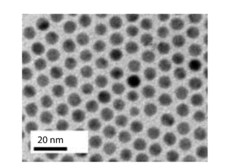
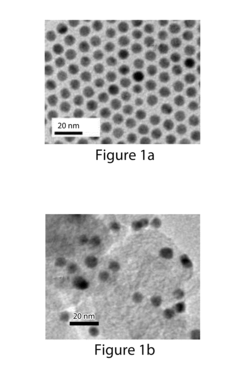
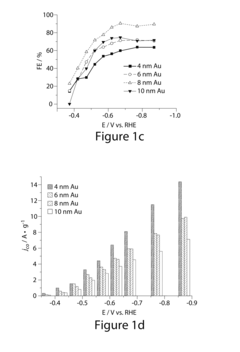
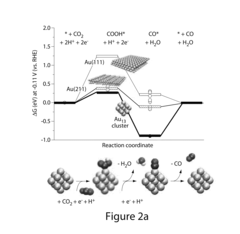
Research on Electrocatalytic CO2 reduction for advanced carbon energy applications
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Technology Background and Objectives
Electrocatalytic CO2 reduction represents a pivotal technology in addressing global climate challenges while simultaneously creating valuable carbon-based products. This technology has evolved significantly since its conceptual introduction in the 1980s, with major breakthroughs occurring in the past decade as climate change mitigation has become increasingly urgent. The fundamental process involves converting carbon dioxide into useful chemicals and fuels using electrical energy, ideally derived from renewable sources, thus creating a sustainable carbon cycle.
The evolution of CO2 reduction technology has been marked by several key developments, including the discovery of copper-based catalysts in the 1980s, the emergence of nanostructured materials in the 2000s, and recent advances in single-atom catalysts and molecular engineering. These developments have progressively improved reaction efficiency, selectivity, and stability—three critical parameters that determine the commercial viability of the technology.
Current technological trends point toward integrated systems that combine CO2 capture and conversion, the development of novel electrolytes to enhance reaction kinetics, and the scaling of laboratory processes to industrial applications. The field is witnessing a convergence of electrochemistry, materials science, and process engineering to overcome existing limitations.
The primary objective of electrocatalytic CO2 reduction research is to develop economically viable processes that can operate at industrial scales with minimal energy input while producing high-value products. Specific technical goals include achieving Faradaic efficiencies exceeding 90% for target products, maintaining catalyst stability for thousands of hours, and reducing the overall energy consumption to levels competitive with conventional production methods.
Secondary objectives include the development of catalysts from earth-abundant materials to ensure sustainability and scalability, the integration with renewable energy sources to enable carbon-neutral or carbon-negative processes, and the design of modular systems that can be deployed across various industrial settings.
From an environmental perspective, the technology aims to contribute significantly to carbon neutrality goals by recycling industrial CO2 emissions and potentially achieving negative emissions through direct air capture coupled with electrocatalytic reduction. The ultimate vision is to transform CO2 from a problematic waste product into a valuable feedstock for the chemical industry, creating a circular carbon economy.
The technological roadmap envisions progressive improvements in catalyst design, reactor engineering, and system integration over the next decade, with early commercial applications expected in high-value chemical production, followed by fuel synthesis as efficiency improves and costs decrease.
The evolution of CO2 reduction technology has been marked by several key developments, including the discovery of copper-based catalysts in the 1980s, the emergence of nanostructured materials in the 2000s, and recent advances in single-atom catalysts and molecular engineering. These developments have progressively improved reaction efficiency, selectivity, and stability—three critical parameters that determine the commercial viability of the technology.
Current technological trends point toward integrated systems that combine CO2 capture and conversion, the development of novel electrolytes to enhance reaction kinetics, and the scaling of laboratory processes to industrial applications. The field is witnessing a convergence of electrochemistry, materials science, and process engineering to overcome existing limitations.
The primary objective of electrocatalytic CO2 reduction research is to develop economically viable processes that can operate at industrial scales with minimal energy input while producing high-value products. Specific technical goals include achieving Faradaic efficiencies exceeding 90% for target products, maintaining catalyst stability for thousands of hours, and reducing the overall energy consumption to levels competitive with conventional production methods.
Secondary objectives include the development of catalysts from earth-abundant materials to ensure sustainability and scalability, the integration with renewable energy sources to enable carbon-neutral or carbon-negative processes, and the design of modular systems that can be deployed across various industrial settings.
From an environmental perspective, the technology aims to contribute significantly to carbon neutrality goals by recycling industrial CO2 emissions and potentially achieving negative emissions through direct air capture coupled with electrocatalytic reduction. The ultimate vision is to transform CO2 from a problematic waste product into a valuable feedstock for the chemical industry, creating a circular carbon economy.
The technological roadmap envisions progressive improvements in catalyst design, reactor engineering, and system integration over the next decade, with early commercial applications expected in high-value chemical production, followed by fuel synthesis as efficiency improves and costs decrease.
Market Analysis for Carbon Capture Utilization
The global carbon capture, utilization, and storage (CCUS) market is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. As of 2023, the market was valued at approximately $7.5 billion and is projected to reach $15.3 billion by 2030, representing a compound annual growth rate (CAGR) of 10.7%. This growth trajectory is particularly relevant for electrocatalytic CO2 reduction technologies, which offer promising pathways for converting captured carbon into valuable products.
The market for carbon capture utilization can be segmented into several key application areas, including enhanced oil recovery (EOR), food and beverage production, chemical synthesis, fuel production, and building materials. Among these, the conversion of CO2 into fuels and chemical feedstocks through electrocatalytic processes represents one of the fastest-growing segments, with projected growth rates exceeding 15% annually through 2028.
Geographically, North America currently dominates the CCUS market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to rapid industrialization, increasing carbon emissions, and strengthening regulatory frameworks in countries like China, Japan, and South Korea.
From an industry perspective, the energy sector remains the largest end-user of carbon capture technologies, accounting for roughly 65% of the total market. This is followed by the industrial sector (20%) and the transportation sector (10%). The remaining 5% is distributed across various other applications including agriculture and construction.
Key market drivers include increasingly stringent carbon emission regulations, growing corporate commitments to carbon neutrality, and the emergence of carbon pricing mechanisms in major economies. The European Union's Carbon Border Adjustment Mechanism (CBAM) and similar policies are creating economic incentives for carbon capture and utilization technologies.
Market challenges include high capital costs, energy intensity of current processes, and technological limitations in scalability. The levelized cost of carbon capture currently ranges from $40-120 per ton of CO2, depending on the source and technology used, which remains a significant barrier to widespread adoption.
Emerging opportunities include the integration of electrocatalytic CO2 reduction with renewable energy systems, creating pathways for seasonal energy storage and grid balancing services. Additionally, the production of high-value chemicals and materials from captured CO2 is attracting significant investment, with venture capital funding in this space reaching $1.9 billion in 2022 alone.
The market for carbon capture utilization can be segmented into several key application areas, including enhanced oil recovery (EOR), food and beverage production, chemical synthesis, fuel production, and building materials. Among these, the conversion of CO2 into fuels and chemical feedstocks through electrocatalytic processes represents one of the fastest-growing segments, with projected growth rates exceeding 15% annually through 2028.
Geographically, North America currently dominates the CCUS market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to rapid industrialization, increasing carbon emissions, and strengthening regulatory frameworks in countries like China, Japan, and South Korea.
From an industry perspective, the energy sector remains the largest end-user of carbon capture technologies, accounting for roughly 65% of the total market. This is followed by the industrial sector (20%) and the transportation sector (10%). The remaining 5% is distributed across various other applications including agriculture and construction.
Key market drivers include increasingly stringent carbon emission regulations, growing corporate commitments to carbon neutrality, and the emergence of carbon pricing mechanisms in major economies. The European Union's Carbon Border Adjustment Mechanism (CBAM) and similar policies are creating economic incentives for carbon capture and utilization technologies.
Market challenges include high capital costs, energy intensity of current processes, and technological limitations in scalability. The levelized cost of carbon capture currently ranges from $40-120 per ton of CO2, depending on the source and technology used, which remains a significant barrier to widespread adoption.
Emerging opportunities include the integration of electrocatalytic CO2 reduction with renewable energy systems, creating pathways for seasonal energy storage and grid balancing services. Additionally, the production of high-value chemicals and materials from captured CO2 is attracting significant investment, with venture capital funding in this space reaching $1.9 billion in 2022 alone.
Electrocatalytic CO2 Reduction: Current Status and Challenges
Electrocatalytic CO2 reduction (ECR) has emerged as a promising technology for carbon capture and utilization, offering a sustainable pathway to convert CO2 into value-added chemicals and fuels. Currently, the field has witnessed significant advancements in catalyst design, reaction mechanisms understanding, and system integration. However, several critical challenges remain unresolved, limiting its widespread commercial implementation.
The state-of-the-art catalysts for ECR include copper-based materials, which demonstrate unique capabilities for C-C coupling to produce multi-carbon products. Recent breakthroughs have also highlighted the potential of bimetallic catalysts, single-atom catalysts, and metal-organic frameworks, each offering distinct advantages in selectivity and efficiency. Despite these advances, current catalytic systems still suffer from low Faradaic efficiency, poor product selectivity, and insufficient stability during long-term operation.
From a global perspective, research efforts are concentrated in North America, Europe, and East Asia, with the United States, China, and Germany leading in patent applications and scientific publications. This geographical distribution reflects both the technological capabilities and policy priorities regarding carbon neutrality goals across different regions.
A major technical bottleneck is the competitive hydrogen evolution reaction, which significantly reduces the efficiency of CO2 conversion. Additionally, the multi-electron transfer process required for CO2 reduction presents kinetic barriers that limit reaction rates and product yields. Mass transport limitations, particularly in gas-diffusion electrode configurations, further complicate the scaling of laboratory results to industrial applications.
Energy efficiency remains suboptimal, with current systems requiring substantial electrical input relative to the chemical energy stored in the products. Most laboratory demonstrations operate at current densities below 200 mA/cm², whereas commercial viability would require at least 500 mA/cm² while maintaining high selectivity and durability.
The reaction environment, including electrolyte composition, pH, and temperature, significantly influences product distribution and catalyst stability. Recent studies have shown that local CO2 concentration near the catalyst surface often becomes a limiting factor, necessitating advanced electrode designs that facilitate efficient mass transport.
Durability issues persist across all catalyst types, with performance degradation observed after several hours or days of operation. This degradation stems from multiple factors including catalyst poisoning, structural changes, and electrolyte degradation. Understanding and mitigating these degradation mechanisms represents a critical research direction for enabling practical applications.
The techno-economic analysis indicates that current ECR technologies remain far from commercial viability, with estimated production costs significantly higher than conventional routes. Bridging this gap requires not only fundamental breakthroughs in catalysis but also innovations in system design, process integration, and renewable electricity utilization.
The state-of-the-art catalysts for ECR include copper-based materials, which demonstrate unique capabilities for C-C coupling to produce multi-carbon products. Recent breakthroughs have also highlighted the potential of bimetallic catalysts, single-atom catalysts, and metal-organic frameworks, each offering distinct advantages in selectivity and efficiency. Despite these advances, current catalytic systems still suffer from low Faradaic efficiency, poor product selectivity, and insufficient stability during long-term operation.
From a global perspective, research efforts are concentrated in North America, Europe, and East Asia, with the United States, China, and Germany leading in patent applications and scientific publications. This geographical distribution reflects both the technological capabilities and policy priorities regarding carbon neutrality goals across different regions.
A major technical bottleneck is the competitive hydrogen evolution reaction, which significantly reduces the efficiency of CO2 conversion. Additionally, the multi-electron transfer process required for CO2 reduction presents kinetic barriers that limit reaction rates and product yields. Mass transport limitations, particularly in gas-diffusion electrode configurations, further complicate the scaling of laboratory results to industrial applications.
Energy efficiency remains suboptimal, with current systems requiring substantial electrical input relative to the chemical energy stored in the products. Most laboratory demonstrations operate at current densities below 200 mA/cm², whereas commercial viability would require at least 500 mA/cm² while maintaining high selectivity and durability.
The reaction environment, including electrolyte composition, pH, and temperature, significantly influences product distribution and catalyst stability. Recent studies have shown that local CO2 concentration near the catalyst surface often becomes a limiting factor, necessitating advanced electrode designs that facilitate efficient mass transport.
Durability issues persist across all catalyst types, with performance degradation observed after several hours or days of operation. This degradation stems from multiple factors including catalyst poisoning, structural changes, and electrolyte degradation. Understanding and mitigating these degradation mechanisms represents a critical research direction for enabling practical applications.
The techno-economic analysis indicates that current ECR technologies remain far from commercial viability, with estimated production costs significantly higher than conventional routes. Bridging this gap requires not only fundamental breakthroughs in catalysis but also innovations in system design, process integration, and renewable electricity utilization.
Current Electrocatalyst Design Strategies
01 Metal-based catalysts for CO2 reduction
Various metal-based catalysts can be used for electrocatalytic CO2 reduction to produce valuable chemicals. These catalysts include transition metals like copper, silver, gold, and their alloys or compounds. The catalytic performance can be enhanced by controlling the morphology, crystal structure, and surface properties of these metals. These catalysts facilitate the electron transfer process required for CO2 reduction and can be tuned to improve selectivity towards specific products.- Metal-based catalysts for CO2 reduction: Various metal-based catalysts can be used for electrocatalytic CO2 reduction to produce valuable chemicals. These catalysts include noble metals, transition metals, and their alloys or composites. The catalytic performance can be enhanced by controlling the morphology, crystal structure, and surface properties of the metal catalysts. These catalysts can efficiently convert CO2 into products such as carbon monoxide, formate, methanol, and hydrocarbons under specific electrochemical conditions.
- Carbon-based materials as electrocatalysts: Carbon-based materials, including graphene, carbon nanotubes, and doped carbon structures, serve as effective electrocatalysts for CO2 reduction. These materials can be functionalized or doped with heteroatoms like nitrogen, boron, or sulfur to enhance their catalytic activity. Carbon-based catalysts offer advantages such as high surface area, good electrical conductivity, and tunable surface chemistry, making them promising candidates for sustainable CO2 conversion technologies.
- Electrode design and reactor configurations: The design of electrodes and reactor configurations plays a crucial role in improving the efficiency of electrocatalytic CO2 reduction. Various approaches include the development of gas diffusion electrodes, flow cells, and microfluidic reactors that enhance mass transport and reaction kinetics. Advanced electrode architectures with optimized porosity, wettability, and catalyst loading can significantly improve CO2 conversion rates and product selectivity while reducing energy consumption.
- Bimetallic and multi-component catalyst systems: Bimetallic and multi-component catalyst systems offer enhanced performance for electrocatalytic CO2 reduction through synergistic effects. These systems combine different metals, metal oxides, or metal-organic frameworks to achieve improved activity, selectivity, and stability. The interaction between different components can create unique active sites, modify electronic properties, and optimize binding energies for CO2 and reaction intermediates, leading to more efficient conversion processes.
- Process optimization and reaction conditions: Optimizing reaction conditions is essential for efficient electrocatalytic CO2 reduction. Parameters such as electrolyte composition, pH, temperature, pressure, and applied potential significantly influence the reaction pathways and product distribution. Advanced techniques for in-situ monitoring and control of these parameters can enhance the performance of CO2 reduction systems. Additionally, the integration of renewable energy sources for powering the electrochemical process contributes to the overall sustainability of CO2 conversion technologies.
02 Carbon-based materials as electrocatalysts
Carbon-based materials such as graphene, carbon nanotubes, and doped carbon structures serve as effective electrocatalysts for CO2 reduction. These materials offer advantages including high surface area, excellent electrical conductivity, and tunable surface chemistry. Nitrogen, boron, or other heteroatom doping can create active sites on carbon materials that enhance catalytic activity and selectivity. Carbon-based catalysts are also valued for their stability and cost-effectiveness compared to precious metal catalysts.Expand Specific Solutions03 Electrode design and reactor configurations
The design of electrodes and reactor configurations plays a crucial role in electrocatalytic CO2 reduction efficiency. Gas diffusion electrodes, flow cells, and microfluidic reactors can enhance mass transport and reaction kinetics. Optimized electrode structures with high surface area and controlled porosity improve catalyst utilization and product selectivity. Advanced reactor designs also address challenges such as CO2 solubility limitations and product separation, leading to improved overall system performance.Expand Specific Solutions04 Electrolyte composition and reaction conditions
The composition of electrolytes and reaction conditions significantly influence the efficiency and selectivity of electrocatalytic CO2 reduction. Factors such as pH, ionic strength, buffer capacity, and the presence of specific ions can alter reaction pathways and product distribution. Temperature, pressure, and applied potential are also critical parameters that affect reaction kinetics and thermodynamics. Optimizing these conditions can enhance conversion efficiency, reduce energy consumption, and improve the selectivity towards desired products.Expand Specific Solutions05 Bimetallic and hybrid catalyst systems
Bimetallic and hybrid catalyst systems combine the advantages of different materials to achieve superior performance in CO2 reduction. These systems may include combinations of metals, metal oxides, and carbon-based materials that create synergistic effects. The interface between different components often serves as active sites with unique electronic properties. These hybrid catalysts can be designed to overcome limitations of single-component catalysts, offering improved activity, selectivity, and stability for long-term operation in electrocatalytic CO2 reduction processes.Expand Specific Solutions
Leading Organizations in Electrocatalytic CO2 Conversion
Electrocatalytic CO2 reduction technology is currently in the early growth phase, characterized by increasing research intensity but limited commercial deployment. The global market is projected to expand significantly as carbon neutrality goals drive demand for carbon capture and utilization solutions. Technologically, the field shows moderate maturity with academic institutions (Harbin Institute of Technology, University of Toronto, Columbia University) leading fundamental research, while energy companies (TotalEnergies, Siemens Energy) are advancing practical applications. Research centers like CNRS and national laboratories are bridging the gap between theory and application. The competitive landscape features collaboration between academic and industrial players, with energy majors investing strategically to position themselves in this emerging clean energy sector.
TotalEnergies SE
Technical Solution: TotalEnergies has developed an integrated CO2 electroreduction system that combines renewable electricity with proprietary catalyst technology for carbon-neutral fuel and chemical production. Their approach utilizes hierarchically structured copper-silver bimetallic catalysts that demonstrate enhanced selectivity toward multi-carbon products while maintaining high current densities (>300 mA/cm²). The company has engineered membrane electrode assemblies that minimize the anode-cathode gap, reducing ohmic losses and improving energy efficiency by approximately 25% compared to conventional designs. Their system incorporates real-time product analysis and adaptive control algorithms that adjust operating parameters to maintain optimal performance despite fluctuating renewable energy inputs. TotalEnergies has successfully demonstrated continuous operation at the pilot scale (>500 hours) with minimal performance degradation, addressing one of the key challenges in CO2 electroreduction technology commercialization.
Strengths: Strong integration capabilities across the entire value chain; substantial financial resources for scaling technology; existing infrastructure for product distribution and utilization. Weaknesses: As an energy company, may face internal resistance to technologies that compete with traditional hydrocarbon business; relatively recent entry into electrocatalysis compared to academic institutions; potential regulatory challenges in different markets.
Honda Motor Co., Ltd.
Technical Solution: Honda has developed a proprietary CO2 electroreduction system focused on integration with hydrogen fuel cell technology. Their approach combines CO2 capture from vehicle exhaust with on-board electrochemical reduction to produce carbon-neutral synthetic fuels. The company's catalyst design features nanostructured copper-zinc alloys deposited on gas diffusion electrodes that achieve CO2-to-formate conversion with Faradaic efficiencies exceeding 85% at moderate overpotentials. Honda's system architecture incorporates waste heat recovery from fuel cells to maintain optimal temperature conditions for CO2 reduction, improving overall energy efficiency by approximately 30% compared to standalone systems. Their modular design allows for scalability from small mobile applications to larger stationary power generation units, with integrated power management systems that can operate under variable load conditions. Honda has demonstrated this technology in prototype vehicles where captured CO2 is partially recycled into usable energy carriers, creating a semi-closed carbon loop system.
Strengths: Extensive experience in fuel cell technology that complements CO2 reduction systems; strong manufacturing capabilities for system integration; established global distribution network. Weaknesses: Primary focus on mobile applications may limit optimization for large-scale stationary systems; technology heavily dependent on parallel advances in hydrogen infrastructure; potential challenges in achieving cost targets for consumer applications.
Key Innovations in Catalyst Materials and Reaction Mechanisms
Method for Electrocatalytic Reduction using Au Nanoparticles Tuned or Optimized for Reduction of CO2 to CO
PatentInactiveUS20160230295A1
Innovation
- The use of gold nanoparticles with a diameter of approximately 8 nm, supported on Ketjen carbon, which presents a surface structure with a higher density of CO-converting edge sites and fewer hydrogen-evolving corner sites, facilitating efficient CO2 reduction to carbon monoxide in an alkaline solution.
Gas diffusion electrode for electrocatalytic co 2 reduction to co, preparation method and use
PatentWO2025019964A1
Innovation
- The conductive polymer solution of a conductive polymer with a Moore is 100: 1 ~ 1: 100 mixes the metal salt solution, and adds a dispersant to form a hybrid solution to transfer it to the base. , Formation of metal-conductive polymer composite electrode.
Policy Framework for Carbon Neutrality Technologies
The global commitment to carbon neutrality has catalyzed the development of comprehensive policy frameworks that support innovative technologies like electrocatalytic CO2 reduction. These frameworks operate at multiple levels, from international agreements to national strategies and local implementation plans. The Paris Agreement serves as the cornerstone of international climate policy, establishing the goal of limiting global warming to well below 2°C above pre-industrial levels. This agreement has prompted nations to develop Nationally Determined Contributions (NDCs) that increasingly recognize carbon capture and utilization technologies as essential components of their climate strategies.
At the national level, countries are implementing diverse policy instruments to accelerate the transition to carbon neutrality. Carbon pricing mechanisms, including carbon taxes and emissions trading systems, create economic incentives for CO2 reduction technologies by internalizing the external costs of carbon emissions. The European Union's Emissions Trading System (EU ETS) and China's national carbon market represent significant examples that directly influence investment in electrocatalytic CO2 reduction research and commercialization.
Research and development subsidies constitute another critical policy tool, with governments worldwide allocating substantial funding to advance carbon neutrality technologies. The United States Department of Energy's ARPA-E program and the EU's Horizon Europe initiative exemplify targeted funding mechanisms that support breakthrough innovations in electrocatalytic processes. These programs often emphasize public-private partnerships to bridge the gap between laboratory research and commercial deployment.
Regulatory frameworks also play a pivotal role in technology adoption. Product standards, certification systems, and performance requirements create market pull for low-carbon alternatives produced through electrocatalytic CO2 reduction. For instance, renewable fuel standards in transportation sectors can create immediate markets for electrocatalytically produced fuels and chemicals.
Industry-specific policies further shape the landscape for carbon neutrality technologies. Sectoral approaches targeting hard-to-abate industries like cement, steel, and chemicals provide tailored support for implementing CO2 reduction technologies where they can have the most significant impact. These policies often combine technology mandates with financial incentives to overcome adoption barriers.
Looking forward, policy integration across energy, industry, and climate domains will be essential for creating coherent frameworks that support electrocatalytic CO2 reduction. Successful policy approaches will need to address the entire innovation chain, from basic research to market deployment, while ensuring that regulatory certainty provides investors with the confidence to support these emerging technologies at scale.
At the national level, countries are implementing diverse policy instruments to accelerate the transition to carbon neutrality. Carbon pricing mechanisms, including carbon taxes and emissions trading systems, create economic incentives for CO2 reduction technologies by internalizing the external costs of carbon emissions. The European Union's Emissions Trading System (EU ETS) and China's national carbon market represent significant examples that directly influence investment in electrocatalytic CO2 reduction research and commercialization.
Research and development subsidies constitute another critical policy tool, with governments worldwide allocating substantial funding to advance carbon neutrality technologies. The United States Department of Energy's ARPA-E program and the EU's Horizon Europe initiative exemplify targeted funding mechanisms that support breakthrough innovations in electrocatalytic processes. These programs often emphasize public-private partnerships to bridge the gap between laboratory research and commercial deployment.
Regulatory frameworks also play a pivotal role in technology adoption. Product standards, certification systems, and performance requirements create market pull for low-carbon alternatives produced through electrocatalytic CO2 reduction. For instance, renewable fuel standards in transportation sectors can create immediate markets for electrocatalytically produced fuels and chemicals.
Industry-specific policies further shape the landscape for carbon neutrality technologies. Sectoral approaches targeting hard-to-abate industries like cement, steel, and chemicals provide tailored support for implementing CO2 reduction technologies where they can have the most significant impact. These policies often combine technology mandates with financial incentives to overcome adoption barriers.
Looking forward, policy integration across energy, industry, and climate domains will be essential for creating coherent frameworks that support electrocatalytic CO2 reduction. Successful policy approaches will need to address the entire innovation chain, from basic research to market deployment, while ensuring that regulatory certainty provides investors with the confidence to support these emerging technologies at scale.
Economic Viability of Electrocatalytic CO2 Conversion Systems
The economic viability of electrocatalytic CO2 conversion systems represents a critical factor in determining their potential for widespread industrial adoption. Current cost analyses indicate that capital expenditures for these systems range between $1,500-2,500 per kilowatt, significantly higher than conventional carbon-based energy technologies. This cost differential presents a substantial barrier to market entry despite the environmental benefits offered by CO2 conversion.
Operating expenses are similarly challenging, with electricity consumption accounting for 60-70% of total operational costs. At current industrial electricity rates averaging $0.07-0.12 per kWh in major markets, the economics remain unfavorable compared to traditional fossil fuel processes for producing similar chemical products.
Faradaic efficiency and energy conversion rates directly impact economic performance. Recent laboratory demonstrations have achieved efficiencies of 85-95% for CO and formate production, but these results have not been consistently replicated at industrial scales where efficiencies typically drop to 60-75%, negatively affecting production economics.
Product value streams present varying economic prospects. High-value chemicals like ethylene and ethanol (market values of $1,000-1,500/ton) offer better economic returns than lower-value products like carbon monoxide ($300-500/ton). This differential suggests that initial commercialization efforts should focus on higher-value chemical pathways to improve economic viability.
Integration with renewable energy sources presents a pathway to improved economics. Systems coupled with dedicated solar or wind installations can reduce operational costs by 30-40% in optimal locations, though intermittency issues require additional consideration for continuous industrial operations.
Policy incentives significantly influence economic viability. Carbon pricing mechanisms (currently ranging from $15-50/ton CO2 in various markets), tax credits for carbon utilization, and renewable energy subsidies collectively improve the business case. Models suggest that a carbon price of approximately $80-100/ton CO2 would make many electrocatalytic conversion processes competitive with conventional alternatives.
Scale-up economics demonstrate potential for cost reduction through economies of scale. Industry projections indicate that capital costs could decrease by 40-50% with gigawatt-scale deployment, while operational efficiencies could improve by 15-25%, substantially enhancing long-term economic viability as the technology matures and manufacturing processes are optimized.
Operating expenses are similarly challenging, with electricity consumption accounting for 60-70% of total operational costs. At current industrial electricity rates averaging $0.07-0.12 per kWh in major markets, the economics remain unfavorable compared to traditional fossil fuel processes for producing similar chemical products.
Faradaic efficiency and energy conversion rates directly impact economic performance. Recent laboratory demonstrations have achieved efficiencies of 85-95% for CO and formate production, but these results have not been consistently replicated at industrial scales where efficiencies typically drop to 60-75%, negatively affecting production economics.
Product value streams present varying economic prospects. High-value chemicals like ethylene and ethanol (market values of $1,000-1,500/ton) offer better economic returns than lower-value products like carbon monoxide ($300-500/ton). This differential suggests that initial commercialization efforts should focus on higher-value chemical pathways to improve economic viability.
Integration with renewable energy sources presents a pathway to improved economics. Systems coupled with dedicated solar or wind installations can reduce operational costs by 30-40% in optimal locations, though intermittency issues require additional consideration for continuous industrial operations.
Policy incentives significantly influence economic viability. Carbon pricing mechanisms (currently ranging from $15-50/ton CO2 in various markets), tax credits for carbon utilization, and renewable energy subsidies collectively improve the business case. Models suggest that a carbon price of approximately $80-100/ton CO2 would make many electrocatalytic conversion processes competitive with conventional alternatives.
Scale-up economics demonstrate potential for cost reduction through economies of scale. Industry projections indicate that capital costs could decrease by 40-50% with gigawatt-scale deployment, while operational efficiencies could improve by 15-25%, substantially enhancing long-term economic viability as the technology matures and manufacturing processes are optimized.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!