies.
Key market drivers include stringent carbon emission regulations, increasing carbon pricing mechanisms, and growing corporate commitments to carbon neutrality. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for CO2 conversion technologies. Additionally, the declining costs of renewable electricity are improving the economic feasibility of electrocatalytic approaches.
Market segmentation reveals diverse applications across chemical manufacturing, fuel production, and mineralization processes. The chemical manufacturing segment currently dominates with 45% market share, while fuel production is expected to grow most rapidly at 16.2% annually through 2030.
End-user industries showing the strongest demand include petrochemicals, cement, steel, and power generation. These carbon-intensive sectors are increasingly investing in CO2 conversion technologies to reduce their carbon footprint and comply with emissions regulations.
Challenges limiting market growth include high capital costs, energy efficiency concerns, and scalability issues. The average capital expenditure for commercial-scale electrocatalytic CO2 reduction facilities remains high at $800-1,200 per ton of CO2 processing capacity, though this represents a 30% decrease from 2018 levels.
Electrocatalytic CO2 reduction represents a particularly promising segment within this market, with increasing commercial interest due to its potential for producing value-added chemicals and fuels from waste carbon dioxide. The market for electrocatalytic CO2 reduction specifically is expected to grow from $420 million in 2023 to over $1.2 billion by 2030, reflecting the technology's increasing maturity and commercial viability.
Regional analysis indicates that North America currently leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.7% annually, primarily driven by China's aggressive carbon neutrality targets and substantial investments in green technologies.
Key market drivers include stringent carbon emission regulations, increasing carbon pricing mechanisms, and growing corporate commitments to carbon neutrality. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for CO2 conversion technologies. Additionally, the declining costs of renewable electricity are improving the economic feasibility of electrocatalytic approaches.
Market segmentation reveals diverse applications across chemical manufacturing, fuel production, and mineralization processes. The chemical manufacturing segment currently dominates with 45% market share, while fuel production is expected to grow most rapidly at 16.2% annually through 2030.
End-user industries showing the strongest demand include petrochemicals, cement, steel, and power generation. These carbon-intensive sectors are increasingly investing in CO2 conversion technologies to reduce their carbon footprint and comply with emissions regulations.
Challenges limiting market growth include high capital costs, energy efficiency concerns, and scalability issues. The average capital expenditure for commercial-scale electrocatalytic CO2 reduction facilities remains high at $800-1,200 per ton of CO2 processing capacity, though this represents a 30% decrease from 2018 levels.
Current Challenges in Electrocatalytic CO2 Reduction
Despite significant advancements in electrocatalytic CO2 reduction technology, several critical challenges continue to impede its widespread implementation and commercial viability. The comparative evaluation of thermal and electrochemical properties reveals fundamental limitations that researchers worldwide are actively addressing.
One of the primary challenges lies in catalyst selectivity and efficiency. Current catalysts often produce a mixture of products rather than selectively generating high-value chemicals, resulting in costly separation processes. The Faradaic efficiency for desired products rarely exceeds 80% under industrially relevant conditions, with competing hydrogen evolution reactions consuming significant energy without contributing to carbon conversion.
Catalyst stability presents another formidable obstacle. Many promising materials exhibit performance degradation during extended operation periods, particularly when exposed to the complex chemical environment of CO2 reduction. Copper-based catalysts, while showing excellent product diversity, suffer from surface reconstruction and poisoning effects that alter their selectivity over time. Noble metal catalysts demonstrate better stability but face economic constraints for large-scale deployment.
The energy efficiency of the overall process remains suboptimal, with high overpotentials required to drive CO2 reduction reactions. This translates to excessive energy consumption, undermining the sustainability benefits of the technology. Current systems typically operate at energy efficiencies below 50%, far from the theoretical maximum and insufficient for economic viability without substantial subsidies.
Mass transport limitations significantly affect reaction kinetics and product distribution. The low solubility of CO2 in aqueous electrolytes (approximately 33 mM at ambient conditions) creates concentration gradients near catalyst surfaces, limiting reaction rates and favoring hydrogen evolution. Advanced gas diffusion electrode designs have partially addressed this issue but introduce additional complexity and durability concerns.
Scale-up challenges persist as laboratory-scale successes often fail to translate to industrial settings. The thermal management of electrochemical cells becomes increasingly problematic at larger scales, affecting reaction selectivity and efficiency. Additionally, the integration of renewable electricity sources introduces variability that current catalyst systems are not optimized to handle.
Mechanistic understanding remains incomplete, hampering rational catalyst design. The complex interplay between thermal effects and electrochemical properties during CO2 reduction is particularly poorly understood. Reaction intermediates and pathways vary significantly with operating conditions, electrode potential, and local pH, making predictive catalyst development challenging.
Standardization of testing protocols and performance metrics represents another significant challenge, complicating direct comparisons between different research efforts and slowing collective progress in the field. The diversity of reactor designs, operating conditions, and analytical methods creates a fragmented knowledge landscape that impedes systematic advancement.
One of the primary challenges lies in catalyst selectivity and efficiency. Current catalysts often produce a mixture of products rather than selectively generating high-value chemicals, resulting in costly separation processes. The Faradaic efficiency for desired products rarely exceeds 80% under industrially relevant conditions, with competing hydrogen evolution reactions consuming significant energy without contributing to carbon conversion.
Catalyst stability presents another formidable obstacle. Many promising materials exhibit performance degradation during extended operation periods, particularly when exposed to the complex chemical environment of CO2 reduction. Copper-based catalysts, while showing excellent product diversity, suffer from surface reconstruction and poisoning effects that alter their selectivity over time. Noble metal catalysts demonstrate better stability but face economic constraints for large-scale deployment.
The energy efficiency of the overall process remains suboptimal, with high overpotentials required to drive CO2 reduction reactions. This translates to excessive energy consumption, undermining the sustainability benefits of the technology. Current systems typically operate at energy efficiencies below 50%, far from the theoretical maximum and insufficient for economic viability without substantial subsidies.
Mass transport limitations significantly affect reaction kinetics and product distribution. The low solubility of CO2 in aqueous electrolytes (approximately 33 mM at ambient conditions) creates concentration gradients near catalyst surfaces, limiting reaction rates and favoring hydrogen evolution. Advanced gas diffusion electrode designs have partially addressed this issue but introduce additional complexity and durability concerns.
Scale-up challenges persist as laboratory-scale successes often fail to translate to industrial settings. The thermal management of electrochemical cells becomes increasingly problematic at larger scales, affecting reaction selectivity and efficiency. Additionally, the integration of renewable electricity sources introduces variability that current catalyst systems are not optimized to handle.
Mechanistic understanding remains incomplete, hampering rational catalyst design. The complex interplay between thermal effects and electrochemical properties during CO2 reduction is particularly poorly understood. Reaction intermediates and pathways vary significantly with operating conditions, electrode potential, and local pH, making predictive catalyst development challenging.
Standardization of testing protocols and performance metrics represents another significant challenge, complicating direct comparisons between different research efforts and slowing collective progress in the field. The diversity of reactor designs, operating conditions, and analytical methods creates a fragmented knowledge landscape that impedes systematic advancement.
Comparative Analysis of Current Electrocatalytic Methods
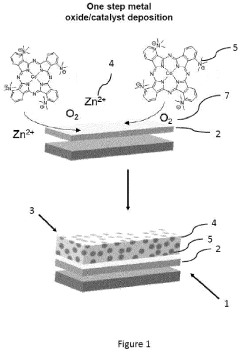
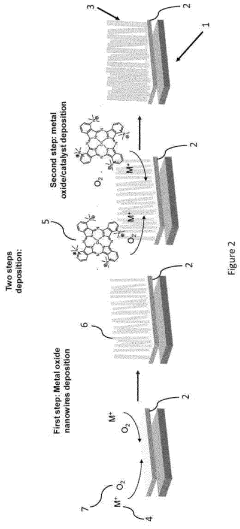
01 Catalyst materials for electrocatalytic CO2 reduction
Various catalyst materials can be used for electrocatalytic CO2 reduction, including metal-based catalysts, metal oxides, and composite materials. These catalysts facilitate the conversion of CO2 into valuable products such as carbon monoxide, formate, or hydrocarbons. The selection of catalyst material significantly impacts the efficiency, selectivity, and stability of the CO2 reduction process. Catalyst design focuses on optimizing active sites, surface area, and electronic properties to enhance catalytic performance.- Catalyst materials for electrocatalytic CO2 reduction: Various catalyst materials can be used for electrocatalytic CO2 reduction, including metal-based catalysts, metal oxides, and composite materials. These catalysts facilitate the conversion of CO2 to valuable products such as carbon monoxide, formic acid, or hydrocarbons. The choice of catalyst material significantly affects the efficiency, selectivity, and stability of the CO2 reduction process, with different materials showing varying thermal and electrochemical properties.
- Electrode design and structure for enhanced CO2 reduction: The design and structure of electrodes play a crucial role in electrocatalytic CO2 reduction. Factors such as porosity, surface area, and morphology affect the catalytic performance. Nanostructured electrodes, hierarchical structures, and supported catalysts can enhance the contact between the catalyst and CO2 molecules, improving reaction kinetics and thermal stability. Optimized electrode designs can also facilitate mass transport and electron transfer during the electrochemical process.
- Electrolyte composition and reaction conditions: The composition of the electrolyte and reaction conditions significantly influence the thermal and electrochemical properties of CO2 reduction systems. Parameters such as pH, temperature, pressure, and electrolyte concentration affect the reaction pathways and product selectivity. Ionic liquids, aqueous solutions with specific additives, and buffer systems can be used to optimize the electrochemical environment, enhancing stability and efficiency while managing heat generation during the reaction.
- Reactor design and system integration: The design of reactors and integration of system components are essential for efficient electrocatalytic CO2 reduction. Considerations include heat management, gas diffusion layers, flow field designs, and separation systems for products. Advanced reactor configurations can improve mass transfer, thermal management, and energy efficiency. Integrated systems that combine CO2 capture with electrochemical reduction offer advantages for industrial applications, with careful attention to thermal stability and electrochemical performance.
- Performance enhancement and stability strategies: Various strategies can be employed to enhance the performance and stability of electrocatalytic CO2 reduction systems. These include surface modification of catalysts, incorporation of promoters or co-catalysts, development of protective coatings, and optimization of operational parameters. Addressing challenges such as catalyst deactivation, electrode fouling, and thermal degradation is crucial for long-term operation. Advanced characterization techniques help understand the relationship between thermal properties and electrochemical performance for continuous improvement.
02 Thermal stability and temperature effects on CO2 reduction
The thermal properties of electrocatalysts play a crucial role in CO2 reduction performance. Temperature affects reaction kinetics, catalyst stability, and product selectivity. Higher temperatures can enhance reaction rates but may reduce selectivity or cause catalyst degradation. Thermal management systems are essential for maintaining optimal operating conditions and preventing thermal runaway. Understanding the thermal behavior of catalysts under reaction conditions is vital for designing efficient and stable CO2 reduction systems.Expand Specific Solutions03 Electrochemical properties and reaction mechanisms
The electrochemical properties of catalysts determine their effectiveness in CO2 reduction. Key properties include conductivity, electron transfer capability, overpotential requirements, and faradaic efficiency. Understanding reaction mechanisms, including proton-coupled electron transfer processes and intermediate formation, is crucial for catalyst design. Electrochemical characterization techniques such as cyclic voltammetry, impedance spectroscopy, and chronoamperometry are used to evaluate catalyst performance and elucidate reaction pathways.Expand Specific Solutions04 Nanostructured catalysts and surface engineering
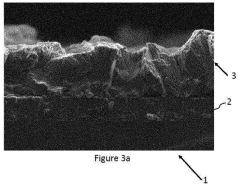
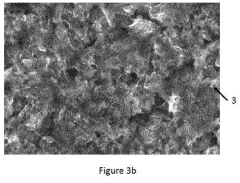
Nanostructured catalysts offer enhanced performance for CO2 reduction due to their high surface area, abundant active sites, and tunable properties. Surface engineering techniques, including defect creation, doping, and functionalization, can optimize catalyst activity and selectivity. Controlled synthesis methods enable precise manipulation of catalyst morphology, composition, and electronic structure. Nanostructured catalysts often demonstrate improved stability and efficiency compared to their bulk counterparts.Expand Specific Solutions05 Reactor design and system integration
Reactor design significantly impacts the performance of electrocatalytic CO2 reduction systems. Factors such as electrode configuration, electrolyte composition, mass transport, and gas diffusion affect conversion efficiency and product distribution. Advanced reactor designs incorporate features for enhanced CO2 dissolution, improved mass transfer, and efficient product separation. System integration considerations include coupling with renewable energy sources, heat management, and product recovery systems to create sustainable and economically viable CO2 conversion technologies.Expand Specific Solutions
Leading Research Groups and Companies in CO2 Electrocatalysis
The electrocatalytic CO2 reduction market is currently in an early growth phase, characterized by intensive R&D activities and emerging commercial applications. The global market size is projected to expand significantly as carbon utilization technologies gain traction in decarbonization efforts. Leading research institutions like Caltech, CNRS, and Kyushu University are advancing fundamental science, while industrial players such as Siemens, Topsoe, and Saudi Aramco are scaling up technologies toward commercialization. Technical maturity varies across approaches, with companies like Faraday Technology and Carbon Energy Technology (Beijing) focusing on electrochemical process optimization, while Siemens Energy and Samsung Electronics leverage their manufacturing capabilities to address system integration challenges. The competitive landscape reflects a blend of academic innovation and industrial implementation, with increasing collaboration between sectors to overcome efficiency and cost barriers.
California Institute of Technology
Technical Solution: Caltech has pioneered molecular-level approaches to electrocatalytic CO2 reduction, focusing on mechanistic understanding and catalyst design principles. Their research teams have developed novel transition metal complexes and hybrid materials that can selectively reduce CO2 to specific products like CO, formate, or methanol. A distinctive aspect of Caltech's approach is the integration of computational modeling with experimental validation, enabling prediction of catalytic activity based on electronic structure calculations. Their researchers have demonstrated the importance of local pH effects and mass transport phenomena in determining product selectivity. Caltech has also made significant advances in understanding the role of the electrode-electrolyte interface in CO2 reduction, developing specialized techniques to probe the double layer environment during catalysis. Their work has established fundamental correlations between catalyst structure, binding energies, and product distribution that guide rational catalyst design across the field.
Strengths: Exceptional fundamental understanding of reaction mechanisms; integration of computational and experimental approaches; innovative catalyst design principles. Weaknesses: Focus primarily on fundamental science rather than industrial applications; catalysts often require expensive noble metals; lower current densities compared to some industrial systems.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced electrocatalysts for CO2 reduction featuring copper-based materials with precisely controlled morphology and composition. Their approach involves synthesizing Cu nanomaterials with specific facets and introducing dopants to modify electronic properties. DICP researchers have achieved significant breakthroughs in selective CO2 conversion to multi-carbon products with Faradaic efficiencies exceeding 60% for C2+ products. Their technology employs flow-cell configurations that address mass transport limitations and enable higher current densities (>200 mA/cm²). The institute has also pioneered in-situ/operando characterization techniques to understand reaction mechanisms and catalyst degradation pathways during CO2 electroreduction, allowing for rational catalyst design based on structure-property relationships. DICP's integrated systems combine CO2 capture with electrochemical reduction, improving overall energy efficiency by eliminating intermediate separation steps.
Strengths: Superior selectivity toward valuable C2+ products; advanced in-situ characterization capabilities; integrated system approach combining capture and conversion. Weaknesses: Higher energy consumption compared to thermal processes; catalyst stability issues under industrial conditions; challenges in scaling up laboratory results to commercial applications.
Key Thermal and Electrochemical Property Innovations
Porous electrode with a catalytic activity towards co2 or co electrochemical and photo-electrochemical reduction
PatentPendingEP4227441A1
Innovation
- Development of porous electrodes with a conductive nanoporous catalytic matrix that encapsulates molecular catalysts within structured oxide nanolayers, enhancing catalytic activity and stability while using minimal molecular material, and allowing for easy tuning of the oxide and catalyst nature.
Electrochemical reduction of carbon dioxide catalyzed by polyoxometalates
PatentPendingUS20240287693A1
Innovation
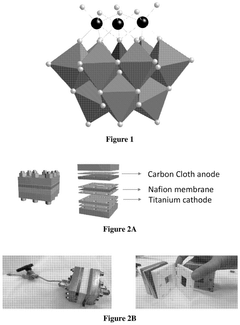
- The use of polyoxometalate compounds, specifically represented by the formula (Q)n[XMaMbMc(La)(Lb)(Lc)W9O37], as electrocatalysts for the reduction of CO2 to carbon monoxide, formate, formic acid, formaldehyde, methanol, ethane, or ethylene, which are thermally and oxidatively stable, and can facilitate proton-coupled electron transfer reactions with reduced overpotentials.
Environmental Impact and Sustainability Assessment
The environmental impact of electrocatalytic CO2 reduction technologies extends far beyond their immediate technical performance. These systems represent a promising approach to carbon capture and utilization, potentially transforming a greenhouse gas into valuable chemical feedstocks and fuels while reducing atmospheric carbon levels.
Life cycle assessment (LCA) studies of various electrocatalytic CO2 reduction systems reveal significant variations in environmental footprints. Systems utilizing renewable energy sources demonstrate substantially lower carbon emissions compared to those powered by conventional grid electricity. Research indicates that solar-powered electrocatalytic systems can achieve carbon neutrality or even negative emissions when operational efficiency exceeds certain thresholds, typically around 60-70% Faradaic efficiency for high-value products.
Water consumption presents another critical environmental consideration, particularly for large-scale implementation. Current electrocatalytic systems require substantial water inputs both for reaction media and cooling processes. Advanced membrane-based systems have shown potential to reduce water requirements by 30-45% compared to conventional setups, though further optimization remains necessary for water-stressed regions.
The sustainability of catalyst materials poses significant challenges, particularly regarding rare earth elements and precious metals commonly employed in high-performance catalysts. Recent advances in earth-abundant catalysts, including nitrogen-doped carbon structures and transition metal complexes, demonstrate promising electrochemical properties while reducing dependence on scarce resources. However, these alternatives typically exhibit lower thermal stability, necessitating careful operational parameter control.
Waste management considerations include spent catalyst disposal and electrolyte recycling. Copper-based catalysts, while effective for CO2 reduction, present potential environmental hazards if improperly disposed. Closed-loop electrolyte systems have demonstrated 85-95% recovery rates for key components, substantially reducing waste streams and environmental contamination risks.
Energy efficiency metrics reveal that current electrocatalytic CO2 reduction systems require 2-5 times more energy input than the theoretical minimum, highlighting significant room for improvement. Thermal management innovations, including waste heat recovery systems, have demonstrated potential to improve overall energy efficiency by 15-25% in laboratory settings, though commercial-scale implementation remains limited.
The scalability of these technologies will ultimately determine their environmental impact. Modular designs show promise for distributed implementation, potentially reducing transmission losses and infrastructure requirements while enabling gradual capacity expansion aligned with renewable energy availability.
Life cycle assessment (LCA) studies of various electrocatalytic CO2 reduction systems reveal significant variations in environmental footprints. Systems utilizing renewable energy sources demonstrate substantially lower carbon emissions compared to those powered by conventional grid electricity. Research indicates that solar-powered electrocatalytic systems can achieve carbon neutrality or even negative emissions when operational efficiency exceeds certain thresholds, typically around 60-70% Faradaic efficiency for high-value products.
Water consumption presents another critical environmental consideration, particularly for large-scale implementation. Current electrocatalytic systems require substantial water inputs both for reaction media and cooling processes. Advanced membrane-based systems have shown potential to reduce water requirements by 30-45% compared to conventional setups, though further optimization remains necessary for water-stressed regions.
The sustainability of catalyst materials poses significant challenges, particularly regarding rare earth elements and precious metals commonly employed in high-performance catalysts. Recent advances in earth-abundant catalysts, including nitrogen-doped carbon structures and transition metal complexes, demonstrate promising electrochemical properties while reducing dependence on scarce resources. However, these alternatives typically exhibit lower thermal stability, necessitating careful operational parameter control.
Waste management considerations include spent catalyst disposal and electrolyte recycling. Copper-based catalysts, while effective for CO2 reduction, present potential environmental hazards if improperly disposed. Closed-loop electrolyte systems have demonstrated 85-95% recovery rates for key components, substantially reducing waste streams and environmental contamination risks.
Energy efficiency metrics reveal that current electrocatalytic CO2 reduction systems require 2-5 times more energy input than the theoretical minimum, highlighting significant room for improvement. Thermal management innovations, including waste heat recovery systems, have demonstrated potential to improve overall energy efficiency by 15-25% in laboratory settings, though commercial-scale implementation remains limited.
The scalability of these technologies will ultimately determine their environmental impact. Modular designs show promise for distributed implementation, potentially reducing transmission losses and infrastructure requirements while enabling gradual capacity expansion aligned with renewable energy availability.
Techno-economic Analysis of CO2 Reduction Systems
The techno-economic analysis of CO2 reduction systems reveals significant challenges and opportunities in scaling these technologies for commercial viability. Current capital expenditure (CAPEX) for electrochemical CO2 reduction systems ranges from $1,500-3,000 per kilowatt, substantially higher than established technologies like water electrolysis ($500-1,000/kW). This cost differential primarily stems from the complexity of catalyst materials, specialized membranes, and precision control systems required for selective CO2 conversion.
Operating expenses (OPEX) present another critical economic consideration, with electricity consumption representing 60-75% of ongoing costs. Systems operating at ambient temperatures typically demonstrate energy efficiencies of 30-45%, while thermal-assisted variants can achieve 50-65% efficiency but require additional heating infrastructure. The economic viability threshold appears to be approximately $0.04-0.06/kWh for electricity pricing to enable competitive production of carbon-neutral fuels and chemicals.
Product selectivity significantly impacts economic returns, with high-value products like ethylene and ethanol offering theoretical profit margins 3-5 times greater than carbon monoxide or formate. However, achieving consistent selectivity above 80% remains technically challenging at industrially relevant current densities (>200 mA/cm²).
Sensitivity analysis indicates that catalyst durability represents the most influential factor in long-term economics. Current catalyst degradation rates of 1-2% per 1,000 operating hours must improve to <0.1% to achieve the 5+ year operational lifetimes necessary for favorable return on investment calculations.
Scale-up economics demonstrate promising trends, with modeled cost reductions of 30-40% achievable at megawatt-scale implementations through manufacturing optimization and economies of scale. However, these projections assume continued improvements in catalyst performance and membrane durability.
Integration with renewable energy sources presents both challenges and opportunities. While intermittent operation impacts system efficiency, modeling suggests that dedicated solar or wind installations with appropriate buffering could provide electricity at $0.03-0.05/kWh, potentially bringing total production costs below the critical threshold for market competitiveness against conventional petrochemical routes.
Operating expenses (OPEX) present another critical economic consideration, with electricity consumption representing 60-75% of ongoing costs. Systems operating at ambient temperatures typically demonstrate energy efficiencies of 30-45%, while thermal-assisted variants can achieve 50-65% efficiency but require additional heating infrastructure. The economic viability threshold appears to be approximately $0.04-0.06/kWh for electricity pricing to enable competitive production of carbon-neutral fuels and chemicals.
Product selectivity significantly impacts economic returns, with high-value products like ethylene and ethanol offering theoretical profit margins 3-5 times greater than carbon monoxide or formate. However, achieving consistent selectivity above 80% remains technically challenging at industrially relevant current densities (>200 mA/cm²).
Sensitivity analysis indicates that catalyst durability represents the most influential factor in long-term economics. Current catalyst degradation rates of 1-2% per 1,000 operating hours must improve to <0.1% to achieve the 5+ year operational lifetimes necessary for favorable return on investment calculations.
Scale-up economics demonstrate promising trends, with modeled cost reductions of 30-40% achievable at megawatt-scale implementations through manufacturing optimization and economies of scale. However, these projections assume continued improvements in catalyst performance and membrane durability.
Integration with renewable energy sources presents both challenges and opportunities. While intermittent operation impacts system efficiency, modeling suggests that dedicated solar or wind installations with appropriate buffering could provide electricity at $0.03-0.05/kWh, potentially bringing total production costs below the critical threshold for market competitiveness against conventional petrochemical routes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!