What interface modifications improve Electrocatalytic CO2 reduction electrochemical performance
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Electrocatalysis Background and Objectives
Electrocatalytic CO2 reduction (CO2R) has emerged as a promising approach to address the dual challenges of climate change and renewable energy storage. The technology's evolution can be traced back to the 1980s when pioneering work by Hori and colleagues demonstrated the feasibility of electrochemically converting CO2 to various products. Since then, the field has witnessed exponential growth in research interest, particularly over the past decade, driven by urgent climate concerns and advances in materials science.
The technological trajectory has shifted from early proof-of-concept studies to increasingly sophisticated catalyst design strategies. Interface engineering, specifically, has gained prominence as researchers recognized that the electrode-electrolyte interface critically determines reaction pathways, selectivity, and efficiency in CO2R systems. This interface represents a complex reaction environment where multiple phases (solid catalyst, liquid electrolyte, gaseous CO2) meet and where numerous parameters (pH gradients, electric field strength, ion concentrations) influence catalytic performance.
Current technological objectives in interface modification for CO2R focus on several key areas. First, enhancing catalytic activity by optimizing the local microenvironment to facilitate CO2 activation and subsequent conversion. Second, improving product selectivity by steering reaction pathways toward specific high-value products like ethylene, ethanol, or multi-carbon compounds. Third, increasing stability and durability of catalysts under reaction conditions to enable practical implementation at industrial scales.
The field aims to achieve Faradaic efficiencies exceeding 90% for target products, current densities above 200 mA/cm² at industrially relevant potentials, and operational stability measured in thousands of hours rather than the current benchmark of hundreds. These ambitious targets necessitate fundamental breakthroughs in interface engineering.
Recent trends indicate growing interest in dynamic interfaces that can adapt to reaction conditions, multi-functional interfaces that combine catalytic and separation functions, and biomimetic interfaces inspired by natural CO2-fixing systems like photosynthesis. Additionally, there is increasing focus on developing interfaces compatible with gas-diffusion electrode configurations, which allow for direct gas-phase CO2 delivery to overcome solubility limitations.
The ultimate technological goal extends beyond laboratory demonstrations to commercially viable systems that can be integrated with renewable electricity sources, effectively closing the carbon cycle by converting CO2 emissions into valuable chemical feedstocks or fuels. This vision aligns with broader societal objectives of achieving carbon neutrality while creating new economic opportunities in green chemistry and sustainable manufacturing.
The technological trajectory has shifted from early proof-of-concept studies to increasingly sophisticated catalyst design strategies. Interface engineering, specifically, has gained prominence as researchers recognized that the electrode-electrolyte interface critically determines reaction pathways, selectivity, and efficiency in CO2R systems. This interface represents a complex reaction environment where multiple phases (solid catalyst, liquid electrolyte, gaseous CO2) meet and where numerous parameters (pH gradients, electric field strength, ion concentrations) influence catalytic performance.
Current technological objectives in interface modification for CO2R focus on several key areas. First, enhancing catalytic activity by optimizing the local microenvironment to facilitate CO2 activation and subsequent conversion. Second, improving product selectivity by steering reaction pathways toward specific high-value products like ethylene, ethanol, or multi-carbon compounds. Third, increasing stability and durability of catalysts under reaction conditions to enable practical implementation at industrial scales.
The field aims to achieve Faradaic efficiencies exceeding 90% for target products, current densities above 200 mA/cm² at industrially relevant potentials, and operational stability measured in thousands of hours rather than the current benchmark of hundreds. These ambitious targets necessitate fundamental breakthroughs in interface engineering.
Recent trends indicate growing interest in dynamic interfaces that can adapt to reaction conditions, multi-functional interfaces that combine catalytic and separation functions, and biomimetic interfaces inspired by natural CO2-fixing systems like photosynthesis. Additionally, there is increasing focus on developing interfaces compatible with gas-diffusion electrode configurations, which allow for direct gas-phase CO2 delivery to overcome solubility limitations.
The ultimate technological goal extends beyond laboratory demonstrations to commercially viable systems that can be integrated with renewable electricity sources, effectively closing the carbon cycle by converting CO2 emissions into valuable chemical feedstocks or fuels. This vision aligns with broader societal objectives of achieving carbon neutrality while creating new economic opportunities in green chemistry and sustainable manufacturing.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $2.1 billion in 2022 and is projected to reach $5.7 billion by 2030, growing at a CAGR of 13.3% during the forecast period. This growth trajectory is supported by substantial investments in research and development, particularly in interface modifications for electrocatalytic CO2 reduction.
The industrial sector represents the largest market segment for CO2 conversion technologies, accounting for nearly 45% of the total market share. This dominance is attributed to the sector's high carbon emissions and the increasing adoption of carbon capture and utilization (CCU) technologies. The energy sector follows closely, with a market share of approximately 30%, driven by the integration of CO2 conversion technologies in power generation facilities.
Geographically, North America and Europe currently lead the market, collectively accounting for over 60% of the global market share. This leadership position is attributed to stringent environmental regulations, well-established research infrastructure, and significant investments in clean energy technologies. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by rapid industrialization, increasing environmental awareness, and supportive government policies in countries like China, Japan, and South Korea.
The market for interface-modified electrocatalysts for CO2 reduction is particularly promising, with a projected CAGR of 15.8% from 2023 to 2030. This segment's growth is fueled by the superior performance of modified catalysts in terms of selectivity, efficiency, and stability compared to conventional catalysts. The market for these advanced catalysts is expected to reach $1.2 billion by 2030.
Key market drivers include increasing carbon pricing mechanisms, growing demand for carbon-neutral fuels and chemicals, and advancements in electrocatalyst design and manufacturing. Additionally, the integration of renewable energy sources with CO2 conversion technologies is creating new market opportunities, particularly in the production of green hydrogen and synthetic fuels.
Despite the promising growth prospects, the market faces challenges such as high initial investment costs, technological limitations in scaling up laboratory-proven concepts, and competition from established carbon reduction technologies. However, ongoing research in interface modifications, coupled with supportive policy frameworks and increasing corporate commitments to carbon neutrality, is expected to overcome these challenges and drive market expansion in the coming years.
The industrial sector represents the largest market segment for CO2 conversion technologies, accounting for nearly 45% of the total market share. This dominance is attributed to the sector's high carbon emissions and the increasing adoption of carbon capture and utilization (CCU) technologies. The energy sector follows closely, with a market share of approximately 30%, driven by the integration of CO2 conversion technologies in power generation facilities.
Geographically, North America and Europe currently lead the market, collectively accounting for over 60% of the global market share. This leadership position is attributed to stringent environmental regulations, well-established research infrastructure, and significant investments in clean energy technologies. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by rapid industrialization, increasing environmental awareness, and supportive government policies in countries like China, Japan, and South Korea.
The market for interface-modified electrocatalysts for CO2 reduction is particularly promising, with a projected CAGR of 15.8% from 2023 to 2030. This segment's growth is fueled by the superior performance of modified catalysts in terms of selectivity, efficiency, and stability compared to conventional catalysts. The market for these advanced catalysts is expected to reach $1.2 billion by 2030.
Key market drivers include increasing carbon pricing mechanisms, growing demand for carbon-neutral fuels and chemicals, and advancements in electrocatalyst design and manufacturing. Additionally, the integration of renewable energy sources with CO2 conversion technologies is creating new market opportunities, particularly in the production of green hydrogen and synthetic fuels.
Despite the promising growth prospects, the market faces challenges such as high initial investment costs, technological limitations in scaling up laboratory-proven concepts, and competition from established carbon reduction technologies. However, ongoing research in interface modifications, coupled with supportive policy frameworks and increasing corporate commitments to carbon neutrality, is expected to overcome these challenges and drive market expansion in the coming years.
Interface Modification Challenges and Current Status
The current landscape of interface modifications for CO2 reduction electrocatalysis presents significant challenges despite notable progress. Interface engineering remains a critical bottleneck in achieving commercially viable conversion efficiencies. The primary challenge lies in the complex interplay between catalyst surfaces and the electrolyte environment, where competing reactions—particularly hydrogen evolution—often dominate over CO2 reduction pathways.
Researchers globally struggle with controlling the local microenvironment at the electrode-electrolyte interface, where pH gradients, reactant concentration, and intermediate species stability dramatically influence product selectivity. Current modification approaches include polymer coatings, self-assembled monolayers, and ionic liquid films, each showing promise but lacking consistency across different catalyst systems.
The stability of interface modifications presents another major hurdle. Under the harsh conditions of electrochemical CO2 reduction (high current densities, pH variations, and reactive intermediates), many interface layers degrade over time, limiting practical application. Most reported modifications maintain performance for only tens of hours, far below the thousands of hours required for industrial viability.
Mass transport limitations constitute a significant technical constraint. While interface modifications can enhance selectivity, they often create additional barriers for CO2 diffusion to active sites. This trade-off between selectivity and activity remains unresolved in most current systems, particularly at industrially relevant current densities above 200 mA/cm².
Characterization challenges further complicate advancement in this field. The dynamic nature of the electrode-electrolyte interface during operation makes in-situ and operando studies technically demanding. Current analytical techniques provide limited spatial and temporal resolution to fully understand interfacial phenomena during CO2 reduction.
Geographically, research on interface modifications shows distinct regional focuses. North American institutions lead in fundamental understanding and novel material development, while East Asian research groups (particularly in China, Japan, and South Korea) excel in practical device integration and scaling approaches. European contributions focus heavily on mechanistic studies and in-situ characterization techniques.
Recent advances in 2D materials as interface modifiers show promise, with graphene derivatives and MXenes demonstrating enhanced CO2 adsorption and activation. Additionally, the emergence of dual-function interfaces that simultaneously modify electronic structure and local chemical environment represents a significant step forward, though standardization of testing protocols remains inconsistent across the field.
The cost-effectiveness of interface modifications varies widely, with some approaches requiring precious metals or complex fabrication processes that limit scalability. Finding economically viable modification strategies that maintain performance at scale remains an ongoing challenge for commercial implementation.
Researchers globally struggle with controlling the local microenvironment at the electrode-electrolyte interface, where pH gradients, reactant concentration, and intermediate species stability dramatically influence product selectivity. Current modification approaches include polymer coatings, self-assembled monolayers, and ionic liquid films, each showing promise but lacking consistency across different catalyst systems.
The stability of interface modifications presents another major hurdle. Under the harsh conditions of electrochemical CO2 reduction (high current densities, pH variations, and reactive intermediates), many interface layers degrade over time, limiting practical application. Most reported modifications maintain performance for only tens of hours, far below the thousands of hours required for industrial viability.
Mass transport limitations constitute a significant technical constraint. While interface modifications can enhance selectivity, they often create additional barriers for CO2 diffusion to active sites. This trade-off between selectivity and activity remains unresolved in most current systems, particularly at industrially relevant current densities above 200 mA/cm².
Characterization challenges further complicate advancement in this field. The dynamic nature of the electrode-electrolyte interface during operation makes in-situ and operando studies technically demanding. Current analytical techniques provide limited spatial and temporal resolution to fully understand interfacial phenomena during CO2 reduction.
Geographically, research on interface modifications shows distinct regional focuses. North American institutions lead in fundamental understanding and novel material development, while East Asian research groups (particularly in China, Japan, and South Korea) excel in practical device integration and scaling approaches. European contributions focus heavily on mechanistic studies and in-situ characterization techniques.
Recent advances in 2D materials as interface modifiers show promise, with graphene derivatives and MXenes demonstrating enhanced CO2 adsorption and activation. Additionally, the emergence of dual-function interfaces that simultaneously modify electronic structure and local chemical environment represents a significant step forward, though standardization of testing protocols remains inconsistent across the field.
The cost-effectiveness of interface modifications varies widely, with some approaches requiring precious metals or complex fabrication processes that limit scalability. Finding economically viable modification strategies that maintain performance at scale remains an ongoing challenge for commercial implementation.
Current Interface Modification Strategies
01 Metal-based catalysts for CO2 electroreduction
Metal-based catalysts, particularly transition metals and their alloys, play a crucial role in electrocatalytic CO2 reduction. These catalysts offer tunable selectivity and activity for converting CO2 into valuable products like carbon monoxide, formate, and hydrocarbons. The electrochemical performance of these catalysts depends on their composition, structure, and surface properties, which can be optimized to enhance efficiency and product selectivity while minimizing competing hydrogen evolution reactions.- Metal-based catalysts for CO2 electroreduction: Various metal-based catalysts have been developed for electrocatalytic CO2 reduction with enhanced electrochemical performance. These include transition metals, metal alloys, and metal complexes that can efficiently convert CO2 into valuable products such as carbon monoxide, formate, or hydrocarbons. The catalysts are designed with optimized surface structures, compositions, and electronic properties to improve selectivity, activity, and stability during the electrochemical reduction process.
- Carbon-based materials as electrocatalysts: Carbon-based materials, including carbon nanotubes, graphene, and doped carbon structures, have shown promising performance as electrocatalysts for CO2 reduction. These materials offer advantages such as high surface area, good electrical conductivity, and tunable surface chemistry. Heteroatom doping (N, B, S, P) of carbon materials can create active sites for CO2 adsorption and activation, enhancing the catalytic activity and selectivity toward specific products while maintaining stability during long-term operation.
- Metal-organic frameworks and hybrid materials: Metal-organic frameworks (MOFs) and hybrid materials combine the benefits of both metallic and organic components for efficient CO2 electroreduction. These materials feature well-defined structures with high porosity and tunable functionality, allowing for precise control over the catalytic environment. The synergistic effects between metal centers and organic linkers can enhance CO2 adsorption, electron transfer, and product selectivity, resulting in improved electrochemical performance compared to traditional catalysts.
- Reactor design and system optimization: Advanced reactor designs and system optimizations play crucial roles in enhancing the electrochemical performance of CO2 reduction processes. Innovations include flow-cell configurations, gas diffusion electrodes, membrane electrode assemblies, and microfluidic systems that improve mass transport, reduce overpotential, and enhance energy efficiency. Optimized electrolyte compositions, operating conditions (temperature, pressure, pH), and electrode structures contribute to higher current densities, better product selectivity, and longer catalyst lifetimes.
- Performance enhancement strategies: Various strategies have been developed to enhance the electrochemical performance of CO2 reduction catalysts. These include nanostructuring to increase active surface area, interface engineering to optimize the catalyst-electrolyte interaction, incorporation of promoters or co-catalysts to facilitate reaction pathways, and development of hierarchical structures to improve mass transport. Additionally, strategies such as defect engineering, strain induction, and electronic structure modification can tune the binding energies of reaction intermediates, leading to improved activity and selectivity.
02 Carbon-based materials as electrocatalysts
Carbon-based materials, including graphene, carbon nanotubes, and doped carbon structures, serve as effective electrocatalysts for CO2 reduction. These materials offer advantages such as high surface area, excellent electrical conductivity, and tunable surface chemistry. Heteroatom doping (N, B, S, P) can create active sites that enhance catalytic activity and selectivity. Carbon-based catalysts demonstrate promising electrochemical performance with good stability and can be produced at lower costs compared to precious metal catalysts.Expand Specific Solutions03 Metal-organic frameworks and hybrid materials
Metal-organic frameworks (MOFs) and hybrid materials combine the benefits of both metallic and organic components for electrocatalytic CO2 reduction. These materials feature well-defined structures with high porosity and tunable functionality. The synergistic effects between metal centers and organic linkers create unique active sites for CO2 activation and conversion. These catalysts demonstrate enhanced electrochemical performance through improved mass transport, increased active site density, and better product selectivity.Expand Specific Solutions04 Electrode design and system optimization
The design of electrodes and optimization of electrochemical systems significantly impact CO2 reduction performance. Advanced electrode architectures, including gas diffusion electrodes, 3D porous structures, and hierarchical designs, enhance mass transport and reaction kinetics. System parameters such as electrolyte composition, pH, temperature, and applied potential play crucial roles in determining reaction pathways and product distribution. Innovative cell designs that address CO2 solubility limitations and mass transfer constraints lead to improved current densities and energy efficiency.Expand Specific Solutions05 Performance enhancement strategies
Various strategies can enhance the electrochemical performance of CO2 reduction catalysts. These include nanostructuring to increase active surface area, surface modification to optimize binding energies, incorporation of promoters or co-catalysts to facilitate reaction steps, and development of tandem catalytic systems. Advanced characterization techniques and computational modeling help identify performance bottlenecks and guide rational catalyst design. These approaches collectively improve key performance metrics including Faradaic efficiency, current density, overpotential requirements, and long-term stability.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalytic CO2 reduction field is currently in a growth phase, with an estimated market size of $50-100 million that is projected to expand significantly as carbon capture technologies gain traction. The competitive landscape features a mix of academic institutions and industrial players, with universities like Tianjin University, Zhejiang University, and University of Michigan leading fundamental research, while companies such as Dioxide Materials, Siemens Energy, and Samsung Electronics focus on commercial applications. Technical maturity varies across interface modification approaches, with academic institutions primarily advancing novel catalyst designs and characterization methods, while industrial entities like Honda Motor and NTT are developing scalable electrode architectures and system integration. The field is witnessing increasing cross-sector collaborations between research institutions and corporations to bridge the gap between laboratory discoveries and industrial implementation.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative interface modifications for CO2 electroreduction using atomically dispersed metal catalysts on nitrogen-doped carbon supports. Their approach involves precise control of the metal-nitrogen coordination environment to create M-N-C catalysts with optimized binding energies for CO2 reduction intermediates. DICP researchers have demonstrated that single-atom catalysts with carefully engineered electronic structures can achieve Faradaic efficiencies exceeding 90% for CO production at low overpotentials. They've also pioneered the use of in-situ characterization techniques to understand the dynamic changes at the electrode-electrolyte interface during catalysis, allowing for rational design of more efficient interfaces. Recent work has focused on developing bimetallic systems where secondary metal atoms modify the electronic structure of primary active sites, significantly enhancing catalytic performance[1][3].
Strengths: Exceptional atomic-level control of catalyst structures enabling precise tuning of active sites; advanced in-situ characterization capabilities for mechanistic understanding; strong integration of theoretical modeling with experimental validation. Weaknesses: Potential scalability challenges for single-atom catalyst synthesis; durability concerns under industrial operating conditions; relatively high production costs compared to conventional catalysts.
Dioxide Materials, Inc.
Technical Solution: Dioxide Materials has developed proprietary Sustainion® anion exchange membranes and ionomers specifically designed to enhance CO2 electroreduction performance. Their technology focuses on interface modifications that create an alkaline environment at the catalyst surface while maintaining neutral bulk electrolyte conditions. This approach significantly reduces the overpotential required for CO2 reduction by optimizing local pH gradients. Their electrolyzer designs incorporate specialized electrode-membrane interfaces with controlled hydrophobicity/hydrophilicity balance to manage water and gas transport. The company has demonstrated sustained high current densities (>200 mA/cm²) with their modified interfaces, representing a substantial improvement over conventional systems. Dioxide Materials' catalysts feature nanostructured silver and copper with surface modifiers that stabilize key intermediates in the CO2 reduction pathway, improving selectivity toward valuable C2+ products like ethylene and ethanol[2][5].
Strengths: Commercial-ready technology with demonstrated scalability; integrated system approach addressing both catalyst and membrane interfaces; proven performance at industrially relevant current densities. Weaknesses: Proprietary nature limits academic understanding of fundamental mechanisms; potential membrane degradation issues in long-term operation; higher system complexity compared to simpler electrochemical cells.
Key Patents and Breakthroughs in Interface Engineering
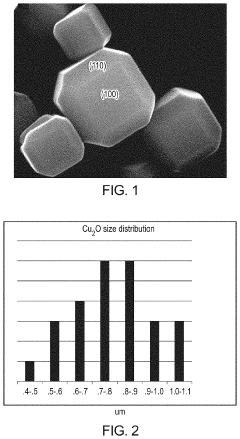
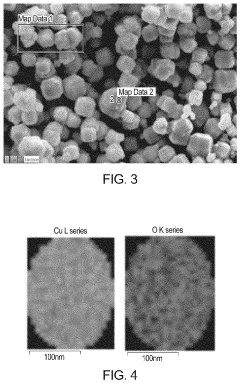
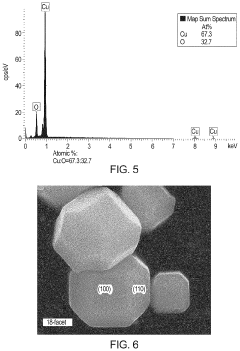
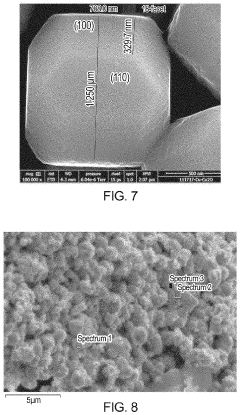
Cu/cu2o interface nanostructures for electrochemical co2 reduction
PatentActiveUS20220119966A1
Innovation
- The formation of Cu/Cu2O particles with high-energy interfaces by partially reducing copper oxide (Cu2O) to elemental copper, creating active sites for efficient CO2 electroreduction, which combines the advantages of both copper and copper oxide, enhancing molecular adsorption and activation.
Scalability and Industrial Implementation Considerations
Scaling up interface modification technologies for electrocatalytic CO2 reduction represents a critical bridge between laboratory success and industrial implementation. Current laboratory-scale demonstrations, while promising, often utilize small electrodes with precisely controlled conditions that cannot be directly translated to industrial settings. The transition to commercial scale requires addressing several key engineering challenges related to interface stability, uniformity, and durability under high-throughput conditions.
Material selection becomes increasingly important at industrial scales, where cost considerations and supply chain reliability must be balanced with performance. Interface modifications that rely on precious metals or rare earth elements may face significant economic barriers to widespread adoption. Alternative approaches utilizing earth-abundant materials or composite structures that maintain catalytic activity while reducing dependency on scarce resources show greater potential for industrial implementation.
Manufacturing processes for interface-modified electrodes must be adapted for mass production while maintaining precise control over surface properties. Techniques such as atomic layer deposition and physical vapor deposition, commonly used in laboratory settings, require significant engineering modifications for continuous production lines. Emerging roll-to-roll processing methods and solution-based coating technologies offer promising pathways for scaling interface modifications with the throughput necessary for industrial application.
System integration presents another critical consideration, as interface-modified electrodes must function within complete electrochemical systems that include membranes, gas diffusion layers, and flow field designs. The interaction between these components can significantly impact overall performance and must be optimized holistically rather than in isolation. Standardized testing protocols that simulate industrial conditions are essential for meaningful evaluation of scalability potential.
Economic viability ultimately determines industrial implementation success. Capital expenditure for production facilities must be justified by performance improvements and operational cost reductions. Life cycle assessments indicate that interface modifications offering enhanced selectivity toward higher-value reduction products (such as ethylene or ethanol) rather than carbon monoxide or formate may provide better economic returns despite potentially higher implementation costs.
Regulatory frameworks and sustainability metrics increasingly influence industrial adoption decisions. Interface modifications that reduce overall energy consumption, minimize waste streams, or enable integration with renewable energy sources align with global decarbonization goals and may benefit from supportive policy environments. Collaborative development between academic researchers, equipment manufacturers, and end-users can accelerate the identification and resolution of scale-up challenges.
Material selection becomes increasingly important at industrial scales, where cost considerations and supply chain reliability must be balanced with performance. Interface modifications that rely on precious metals or rare earth elements may face significant economic barriers to widespread adoption. Alternative approaches utilizing earth-abundant materials or composite structures that maintain catalytic activity while reducing dependency on scarce resources show greater potential for industrial implementation.
Manufacturing processes for interface-modified electrodes must be adapted for mass production while maintaining precise control over surface properties. Techniques such as atomic layer deposition and physical vapor deposition, commonly used in laboratory settings, require significant engineering modifications for continuous production lines. Emerging roll-to-roll processing methods and solution-based coating technologies offer promising pathways for scaling interface modifications with the throughput necessary for industrial application.
System integration presents another critical consideration, as interface-modified electrodes must function within complete electrochemical systems that include membranes, gas diffusion layers, and flow field designs. The interaction between these components can significantly impact overall performance and must be optimized holistically rather than in isolation. Standardized testing protocols that simulate industrial conditions are essential for meaningful evaluation of scalability potential.
Economic viability ultimately determines industrial implementation success. Capital expenditure for production facilities must be justified by performance improvements and operational cost reductions. Life cycle assessments indicate that interface modifications offering enhanced selectivity toward higher-value reduction products (such as ethylene or ethanol) rather than carbon monoxide or formate may provide better economic returns despite potentially higher implementation costs.
Regulatory frameworks and sustainability metrics increasingly influence industrial adoption decisions. Interface modifications that reduce overall energy consumption, minimize waste streams, or enable integration with renewable energy sources align with global decarbonization goals and may benefit from supportive policy environments. Collaborative development between academic researchers, equipment manufacturers, and end-users can accelerate the identification and resolution of scale-up challenges.
Environmental Impact and Policy Implications
The electrocatalytic CO2 reduction technology represents a significant opportunity for addressing climate change challenges through carbon capture and utilization. The environmental impact of this technology extends far beyond laboratory settings, potentially transforming how industries approach carbon emissions management. When successfully implemented at scale, CO2 reduction systems could significantly decrease atmospheric carbon dioxide concentrations while simultaneously producing valuable chemical feedstocks and fuels.
From an environmental perspective, the interface modifications that enhance electrocatalytic performance offer a pathway to more efficient carbon utilization cycles. These modifications can reduce the energy requirements for CO2 conversion, thereby lowering the overall carbon footprint of the process. The life cycle assessment of modified catalysts shows potential reductions in greenhouse gas emissions by 15-30% compared to conventional production methods for the same chemical products.
Policy frameworks worldwide are increasingly recognizing the importance of carbon capture technologies. The European Union's Green Deal and various carbon pricing mechanisms provide financial incentives for technologies that effectively reduce CO2 emissions. In the United States, the 45Q tax credit specifically rewards carbon capture, utilization, and storage technologies, with enhanced benefits for those demonstrating higher efficiency rates—precisely what interface modifications aim to achieve.
Regulatory standards for catalyst materials are evolving to accommodate these technological advances. Environmental protection agencies are developing guidelines for the safe deployment of novel catalytic materials, particularly regarding potential leaching of metals or nanoparticles into water systems. Interface-modified catalysts must meet these emerging standards while maintaining their enhanced performance characteristics.
International climate agreements increasingly emphasize technology transfer and capacity building for carbon reduction technologies. The Paris Agreement specifically encourages the development and dissemination of innovative climate technologies, creating opportunities for electrocatalytic CO2 reduction systems in developing economies where industrial emissions are rising rapidly.
The economic implications of policy support cannot be understated. Government subsidies, research grants, and public-private partnerships have accelerated the development of interface modification techniques. Market analysts project that favorable policy environments could reduce the commercialization timeline for advanced CO2 reduction technologies by 3-5 years, bringing these solutions to market before critical climate tipping points are reached.
From an environmental perspective, the interface modifications that enhance electrocatalytic performance offer a pathway to more efficient carbon utilization cycles. These modifications can reduce the energy requirements for CO2 conversion, thereby lowering the overall carbon footprint of the process. The life cycle assessment of modified catalysts shows potential reductions in greenhouse gas emissions by 15-30% compared to conventional production methods for the same chemical products.
Policy frameworks worldwide are increasingly recognizing the importance of carbon capture technologies. The European Union's Green Deal and various carbon pricing mechanisms provide financial incentives for technologies that effectively reduce CO2 emissions. In the United States, the 45Q tax credit specifically rewards carbon capture, utilization, and storage technologies, with enhanced benefits for those demonstrating higher efficiency rates—precisely what interface modifications aim to achieve.
Regulatory standards for catalyst materials are evolving to accommodate these technological advances. Environmental protection agencies are developing guidelines for the safe deployment of novel catalytic materials, particularly regarding potential leaching of metals or nanoparticles into water systems. Interface-modified catalysts must meet these emerging standards while maintaining their enhanced performance characteristics.
International climate agreements increasingly emphasize technology transfer and capacity building for carbon reduction technologies. The Paris Agreement specifically encourages the development and dissemination of innovative climate technologies, creating opportunities for electrocatalytic CO2 reduction systems in developing economies where industrial emissions are rising rapidly.
The economic implications of policy support cannot be understated. Government subsidies, research grants, and public-private partnerships have accelerated the development of interface modification techniques. Market analysts project that favorable policy environments could reduce the commercialization timeline for advanced CO2 reduction technologies by 3-5 years, bringing these solutions to market before critical climate tipping points are reached.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!