Evaluation of Electrocatalytic CO2 reduction for renewable energy storage and synthesis
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Technology Background and Objectives
Electrocatalytic CO2 reduction represents a transformative technology at the intersection of renewable energy and sustainable chemistry. The process involves converting carbon dioxide, a primary greenhouse gas, into valuable chemical feedstocks and fuels using electrical energy. This technology has evolved significantly since its conceptual introduction in the 1980s, with major breakthroughs occurring in the past decade as climate change concerns and renewable energy integration challenges have intensified.
The historical trajectory of CO2 reduction technology began with fundamental electrochemical studies, progressing through catalyst development phases from simple metal electrodes to today's advanced nanomaterials and molecular catalysts. Recent technological evolution has focused on improving selectivity, efficiency, and stability - the three critical parameters that determine commercial viability.
Current technological trends indicate a convergence of materials science, electrochemistry, and process engineering to overcome persistent challenges. The field is witnessing rapid advancement in computational catalyst design, operando characterization techniques, and system integration approaches that promise to accelerate development cycles.
The primary technical objective of electrocatalytic CO2 reduction is to achieve economically viable conversion of CO2 to value-added products while utilizing renewable electricity. Specific goals include developing catalysts capable of >90% Faradaic efficiency toward single products, reducing overpotentials to <0.5V, maintaining stability for thousands of hours, and scaling systems to industrial relevance.
Secondary objectives encompass the integration of CO2 reduction systems with renewable energy sources to provide grid-balancing services, thereby addressing intermittency challenges. This creates a dual-purpose technology that simultaneously mitigates carbon emissions and enhances renewable energy deployment.
Long-term aspirations for this technology include creating artificial carbon cycles that mimic natural processes but operate at accelerated rates and with precise product selectivity. The ultimate vision involves establishing distributed, modular CO2 conversion facilities powered by renewable electricity that transform waste carbon dioxide into chemical building blocks for a circular economy.
The technology aims to bridge multiple sectors including renewable energy, chemical manufacturing, and carbon management, potentially disrupting conventional petrochemical supply chains while creating new economic opportunities in green chemistry and sustainable manufacturing.
The historical trajectory of CO2 reduction technology began with fundamental electrochemical studies, progressing through catalyst development phases from simple metal electrodes to today's advanced nanomaterials and molecular catalysts. Recent technological evolution has focused on improving selectivity, efficiency, and stability - the three critical parameters that determine commercial viability.
Current technological trends indicate a convergence of materials science, electrochemistry, and process engineering to overcome persistent challenges. The field is witnessing rapid advancement in computational catalyst design, operando characterization techniques, and system integration approaches that promise to accelerate development cycles.
The primary technical objective of electrocatalytic CO2 reduction is to achieve economically viable conversion of CO2 to value-added products while utilizing renewable electricity. Specific goals include developing catalysts capable of >90% Faradaic efficiency toward single products, reducing overpotentials to <0.5V, maintaining stability for thousands of hours, and scaling systems to industrial relevance.
Secondary objectives encompass the integration of CO2 reduction systems with renewable energy sources to provide grid-balancing services, thereby addressing intermittency challenges. This creates a dual-purpose technology that simultaneously mitigates carbon emissions and enhances renewable energy deployment.
Long-term aspirations for this technology include creating artificial carbon cycles that mimic natural processes but operate at accelerated rates and with precise product selectivity. The ultimate vision involves establishing distributed, modular CO2 conversion facilities powered by renewable electricity that transform waste carbon dioxide into chemical building blocks for a circular economy.
The technology aims to bridge multiple sectors including renewable energy, chemical manufacturing, and carbon management, potentially disrupting conventional petrochemical supply chains while creating new economic opportunities in green chemistry and sustainable manufacturing.
Market Analysis for CO2 Conversion Products
The global market for CO2 conversion products is experiencing significant growth, driven by increasing environmental regulations and the push for sustainable industrial practices. The market size for CO2-derived products was valued at approximately $5.8 billion in 2021 and is projected to reach $12.3 billion by 2028, representing a compound annual growth rate (CAGR) of 11.2%. This growth trajectory reflects the expanding applications of CO2 conversion technologies across multiple industries.
Carbon dioxide conversion yields several commercially viable products, each with distinct market dynamics. Methanol, a primary product of CO2 reduction, has a robust global market exceeding $31 billion, with demand primarily from the chemical industry for formaldehyde production and increasingly as a clean fuel alternative. Formic acid, another significant conversion product, has a market value of $2.3 billion with applications in agriculture, textiles, and emerging uses in hydrogen storage systems.
Syngas (CO + H2) derived from CO2 reduction serves as a versatile feedstock for various chemical processes, including Fischer-Tropsch synthesis for producing synthetic fuels. The market for CO2-derived syngas is growing at 9.7% annually, driven by its role as an intermediate in sustainable fuel production pathways. Carbon monoxide as a standalone product has established markets in metallurgical processes and chemical manufacturing.
Ethylene and ethanol, higher-value products from CO2 electroreduction, represent particularly attractive market opportunities due to their widespread use in polymer production and as fuel additives, respectively. The global ethylene market exceeds $230 billion, with growing interest in bio-based and CO2-derived alternatives to petroleum-sourced ethylene.
Regional analysis reveals that Europe currently leads in CO2 conversion product deployment, supported by stringent carbon regulations and substantial government funding for carbon capture and utilization technologies. North America follows closely, with significant private sector investment in CO2 utilization startups. The Asia-Pacific region, particularly China, is experiencing the fastest growth rate in this sector, driven by national decarbonization policies and industrial expansion.
Key market drivers include increasingly stringent carbon pricing mechanisms, corporate sustainability commitments, and consumer preference for environmentally responsible products. The economic viability of CO2-derived products is improving as renewable electricity costs decline and carbon taxes increase, narrowing the price gap with conventional petrochemical routes. Industry analysts project that several CO2-derived chemicals will achieve price parity with fossil-based alternatives by 2030, particularly in regions with favorable regulatory frameworks.
Carbon dioxide conversion yields several commercially viable products, each with distinct market dynamics. Methanol, a primary product of CO2 reduction, has a robust global market exceeding $31 billion, with demand primarily from the chemical industry for formaldehyde production and increasingly as a clean fuel alternative. Formic acid, another significant conversion product, has a market value of $2.3 billion with applications in agriculture, textiles, and emerging uses in hydrogen storage systems.
Syngas (CO + H2) derived from CO2 reduction serves as a versatile feedstock for various chemical processes, including Fischer-Tropsch synthesis for producing synthetic fuels. The market for CO2-derived syngas is growing at 9.7% annually, driven by its role as an intermediate in sustainable fuel production pathways. Carbon monoxide as a standalone product has established markets in metallurgical processes and chemical manufacturing.
Ethylene and ethanol, higher-value products from CO2 electroreduction, represent particularly attractive market opportunities due to their widespread use in polymer production and as fuel additives, respectively. The global ethylene market exceeds $230 billion, with growing interest in bio-based and CO2-derived alternatives to petroleum-sourced ethylene.
Regional analysis reveals that Europe currently leads in CO2 conversion product deployment, supported by stringent carbon regulations and substantial government funding for carbon capture and utilization technologies. North America follows closely, with significant private sector investment in CO2 utilization startups. The Asia-Pacific region, particularly China, is experiencing the fastest growth rate in this sector, driven by national decarbonization policies and industrial expansion.
Key market drivers include increasingly stringent carbon pricing mechanisms, corporate sustainability commitments, and consumer preference for environmentally responsible products. The economic viability of CO2-derived products is improving as renewable electricity costs decline and carbon taxes increase, narrowing the price gap with conventional petrochemical routes. Industry analysts project that several CO2-derived chemicals will achieve price parity with fossil-based alternatives by 2030, particularly in regions with favorable regulatory frameworks.
Current Electrocatalytic CO2 Reduction Challenges
Despite significant advancements in electrocatalytic CO2 reduction (ECR) technology, several critical challenges continue to impede its widespread commercial implementation. The primary obstacle remains catalyst efficiency, with current catalysts exhibiting insufficient selectivity toward valuable multi-carbon products. Most systems still favor hydrogen evolution reaction (HER) over CO2 reduction, resulting in low Faradaic efficiencies for desired products and energy waste.
Stability presents another major hurdle, as many promising catalysts demonstrate rapid performance degradation under industrial operating conditions. Copper-based catalysts, while showing excellent C-C coupling capabilities, suffer from surface reconstruction and poisoning during extended operation periods, typically losing significant activity within hours or days rather than maintaining performance over the months required for commercial viability.
Energy efficiency remains suboptimal, with current systems requiring excessive overpotentials (>1V) to drive the reaction at practical rates. This high energy input significantly reduces the overall process efficiency and economic viability, particularly when considering the renewable energy storage application pathway where energy conservation is paramount.
Scalability challenges persist in electrode design and system engineering. Laboratory-scale demonstrations often utilize expensive materials and complex configurations that prove difficult to scale to industrial levels. The gap between promising lab results and practical implementation continues to widen as catalyst performance frequently deteriorates when moving to larger systems with higher current densities.
Mass transport limitations represent a fundamental challenge due to CO2's low solubility in aqueous electrolytes (approximately 30 mM at ambient conditions). This creates concentration gradients near catalyst surfaces and limits reaction rates, while also contributing to pH management issues as hydroxide accumulation at the cathode interface alters local reaction conditions.
Mechanistic understanding remains incomplete, hampering rational catalyst design. The complex multi-electron, multi-proton transfer processes involved in CO2 reduction to various products follow pathways that are not fully elucidated, making systematic improvement difficult.
Economic barriers further complicate commercialization prospects. Current techno-economic analyses indicate that ECR processes remain 2-5 times more expensive than conventional fossil-based routes for producing equivalent chemicals, with capital costs and energy inputs being the primary contributors to this gap. Without significant breakthroughs in catalyst performance or substantial carbon pricing mechanisms, achieving cost parity remains challenging.
Stability presents another major hurdle, as many promising catalysts demonstrate rapid performance degradation under industrial operating conditions. Copper-based catalysts, while showing excellent C-C coupling capabilities, suffer from surface reconstruction and poisoning during extended operation periods, typically losing significant activity within hours or days rather than maintaining performance over the months required for commercial viability.
Energy efficiency remains suboptimal, with current systems requiring excessive overpotentials (>1V) to drive the reaction at practical rates. This high energy input significantly reduces the overall process efficiency and economic viability, particularly when considering the renewable energy storage application pathway where energy conservation is paramount.
Scalability challenges persist in electrode design and system engineering. Laboratory-scale demonstrations often utilize expensive materials and complex configurations that prove difficult to scale to industrial levels. The gap between promising lab results and practical implementation continues to widen as catalyst performance frequently deteriorates when moving to larger systems with higher current densities.
Mass transport limitations represent a fundamental challenge due to CO2's low solubility in aqueous electrolytes (approximately 30 mM at ambient conditions). This creates concentration gradients near catalyst surfaces and limits reaction rates, while also contributing to pH management issues as hydroxide accumulation at the cathode interface alters local reaction conditions.
Mechanistic understanding remains incomplete, hampering rational catalyst design. The complex multi-electron, multi-proton transfer processes involved in CO2 reduction to various products follow pathways that are not fully elucidated, making systematic improvement difficult.
Economic barriers further complicate commercialization prospects. Current techno-economic analyses indicate that ECR processes remain 2-5 times more expensive than conventional fossil-based routes for producing equivalent chemicals, with capital costs and energy inputs being the primary contributors to this gap. Without significant breakthroughs in catalyst performance or substantial carbon pricing mechanisms, achieving cost parity remains challenging.
State-of-the-Art Electrocatalytic Systems
01 Metal-based catalysts for CO2 reduction
Various metal-based catalysts can be employed for electrocatalytic CO2 reduction to enhance efficiency and selectivity. These include single metal catalysts, bimetallic catalysts, and metal alloys that offer different catalytic pathways. The selection of specific metals such as copper, silver, gold, or zinc significantly influences product distribution, with copper being particularly effective for producing multi-carbon products. Catalyst structure and morphology, including nanoparticles, nanowires, and porous structures, play crucial roles in determining active sites and reaction kinetics.- Catalyst design for improved CO2 reduction efficiency: Advanced catalyst materials can significantly enhance the efficiency of electrocatalytic CO2 reduction. These catalysts are designed with specific structures and compositions to lower the activation energy required for CO2 reduction reactions. Novel metal-based catalysts, including single-atom catalysts, nanostructured materials, and bimetallic systems, have demonstrated superior performance in terms of conversion efficiency and energy utilization during the CO2 reduction process.
- Selectivity enhancement through catalyst modification: Modifying catalysts through doping, surface functionalization, or structural engineering can significantly improve product selectivity in CO2 reduction reactions. These modifications alter the electronic structure and binding properties of the catalyst surface, directing the reaction pathway toward specific products such as CO, formate, methanol, or hydrocarbons. Controlled synthesis methods enable precise tuning of catalyst properties to achieve desired product distributions and suppress competing reactions like hydrogen evolution.
- Electrolyte composition and reaction conditions optimization: The composition of the electrolyte and reaction conditions play crucial roles in determining both efficiency and selectivity of CO2 electroreduction. Parameters such as pH, ionic strength, temperature, pressure, and the presence of specific ions or additives can be optimized to enhance catalytic performance. Buffered electrolytes, ionic liquids, and electrolytes with CO2-solubilizing agents have been developed to increase local CO2 concentration at the electrode surface, thereby improving reaction rates and product selectivity.
- Electrode and reactor design innovations: Advanced electrode architectures and reactor designs can significantly improve mass transport, charge transfer, and product separation in CO2 electroreduction systems. Three-dimensional electrodes, gas diffusion electrodes, and flow-cell configurations enhance CO2 delivery to catalytic sites and facilitate product removal. Integrated systems with optimized flow patterns, membrane separators, and controlled potential distribution enable higher current densities and sustained performance, addressing scale-up challenges for practical applications.
- In-situ characterization and mechanistic understanding: In-situ and operando characterization techniques provide critical insights into reaction mechanisms and catalyst behavior under working conditions. Advanced spectroscopic, microscopic, and electrochemical methods help identify active sites, intermediate species, and degradation pathways during CO2 reduction. This mechanistic understanding guides rational catalyst design and process optimization, enabling the development of more efficient and selective electrocatalytic systems with improved stability and performance for practical CO2 conversion applications.
02 Carbon-based and composite electrocatalysts
Carbon-based materials and composites offer promising platforms for CO2 electroreduction with improved efficiency and selectivity. These include nitrogen-doped carbon, carbon nanotubes, graphene-based materials, and metal-organic frameworks. The incorporation of heteroatoms like nitrogen, sulfur, or phosphorus into carbon structures creates active sites with enhanced catalytic properties. Metal-carbon composites combine the advantages of both components, where carbon materials provide high surface area and conductivity while metal components offer specific catalytic activity.Expand Specific Solutions03 Electrolyte composition and reaction conditions
The composition of the electrolyte and reaction conditions significantly impact the efficiency and selectivity of CO2 electroreduction. Factors such as pH, ionic strength, buffer capacity, and specific ions present in the electrolyte influence reaction pathways and product distribution. Temperature, pressure, and CO2 concentration affect solubility and mass transport. The applied potential and current density are critical parameters that determine which products are formed. Optimizing these conditions can enhance reaction rates and direct selectivity toward desired products.Expand Specific Solutions04 Electrode design and reactor configuration
Advanced electrode designs and reactor configurations can significantly improve CO2 reduction performance. Gas diffusion electrodes facilitate efficient CO2 transport to catalytic sites, overcoming solubility limitations. Flow cells and microfluidic reactors provide better mass transport and reaction control. Three-dimensional electrode structures increase the number of active sites per geometric area. Membrane electrode assemblies separate reaction products and prevent crossover. These engineering approaches address mass transport limitations and improve overall system efficiency.Expand Specific Solutions05 In-situ characterization and mechanistic studies
In-situ characterization techniques and mechanistic studies provide crucial insights for developing more efficient and selective CO2 reduction catalysts. Spectroscopic methods such as X-ray absorption spectroscopy, Raman spectroscopy, and infrared spectroscopy reveal catalyst structure and intermediate species during reaction. Computational methods including density functional theory calculations help understand reaction pathways and energy barriers. These approaches enable rational catalyst design by identifying rate-determining steps and key intermediates that influence product selectivity.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalytic CO2 reduction market is currently in an early growth phase, characterized by intensive research and development activities across academic institutions and industrial players. The global market size is projected to expand significantly as renewable energy storage solutions gain traction, with estimates suggesting a compound annual growth rate exceeding 10% through 2030. Technologically, the field remains in development with varying maturity levels across different catalytic approaches. Leading research institutions like Dalian Institute of Chemical Physics, California Institute of Technology, and Sorbonne Université are advancing fundamental science, while industrial players including Siemens Energy, TotalEnergies, and Saudi Aramco are focusing on scalable applications. University-industry collaborations are accelerating technology transfer, with notable progress from consortia involving CNRS, Brown University, and Korean KAIST, suggesting the technology is approaching commercial viability for specific applications within renewable energy storage and chemical synthesis pathways.
Dalian Institute of Chemical Physics of CAS
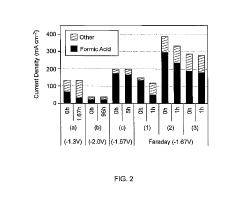
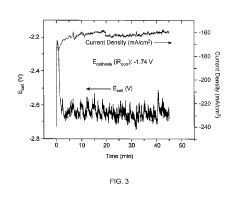
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced copper-based catalysts for electrochemical CO2 reduction with remarkable selectivity toward multi-carbon products. Their innovative approach involves precise control of copper catalyst morphology and electronic structure through atomic-level engineering. DICP researchers have created oxide-derived copper catalysts that demonstrate enhanced C-C coupling capabilities, achieving Faradaic efficiencies exceeding 60% for C2+ products at industrially relevant current densities. Their technology incorporates in-situ spectroscopic techniques to monitor reaction intermediates, enabling rational catalyst design. DICP has also pioneered the development of tandem catalytic systems that integrate CO2 capture and conversion in a single process, significantly improving energy efficiency. Their gas diffusion electrode configurations have demonstrated stable operation for over 1000 hours with minimal performance degradation, addressing key challenges in catalyst stability and durability.
Strengths: Superior selectivity toward multi-carbon products with high Faradaic efficiency; excellent catalyst stability; integrated CO2 capture and conversion systems. Weaknesses: Higher energy requirements compared to thermochemical approaches; potential scalability challenges for industrial implementation; requires further optimization for commercial viability.
Siemens Energy Global GmbH & Co. KG
Technical Solution: Siemens Energy has developed an integrated electrocatalytic CO2 reduction system designed for industrial-scale implementation. Their technology employs a modular electrolyzer architecture that can be scaled from kilowatt to megawatt capacity, addressing key challenges in technology deployment. Siemens' approach utilizes silver-based gas diffusion electrodes optimized for CO production with Faradaic efficiencies exceeding 90% at industrially relevant current densities (>200 mA/cm²). Their system incorporates advanced flow field designs that ensure uniform reactant distribution across large-area electrodes, maintaining performance consistency at scale. Siemens has also developed proprietary membrane electrode assemblies that minimize crossover effects while reducing internal resistance, enabling operation at cell voltages below 3V. Their technology integrates with renewable power sources through sophisticated power electronics that accommodate intermittent electricity supply, making it particularly suitable for energy storage applications. Siemens' pilot installations have demonstrated continuous operation exceeding 10,000 hours with minimal performance degradation.
Strengths: Industrial-scale system integration; robust operation with intermittent power sources; proven long-term stability in pilot demonstrations. Weaknesses: Limited product diversity (primarily CO production); higher capital costs compared to conventional processes; requires high-purity CO2 feedstock for optimal performance.
Key Catalyst Materials and Reaction Mechanisms
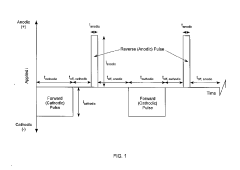
Pulsed current catalyzed gas diffusion electrodes for high rate, efficient co2 conversion reactors
PatentInactiveUS20190112720A1
Innovation
- The development of a gas diffusion electrode (GDE) with a high surface area microporous layer and electrochemically deposited Sn catalyst using pulse/pulse reverse electrodeposition, creating a uniform, adherent nanostructured catalyst layer for enhanced CO2 reduction efficiency and selectivity.
Techno-economic Assessment and Scalability
The techno-economic assessment of electrocatalytic CO2 reduction reveals significant challenges in scaling this technology from laboratory demonstrations to industrial applications. Current cost analyses indicate that capital expenditures for electrolyzer systems range between $500-2,000/kW, with operational costs heavily dependent on electricity prices, which typically constitute 60-80% of total operational expenses. For CO2 reduction to become economically viable, electricity costs must fall below $0.04/kWh, a threshold achievable only in regions with abundant renewable energy resources.
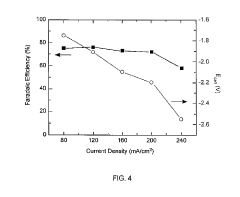
Production scale remains a critical barrier, with most successful demonstrations limited to laboratory scales of less than 100 cm² electrode area. Industrial implementation would require scaling to several square meters per cell, introducing challenges in maintaining reaction selectivity and efficiency across larger electrode surfaces. Current Faradaic efficiencies for valuable products like ethylene and ethanol typically decrease by 15-30% when scaling from laboratory to pilot scale.
Energy efficiency presents another significant challenge, with most systems operating at 30-45% efficiency when considering both electrical input and product value output. To achieve commercial viability, this efficiency must increase to at least 60%, requiring advances in catalyst design and system engineering. The theoretical minimum energy requirement for CO2 reduction is approximately 750 kJ/mol, but practical systems currently require 2-3 times this amount.
Catalyst stability and durability significantly impact economic feasibility. Current state-of-the-art catalysts demonstrate performance degradation of 0.5-2% per 100 hours of operation, whereas commercial viability requires stability over thousands of hours with less than 10% performance loss. This degradation directly affects maintenance costs and system lifetime, which are critical factors in techno-economic models.
Infrastructure requirements present additional scaling challenges. Industrial implementation would necessitate integration with carbon capture systems, renewable energy sources, and product separation technologies. The capital investment for a moderate-scale plant (producing 1,000 tons/year of carbon-based products) is estimated at $15-25 million, with payback periods currently exceeding 8-10 years under most market conditions.
Recent modeling suggests that with technological improvements and favorable policy environments, electrocatalytic CO2 reduction could become economically competitive by 2030-2035 for high-value products like ethylene and ethanol, while commodity chemicals like methanol may require additional technological breakthroughs or carbon pricing mechanisms to achieve market viability.
Production scale remains a critical barrier, with most successful demonstrations limited to laboratory scales of less than 100 cm² electrode area. Industrial implementation would require scaling to several square meters per cell, introducing challenges in maintaining reaction selectivity and efficiency across larger electrode surfaces. Current Faradaic efficiencies for valuable products like ethylene and ethanol typically decrease by 15-30% when scaling from laboratory to pilot scale.
Energy efficiency presents another significant challenge, with most systems operating at 30-45% efficiency when considering both electrical input and product value output. To achieve commercial viability, this efficiency must increase to at least 60%, requiring advances in catalyst design and system engineering. The theoretical minimum energy requirement for CO2 reduction is approximately 750 kJ/mol, but practical systems currently require 2-3 times this amount.
Catalyst stability and durability significantly impact economic feasibility. Current state-of-the-art catalysts demonstrate performance degradation of 0.5-2% per 100 hours of operation, whereas commercial viability requires stability over thousands of hours with less than 10% performance loss. This degradation directly affects maintenance costs and system lifetime, which are critical factors in techno-economic models.
Infrastructure requirements present additional scaling challenges. Industrial implementation would necessitate integration with carbon capture systems, renewable energy sources, and product separation technologies. The capital investment for a moderate-scale plant (producing 1,000 tons/year of carbon-based products) is estimated at $15-25 million, with payback periods currently exceeding 8-10 years under most market conditions.
Recent modeling suggests that with technological improvements and favorable policy environments, electrocatalytic CO2 reduction could become economically competitive by 2030-2035 for high-value products like ethylene and ethanol, while commodity chemicals like methanol may require additional technological breakthroughs or carbon pricing mechanisms to achieve market viability.
Policy Frameworks for Carbon Utilization Technologies
The development of effective policy frameworks is crucial for advancing carbon utilization technologies, particularly electrocatalytic CO2 reduction. Current global policy landscapes exhibit significant variations in their approach to carbon management, with some regions implementing comprehensive carbon pricing mechanisms while others focus on targeted subsidies for renewable technologies.
The European Union leads with its Emissions Trading System (ETS) and the Carbon Border Adjustment Mechanism, creating economic incentives for CO2 reduction technologies. These policies establish a price on carbon emissions, thereby making carbon capture and utilization economically viable for industrial stakeholders. The EU's Renewable Energy Directive further supports electrocatalytic processes by promoting renewable electricity integration with industrial applications.
In North America, policy approaches differ between jurisdictions. The United States has implemented tax credits through the 45Q provision, offering up to $50 per metric ton of CO2 utilized in industrial processes. Canada's Clean Fuel Standard creates market opportunities for low-carbon fuel production through electrocatalytic CO2 reduction. However, both regions lack comprehensive federal frameworks specifically addressing electrochemical carbon conversion technologies.
Asia-Pacific nations demonstrate varied policy maturity. Japan's "Beyond Zero Carbon" initiative and South Korea's Hydrogen Economy Roadmap explicitly support electrocatalytic technologies for energy storage applications. China's inclusion of carbon utilization in its Five-Year Plans signals growing policy attention, though implementation remains fragmented across provinces.
Regulatory barriers persist across all regions, particularly regarding product certification and grid integration. The classification of electrocatalytically produced fuels and chemicals often falls into regulatory gray areas, creating market uncertainty. Additionally, policies governing electricity market access and pricing significantly impact the economic viability of electrocatalytic CO2 reduction systems.
Future policy development requires harmonization of technical standards for electrocatalytic products and processes. International collaboration frameworks, such as the Mission Innovation Carbon Capture Challenge, are beginning to address this need by establishing common metrics for technology assessment. The development of life cycle assessment methodologies specific to electrocatalytic processes will be essential for accurate policy design.
Financial incentive structures need evolution beyond traditional research grants toward commercialization support. Policy mechanisms that recognize both the climate mitigation value and grid balancing capabilities of electrocatalytic CO2 reduction would create stronger market signals. Integrated approaches that combine carbon pricing, renewable energy policies, and industrial strategy offer the most promising pathway for technology advancement.
The European Union leads with its Emissions Trading System (ETS) and the Carbon Border Adjustment Mechanism, creating economic incentives for CO2 reduction technologies. These policies establish a price on carbon emissions, thereby making carbon capture and utilization economically viable for industrial stakeholders. The EU's Renewable Energy Directive further supports electrocatalytic processes by promoting renewable electricity integration with industrial applications.
In North America, policy approaches differ between jurisdictions. The United States has implemented tax credits through the 45Q provision, offering up to $50 per metric ton of CO2 utilized in industrial processes. Canada's Clean Fuel Standard creates market opportunities for low-carbon fuel production through electrocatalytic CO2 reduction. However, both regions lack comprehensive federal frameworks specifically addressing electrochemical carbon conversion technologies.
Asia-Pacific nations demonstrate varied policy maturity. Japan's "Beyond Zero Carbon" initiative and South Korea's Hydrogen Economy Roadmap explicitly support electrocatalytic technologies for energy storage applications. China's inclusion of carbon utilization in its Five-Year Plans signals growing policy attention, though implementation remains fragmented across provinces.
Regulatory barriers persist across all regions, particularly regarding product certification and grid integration. The classification of electrocatalytically produced fuels and chemicals often falls into regulatory gray areas, creating market uncertainty. Additionally, policies governing electricity market access and pricing significantly impact the economic viability of electrocatalytic CO2 reduction systems.
Future policy development requires harmonization of technical standards for electrocatalytic products and processes. International collaboration frameworks, such as the Mission Innovation Carbon Capture Challenge, are beginning to address this need by establishing common metrics for technology assessment. The development of life cycle assessment methodologies specific to electrocatalytic processes will be essential for accurate policy design.
Financial incentive structures need evolution beyond traditional research grants toward commercialization support. Policy mechanisms that recognize both the climate mitigation value and grid balancing capabilities of electrocatalytic CO2 reduction would create stronger market signals. Integrated approaches that combine carbon pricing, renewable energy policies, and industrial strategy offer the most promising pathway for technology advancement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!