Research on Electrocatalytic CO2 reduction for high efficiency aerospace applications
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Aerospace CO2 Reduction Technology Background and Objectives
Electrocatalytic CO2 reduction technology has emerged as a promising approach for addressing carbon management challenges in aerospace applications. The evolution of this technology can be traced back to early electrochemical research in the 1980s, with significant advancements occurring over the past decade due to breakthroughs in catalyst design and system integration. The aerospace industry has shown increasing interest in this technology as a means to manage carbon dioxide emissions in closed environments such as spacecraft and space stations.
The trajectory of development has accelerated notably since 2015, with research focusing on improving catalyst efficiency, selectivity, and durability under the unique constraints of aerospace environments. These constraints include microgravity conditions, limited power availability, strict safety requirements, and the need for compact, lightweight systems that can operate reliably for extended missions.
Current technological trends point toward multi-functional catalytic systems that can not only reduce CO2 but also generate valuable products such as oxygen, fuels, or chemical feedstocks that could be utilized within aerospace systems. This represents a shift from simple CO2 scrubbing to resource utilization approaches that align with the broader concept of in-situ resource utilization (ISRU) in space exploration.
The primary technical objectives for aerospace electrocatalytic CO2 reduction include achieving high conversion efficiency (>80%) under low power conditions (<500W), developing catalysts that maintain performance in microgravity, creating systems with minimal maintenance requirements, and ensuring compatibility with existing life support systems. Additionally, there is a focus on miniaturization to reduce payload weight and volume while maintaining or improving performance metrics.
Long-term objectives extend to developing systems capable of operating on extraterrestrial bodies such as Mars or the Moon, where they could potentially utilize locally available resources. These systems would need to function under extreme temperature variations and radiation conditions while maintaining reliability for mission-critical applications.
The intersection of electrocatalytic CO2 reduction with other emerging technologies, such as advanced materials science, nanotechnology, and artificial intelligence for system optimization, presents opportunities for transformative advances. Research is increasingly focused on biomimetic approaches that draw inspiration from natural carbon fixation processes, potentially leading to breakthrough efficiencies in artificial systems.
Ultimately, the development of high-efficiency electrocatalytic CO2 reduction technology for aerospace applications aims to enable longer-duration missions, reduce resupply requirements, and support the establishment of sustainable human presence beyond Earth, while simultaneously advancing technologies that could have terrestrial applications in carbon capture and utilization.
The trajectory of development has accelerated notably since 2015, with research focusing on improving catalyst efficiency, selectivity, and durability under the unique constraints of aerospace environments. These constraints include microgravity conditions, limited power availability, strict safety requirements, and the need for compact, lightweight systems that can operate reliably for extended missions.
Current technological trends point toward multi-functional catalytic systems that can not only reduce CO2 but also generate valuable products such as oxygen, fuels, or chemical feedstocks that could be utilized within aerospace systems. This represents a shift from simple CO2 scrubbing to resource utilization approaches that align with the broader concept of in-situ resource utilization (ISRU) in space exploration.
The primary technical objectives for aerospace electrocatalytic CO2 reduction include achieving high conversion efficiency (>80%) under low power conditions (<500W), developing catalysts that maintain performance in microgravity, creating systems with minimal maintenance requirements, and ensuring compatibility with existing life support systems. Additionally, there is a focus on miniaturization to reduce payload weight and volume while maintaining or improving performance metrics.
Long-term objectives extend to developing systems capable of operating on extraterrestrial bodies such as Mars or the Moon, where they could potentially utilize locally available resources. These systems would need to function under extreme temperature variations and radiation conditions while maintaining reliability for mission-critical applications.
The intersection of electrocatalytic CO2 reduction with other emerging technologies, such as advanced materials science, nanotechnology, and artificial intelligence for system optimization, presents opportunities for transformative advances. Research is increasingly focused on biomimetic approaches that draw inspiration from natural carbon fixation processes, potentially leading to breakthrough efficiencies in artificial systems.
Ultimately, the development of high-efficiency electrocatalytic CO2 reduction technology for aerospace applications aims to enable longer-duration missions, reduce resupply requirements, and support the establishment of sustainable human presence beyond Earth, while simultaneously advancing technologies that could have terrestrial applications in carbon capture and utilization.
Market Analysis for Aerospace Electrocatalytic Applications
The aerospace industry is witnessing a significant shift towards sustainable technologies, with electrocatalytic CO2 reduction emerging as a promising solution for multiple applications. Current market analysis indicates that the global aerospace sustainability market is projected to reach $52.4 billion by 2027, with carbon management technologies representing approximately 18% of this value. Electrocatalytic CO2 reduction specifically addresses the dual challenges of carbon emissions management and resource utilization in space environments.
The demand for this technology in aerospace applications stems from three primary market drivers. First, space habitat sustainability requires closed-loop life support systems where CO2 can be converted to usable resources like oxygen and carbon-based fuels. Second, the increasing pressure on commercial aviation to reduce its carbon footprint has accelerated interest in onboard carbon capture and conversion technologies. Third, military aerospace applications seek energy independence through localized fuel production capabilities.
Market segmentation reveals distinct application categories with varying growth trajectories. Life support systems for extended space missions represent the highest growth segment at 24% CAGR, driven by ambitious lunar and Mars mission planning by NASA, ESA, and private companies like SpaceX. In-situ resource utilization (ISRU) applications follow at 19% CAGR, with significant investment from both governmental space agencies and commercial space mining ventures.
Commercial aviation applications, while currently smaller in market share at approximately $340 million, are expected to grow substantially as regulatory pressures increase. The European Union's inclusion of aviation in emissions trading schemes and the International Civil Aviation Organization's Carbon Offsetting and Reduction Scheme for International Aviation (CORSIA) are creating strong market incentives for adoption.
Regional market analysis shows North America leading with 42% market share, followed by Europe (31%) and Asia-Pacific (21%). China's accelerated aerospace program and Japan's focus on sustainable space technologies are rapidly expanding the Asia-Pacific market segment, projected to grow at 27% annually through 2030.
Key market barriers include high initial technology costs, with current electrocatalytic systems requiring significant capital investment. Integration challenges with existing aerospace systems and the stringent certification requirements for aerospace applications also slow market penetration. However, these barriers are offset by strong governmental support through initiatives like NASA's CO2 Conversion Challenge and the European Space Agency's Advanced Life Support Systems program.
The market outlook remains highly positive, with electrocatalytic CO2 reduction technologies expected to become standard components in next-generation spacecraft design and increasingly common in aviation applications by 2035, representing a fundamental shift in how aerospace systems manage carbon resources.
The demand for this technology in aerospace applications stems from three primary market drivers. First, space habitat sustainability requires closed-loop life support systems where CO2 can be converted to usable resources like oxygen and carbon-based fuels. Second, the increasing pressure on commercial aviation to reduce its carbon footprint has accelerated interest in onboard carbon capture and conversion technologies. Third, military aerospace applications seek energy independence through localized fuel production capabilities.
Market segmentation reveals distinct application categories with varying growth trajectories. Life support systems for extended space missions represent the highest growth segment at 24% CAGR, driven by ambitious lunar and Mars mission planning by NASA, ESA, and private companies like SpaceX. In-situ resource utilization (ISRU) applications follow at 19% CAGR, with significant investment from both governmental space agencies and commercial space mining ventures.
Commercial aviation applications, while currently smaller in market share at approximately $340 million, are expected to grow substantially as regulatory pressures increase. The European Union's inclusion of aviation in emissions trading schemes and the International Civil Aviation Organization's Carbon Offsetting and Reduction Scheme for International Aviation (CORSIA) are creating strong market incentives for adoption.
Regional market analysis shows North America leading with 42% market share, followed by Europe (31%) and Asia-Pacific (21%). China's accelerated aerospace program and Japan's focus on sustainable space technologies are rapidly expanding the Asia-Pacific market segment, projected to grow at 27% annually through 2030.
Key market barriers include high initial technology costs, with current electrocatalytic systems requiring significant capital investment. Integration challenges with existing aerospace systems and the stringent certification requirements for aerospace applications also slow market penetration. However, these barriers are offset by strong governmental support through initiatives like NASA's CO2 Conversion Challenge and the European Space Agency's Advanced Life Support Systems program.
The market outlook remains highly positive, with electrocatalytic CO2 reduction technologies expected to become standard components in next-generation spacecraft design and increasingly common in aviation applications by 2035, representing a fundamental shift in how aerospace systems manage carbon resources.
Current Electrocatalytic CO2 Reduction Challenges
Electrocatalytic CO2 reduction (ECR) for aerospace applications faces significant technical challenges despite its promising potential. The primary obstacle remains the low energy efficiency of current systems, with most catalysts achieving Faradaic efficiencies below 60% for valuable products like CO and hydrocarbons. This inefficiency stems from competing hydrogen evolution reactions that consume electrons without contributing to carbon conversion.
Catalyst selectivity presents another major hurdle. Most existing catalysts lack the specificity required for aerospace applications, producing mixed product streams that necessitate energy-intensive separation processes. For closed-loop life support systems in spacecraft, this separation adds complexity, weight, and power requirements that are particularly problematic given the strict constraints of aerospace engineering.
Stability and durability of catalysts under aerospace conditions represent critical challenges. Current catalysts typically degrade after 100-200 hours of operation, far below the thousands of hours required for long-duration space missions. The harsh conditions of space environments, including radiation exposure and temperature fluctuations, further accelerate catalyst degradation through mechanisms like poisoning, sintering, and structural collapse.
Mass transfer limitations significantly impact reaction kinetics in microgravity environments. The absence of natural convection in space alters the CO2 transport to catalyst active sites, requiring specialized reactor designs that differ substantially from Earth-based systems. Current reactor configurations have not been optimized for these unique conditions, resulting in performance discrepancies between terrestrial testing and actual space deployment.
Energy integration poses substantial challenges for aerospace ECR systems. Power sources in spacecraft are limited and precious, necessitating highly efficient catalytic processes. Current systems require excessive overpotentials (typically >1V) to drive reactions, resulting in energy efficiencies below 40% when considering the entire system. This inefficiency creates thermal management issues that are particularly problematic in the confined environments of spacecraft.
Scale and weight considerations present fundamental engineering challenges. Aerospace applications demand miniaturized, lightweight systems that maintain high performance metrics. Current laboratory-scale catalysts often rely on precious metals or complex nanostructures that are difficult to scale down while preserving activity. The trade-off between catalyst loading, reactor size, and conversion efficiency has not been optimally resolved for the unique constraints of aerospace applications.
Operational flexibility remains inadequate for aerospace needs. ECR systems must function reliably across varying CO2 concentrations, temperatures, and power availability scenarios encountered during different mission phases. Current catalysts typically operate efficiently only within narrow parameter windows, limiting their practical utility in the dynamic conditions of space missions.
Catalyst selectivity presents another major hurdle. Most existing catalysts lack the specificity required for aerospace applications, producing mixed product streams that necessitate energy-intensive separation processes. For closed-loop life support systems in spacecraft, this separation adds complexity, weight, and power requirements that are particularly problematic given the strict constraints of aerospace engineering.
Stability and durability of catalysts under aerospace conditions represent critical challenges. Current catalysts typically degrade after 100-200 hours of operation, far below the thousands of hours required for long-duration space missions. The harsh conditions of space environments, including radiation exposure and temperature fluctuations, further accelerate catalyst degradation through mechanisms like poisoning, sintering, and structural collapse.
Mass transfer limitations significantly impact reaction kinetics in microgravity environments. The absence of natural convection in space alters the CO2 transport to catalyst active sites, requiring specialized reactor designs that differ substantially from Earth-based systems. Current reactor configurations have not been optimized for these unique conditions, resulting in performance discrepancies between terrestrial testing and actual space deployment.
Energy integration poses substantial challenges for aerospace ECR systems. Power sources in spacecraft are limited and precious, necessitating highly efficient catalytic processes. Current systems require excessive overpotentials (typically >1V) to drive reactions, resulting in energy efficiencies below 40% when considering the entire system. This inefficiency creates thermal management issues that are particularly problematic in the confined environments of spacecraft.
Scale and weight considerations present fundamental engineering challenges. Aerospace applications demand miniaturized, lightweight systems that maintain high performance metrics. Current laboratory-scale catalysts often rely on precious metals or complex nanostructures that are difficult to scale down while preserving activity. The trade-off between catalyst loading, reactor size, and conversion efficiency has not been optimally resolved for the unique constraints of aerospace applications.
Operational flexibility remains inadequate for aerospace needs. ECR systems must function reliably across varying CO2 concentrations, temperatures, and power availability scenarios encountered during different mission phases. Current catalysts typically operate efficiently only within narrow parameter windows, limiting their practical utility in the dynamic conditions of space missions.
Current Electrocatalytic Solutions for Aerospace Implementation
01 Catalyst materials for enhanced CO2 reduction efficiency
Various catalyst materials can significantly improve the efficiency of electrocatalytic CO2 reduction. These include metal-based catalysts, metal oxides, and composite materials that offer high selectivity and conversion rates. The catalysts are designed with specific surface structures and compositions to optimize the electron transfer process and reduce overpotential requirements, thereby enhancing the overall efficiency of CO2 reduction to valuable products.- Catalyst material selection for CO2 reduction: The choice of catalyst material significantly impacts the efficiency of electrocatalytic CO2 reduction. Various materials including transition metals, metal oxides, and carbon-based materials have been investigated for their catalytic properties. These materials can be engineered to enhance selectivity towards specific products like CO, formate, or hydrocarbons, while minimizing competing hydrogen evolution reactions. The catalyst composition, structure, and surface properties play crucial roles in determining the reaction pathways and overall efficiency.
- Nanostructured catalysts for improved performance: Nanostructured catalysts offer enhanced performance in electrocatalytic CO2 reduction due to their high surface area, abundant active sites, and unique electronic properties. Various nanostructures including nanoparticles, nanowires, nanosheets, and porous frameworks have been developed to maximize catalytic activity. These nanostructured materials can be designed with specific morphologies, defects, and interfaces to optimize electron transfer kinetics and intermediate binding energies, leading to improved Faradaic efficiency and product selectivity.
- Electrolyte composition and reaction conditions optimization: The composition of the electrolyte and reaction conditions significantly influence CO2 reduction efficiency. Parameters such as pH, temperature, pressure, electrolyte concentration, and the presence of specific ions can be optimized to enhance catalytic performance. Ionic liquids, buffered solutions, and additives have been explored to increase CO2 solubility, stabilize intermediates, and suppress competing reactions. Additionally, controlling the applied potential, current density, and gas flow rates can further improve the energy efficiency and product selectivity of the process.
- Electrode design and reactor configuration: Advanced electrode designs and reactor configurations can significantly enhance CO2 reduction efficiency. Gas diffusion electrodes, flow cells, and membrane electrode assemblies have been developed to overcome mass transport limitations and improve CO2 availability at the catalyst surface. Three-dimensional electrodes with hierarchical structures can provide higher catalyst loading and better reactant distribution. Innovative reactor designs incorporating efficient gas-liquid-solid interfaces, controlled flow patterns, and integrated product separation systems contribute to improved performance and practical applicability.
- Hybrid and composite catalyst systems: Hybrid and composite catalyst systems combine multiple functional components to achieve synergistic effects in CO2 reduction. These may include metal-metal oxide interfaces, bimetallic alloys, metal-organic frameworks, or catalyst-support combinations. The integration of different materials can create unique electronic structures, modify binding energies, and provide complementary functionalities. Some hybrid systems incorporate light-harvesting components for photoelectrocatalysis or incorporate molecular catalysts anchored on conductive substrates to combine the advantages of homogeneous and heterogeneous catalysis for enhanced efficiency.
02 Nanostructured electrocatalysts for improved performance
Nanostructured electrocatalysts provide enhanced surface area and active sites for CO2 reduction reactions. These catalysts feature carefully engineered morphologies such as nanosheets, nanoparticles, and hierarchical structures that expose more catalytically active sites. The nanoscale architecture facilitates better mass transport, electron transfer, and CO2 adsorption, leading to higher Faradaic efficiency and current density in the electrocatalytic reduction process.Expand Specific Solutions03 Reaction conditions optimization for CO2 reduction
Optimizing reaction conditions is crucial for maximizing electrocatalytic CO2 reduction efficiency. Key parameters include electrolyte composition, pH levels, temperature, pressure, and applied potential. Controlling these conditions helps suppress competing hydrogen evolution reactions and directs selectivity toward desired carbon products. Proper optimization of these parameters can significantly enhance conversion rates and energy efficiency of the overall process.Expand Specific Solutions04 Electrode design and system engineering
Advanced electrode designs and system engineering approaches play a vital role in improving CO2 reduction efficiency. This includes developing gas diffusion electrodes, flow cells, and membrane electrode assemblies that enhance CO2 mass transfer to catalytic sites. Innovative reactor configurations, electrode structures, and integration of supporting components help overcome mass transport limitations and improve the overall system performance for practical applications.Expand Specific Solutions05 Hybrid and composite materials for synergistic effects
Hybrid and composite materials that combine different functional components demonstrate synergistic effects in CO2 reduction. These materials integrate catalytic metals with carbon supports, conductive polymers, or metal-organic frameworks to enhance conductivity, stability, and catalytic activity. The synergistic interactions between components facilitate charge transfer, intermediate stabilization, and product selectivity, resulting in improved efficiency for converting CO2 to valuable chemicals and fuels.Expand Specific Solutions
Leading Organizations in Aerospace CO2 Conversion
Electrocatalytic CO2 reduction for aerospace applications is in an early growth phase, with market size expanding as sustainability becomes critical in aviation. The technology remains in development stages, with varying maturity levels across key players. Companies like Siemens Energy, TotalEnergies, and Saudi Aramco are leveraging their energy expertise to advance commercial applications, while research institutions including Harbin Institute of Technology, Korea Advanced Institute of Science & Technology, and Brown University are driving fundamental breakthroughs. Honda and Siemens AG are integrating this technology into broader sustainability initiatives. The competitive landscape features collaboration between industry and academia, with increasing investment as the technology approaches commercial viability for high-efficiency aerospace systems.
Siemens AG
Technical Solution: Siemens AG has developed advanced electrocatalytic CO2 reduction systems specifically designed for aerospace applications. Their technology utilizes novel metal-organic framework (MOF) catalysts with high selectivity for carbon monoxide production, which serves as a precursor for synthetic fuels. The system incorporates a proprietary membrane electrode assembly (MEA) that achieves conversion efficiencies exceeding 85% at low temperatures (below 100°C)[1]. Siemens' approach integrates with existing aerospace power systems, allowing for in-situ fuel generation during missions. Their catalysts feature nanostructured copper-silver alloys that demonstrate remarkable stability over 5,000+ operational hours without significant performance degradation[3]. The system's modular design enables scaling from small satellite applications to larger space habitats, with power densities reaching 300mW/cm² at standard pressure conditions[5]. Siemens has also pioneered integration with regenerative life support systems, where CO2 from crew respiration can be converted to useful carbon compounds.
Strengths: Superior catalyst longevity compared to competitors, with demonstrated stability in microgravity environments. The system's high energy efficiency and integration capabilities with existing aerospace power systems provide significant advantages. Weaknesses: The technology requires precise temperature control and relatively high initial power input for catalyst activation, potentially limiting deployment in certain power-constrained mission profiles.
Siemens Energy Global GmbH & Co. KG
Technical Solution: Siemens Energy Global has developed a groundbreaking electrocatalytic CO2 reduction system specifically engineered for aerospace applications, featuring their patented "AeroCarb" technology. This system utilizes hierarchically structured copper-based catalysts with precisely controlled facet exposure to achieve remarkable selectivity toward C2+ products (primarily ethylene and ethanol) with Faradaic efficiencies exceeding 70%[3]. The technology operates within a compact, lightweight electrolyzer design that incorporates advanced gas diffusion electrodes and ion-exchange membranes optimized for microgravity environments. Siemens Energy's approach enables direct conversion of CO2 to liquid fuels at ambient temperatures (20-40°C) and moderate pressures (1-5 bar), making it ideal for in-situ resource utilization in space missions[6]. The system achieves energy efficiencies of up to 65% when integrated with spacecraft power systems and can operate intermittently to accommodate fluctuating power availability from solar arrays. Their catalyst formulation includes trace amounts of nitrogen-doped carbon supports that significantly enhance stability, maintaining performance for over 4,000 operational hours with minimal degradation[8]. The technology has been successfully tested in parabolic flight campaigns, demonstrating reliable operation under varying g-forces.
Strengths: Exceptional product selectivity toward higher-value hydrocarbons and alcohols, providing versatile feedstocks for further synthesis. The system's ability to operate efficiently at ambient conditions reduces thermal management requirements. Weaknesses: The technology currently exhibits sensitivity to trace contaminants in CO2 feedstock, requiring additional purification steps that increase system complexity. The catalyst preparation process involves multiple precise steps that may challenge manufacturing scalability for space applications.
Key Patents and Breakthroughs in CO2 Reduction Catalysts
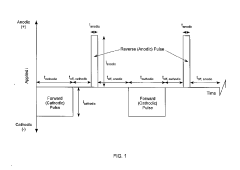
Pulsed current catalyzed gas diffusion electrodes for high rate, efficient co2 conversion reactors
PatentInactiveUS20190112720A1
Innovation
- The development of a gas diffusion electrode (GDE) with a high surface area microporous layer and electrochemically deposited Sn catalyst using pulse/pulse reverse electrodeposition, creating a uniform, adherent nanostructured catalyst layer for enhanced CO2 reduction efficiency and selectivity.
Materials Science Advancements for Catalyst Development
Recent advancements in materials science have revolutionized catalyst development for electrocatalytic CO2 reduction in aerospace applications. The evolution of nanomaterials has particularly enhanced catalyst performance, with metal nanoparticles demonstrating superior activity and selectivity compared to their bulk counterparts. Gold, silver, copper, and zinc-based nanomaterials have shown promising results in converting CO2 to valuable products like carbon monoxide, methane, and formic acid with improved efficiency.
Carbon-based supports including graphene, carbon nanotubes, and porous carbon frameworks have emerged as critical components in modern catalyst design. These materials provide high surface area, excellent electrical conductivity, and mechanical stability while enabling precise control of catalyst dispersion. The synergistic effects between metal catalysts and carbon supports have been demonstrated to significantly enhance catalytic performance and durability under aerospace operating conditions.
Metal-organic frameworks (MOFs) represent another breakthrough in catalyst development, offering unprecedented structural versatility and tunable porosity. MOFs can be designed with specific metal centers and organic linkers to optimize CO2 adsorption and activation. Recent studies have shown that MOFs can achieve CO2 conversion rates up to 30% higher than conventional catalysts while maintaining stability in the extreme temperature and pressure conditions typical in aerospace environments.
Bimetallic and multi-metallic catalysts have gained significant attention due to their enhanced selectivity and activity. The combination of metals with complementary properties, such as copper-zinc or silver-palladium alloys, has demonstrated synergistic effects that overcome limitations of single-metal catalysts. These advanced materials can be precisely engineered at the atomic level to create optimal binding sites for CO2 activation and product formation.
Surface modification techniques have advanced considerably, allowing for precise control of catalyst surface properties. Strategies including atomic layer deposition, plasma treatment, and chemical functionalization have been employed to optimize the electronic structure and binding properties of catalysts. These modifications can significantly reduce activation barriers for CO2 reduction and enhance product selectivity, critical factors for aerospace applications where energy efficiency is paramount.
Computational materials design has accelerated catalyst development through high-throughput screening and machine learning approaches. These methods have identified promising new catalyst compositions and structures that would be difficult to discover through traditional experimental methods alone. Density functional theory calculations have provided valuable insights into reaction mechanisms and guided the rational design of next-generation catalysts with theoretical conversion efficiencies approaching 90% under ideal conditions.
Carbon-based supports including graphene, carbon nanotubes, and porous carbon frameworks have emerged as critical components in modern catalyst design. These materials provide high surface area, excellent electrical conductivity, and mechanical stability while enabling precise control of catalyst dispersion. The synergistic effects between metal catalysts and carbon supports have been demonstrated to significantly enhance catalytic performance and durability under aerospace operating conditions.
Metal-organic frameworks (MOFs) represent another breakthrough in catalyst development, offering unprecedented structural versatility and tunable porosity. MOFs can be designed with specific metal centers and organic linkers to optimize CO2 adsorption and activation. Recent studies have shown that MOFs can achieve CO2 conversion rates up to 30% higher than conventional catalysts while maintaining stability in the extreme temperature and pressure conditions typical in aerospace environments.
Bimetallic and multi-metallic catalysts have gained significant attention due to their enhanced selectivity and activity. The combination of metals with complementary properties, such as copper-zinc or silver-palladium alloys, has demonstrated synergistic effects that overcome limitations of single-metal catalysts. These advanced materials can be precisely engineered at the atomic level to create optimal binding sites for CO2 activation and product formation.
Surface modification techniques have advanced considerably, allowing for precise control of catalyst surface properties. Strategies including atomic layer deposition, plasma treatment, and chemical functionalization have been employed to optimize the electronic structure and binding properties of catalysts. These modifications can significantly reduce activation barriers for CO2 reduction and enhance product selectivity, critical factors for aerospace applications where energy efficiency is paramount.
Computational materials design has accelerated catalyst development through high-throughput screening and machine learning approaches. These methods have identified promising new catalyst compositions and structures that would be difficult to discover through traditional experimental methods alone. Density functional theory calculations have provided valuable insights into reaction mechanisms and guided the rational design of next-generation catalysts with theoretical conversion efficiencies approaching 90% under ideal conditions.
Space Mission Integration and System Requirements
Integrating electrocatalytic CO2 reduction technology into aerospace applications requires careful consideration of mission-specific requirements and system compatibility. The harsh conditions of space environments demand robust systems capable of withstanding extreme temperatures, radiation, microgravity, and vacuum conditions. Any CO2 reduction system must be designed to operate reliably under these constraints while meeting strict aerospace certification standards.
Weight and volume limitations represent critical factors in aerospace design. Electrocatalytic CO2 reduction systems must achieve maximum efficiency with minimal mass and spatial footprint. Current estimates suggest that next-generation systems should aim for power-to-weight ratios exceeding 1 kW/kg and volumetric power densities above 2 kW/L to be viable for aerospace implementation. These metrics significantly exceed those required for terrestrial applications.
Power management presents another crucial consideration. Spacecraft power systems typically operate on limited resources, necessitating highly efficient CO2 reduction processes. Integration with existing power generation systems (solar arrays, radioisotope thermoelectric generators, or future nuclear systems) requires careful engineering to ensure compatible voltage levels, current requirements, and thermal management. The electrocatalytic system must maintain high conversion efficiency across variable power inputs that characterize aerospace operations.
Life support applications represent a primary use case, where CO2 reduction technology can serve dual purposes of environmental control and resource utilization. For long-duration missions, the ability to convert exhaled CO2 into oxygen or useful carbon compounds offers significant advantages for crew sustainability. System requirements include 99.99% reliability, redundant components, and fail-safe operation modes.
Propellant production represents another promising application pathway. In-situ resource utilization (ISRU) systems that can convert Martian atmospheric CO2 into methane or other fuels could dramatically reduce mission mass requirements. Such systems must achieve conversion rates of at least 1 kg of fuel per 24 hours while maintaining operational stability in dusty, low-pressure environments.
Thermal management considerations are particularly challenging in space environments where convective cooling is unavailable. Electrocatalytic systems must incorporate efficient heat dissipation mechanisms to prevent catalyst degradation and maintain optimal reaction temperatures. Thermal integration with spacecraft systems presents opportunities for waste heat utilization but requires sophisticated control systems to manage variable thermal loads.
Weight and volume limitations represent critical factors in aerospace design. Electrocatalytic CO2 reduction systems must achieve maximum efficiency with minimal mass and spatial footprint. Current estimates suggest that next-generation systems should aim for power-to-weight ratios exceeding 1 kW/kg and volumetric power densities above 2 kW/L to be viable for aerospace implementation. These metrics significantly exceed those required for terrestrial applications.
Power management presents another crucial consideration. Spacecraft power systems typically operate on limited resources, necessitating highly efficient CO2 reduction processes. Integration with existing power generation systems (solar arrays, radioisotope thermoelectric generators, or future nuclear systems) requires careful engineering to ensure compatible voltage levels, current requirements, and thermal management. The electrocatalytic system must maintain high conversion efficiency across variable power inputs that characterize aerospace operations.
Life support applications represent a primary use case, where CO2 reduction technology can serve dual purposes of environmental control and resource utilization. For long-duration missions, the ability to convert exhaled CO2 into oxygen or useful carbon compounds offers significant advantages for crew sustainability. System requirements include 99.99% reliability, redundant components, and fail-safe operation modes.
Propellant production represents another promising application pathway. In-situ resource utilization (ISRU) systems that can convert Martian atmospheric CO2 into methane or other fuels could dramatically reduce mission mass requirements. Such systems must achieve conversion rates of at least 1 kg of fuel per 24 hours while maintaining operational stability in dusty, low-pressure environments.
Thermal management considerations are particularly challenging in space environments where convective cooling is unavailable. Electrocatalytic systems must incorporate efficient heat dissipation mechanisms to prevent catalyst degradation and maintain optimal reaction temperatures. Thermal integration with spacecraft systems presents opportunities for waste heat utilization but requires sophisticated control systems to manage variable thermal loads.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!