Electrocatalytic CO2 reduction interface design for enhanced catalytic activity
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Electrocatalysis Background and Objectives
Electrocatalytic CO2 reduction has emerged as a promising approach to address the dual challenges of climate change and sustainable energy production. This technology enables the conversion of carbon dioxide, a major greenhouse gas, into value-added chemicals and fuels using renewable electricity. The concept dates back to the 1980s, but significant advancements have only materialized in the past decade with the development of novel catalysts and improved understanding of interfacial phenomena.
The evolution of CO2 reduction electrocatalysis has progressed through several distinct phases. Initially, metal electrodes such as copper, silver, and gold were investigated for their CO2 reduction capabilities. Subsequently, research expanded to include metal alloys, oxide-derived metals, and more recently, single-atom catalysts and molecular catalysts. Each advancement has contributed to improved selectivity, activity, and energy efficiency of the CO2 reduction process.
Current technological trends point toward interface engineering as a critical frontier in this field. The electrode-electrolyte interface represents the reaction zone where CO2 reduction occurs, and its properties significantly influence catalytic performance. Recent studies have demonstrated that manipulating the local environment at this interface—through the introduction of specific functional groups, controlling hydrophobicity/hydrophilicity, or creating hierarchical structures—can dramatically enhance catalytic activity and product selectivity.
The primary technical objectives in this domain include achieving higher Faradaic efficiencies toward specific products, reducing overpotentials, enhancing reaction rates, and maintaining catalyst stability during long-term operation. Additionally, there is growing interest in developing systems capable of producing multi-carbon products, which typically have higher economic value but present greater catalytic challenges due to the multiple electron transfer steps required.
Beyond laboratory-scale demonstrations, researchers aim to develop scalable electrode architectures and reactor designs that can maintain high performance under industrially relevant conditions. This includes addressing challenges related to mass transport limitations, CO2 solubility in aqueous electrolytes, and integration with renewable energy sources to ensure the process remains carbon-negative overall.
The ultimate goal of electrocatalytic CO2 reduction research is to establish economically viable pathways for closing the carbon cycle, effectively transforming CO2 from an environmental liability into a valuable feedstock for chemical production. Success in this endeavor could revolutionize how we approach both carbon management and chemical manufacturing, creating a more sustainable industrial ecosystem while mitigating climate change impacts.
The evolution of CO2 reduction electrocatalysis has progressed through several distinct phases. Initially, metal electrodes such as copper, silver, and gold were investigated for their CO2 reduction capabilities. Subsequently, research expanded to include metal alloys, oxide-derived metals, and more recently, single-atom catalysts and molecular catalysts. Each advancement has contributed to improved selectivity, activity, and energy efficiency of the CO2 reduction process.
Current technological trends point toward interface engineering as a critical frontier in this field. The electrode-electrolyte interface represents the reaction zone where CO2 reduction occurs, and its properties significantly influence catalytic performance. Recent studies have demonstrated that manipulating the local environment at this interface—through the introduction of specific functional groups, controlling hydrophobicity/hydrophilicity, or creating hierarchical structures—can dramatically enhance catalytic activity and product selectivity.
The primary technical objectives in this domain include achieving higher Faradaic efficiencies toward specific products, reducing overpotentials, enhancing reaction rates, and maintaining catalyst stability during long-term operation. Additionally, there is growing interest in developing systems capable of producing multi-carbon products, which typically have higher economic value but present greater catalytic challenges due to the multiple electron transfer steps required.
Beyond laboratory-scale demonstrations, researchers aim to develop scalable electrode architectures and reactor designs that can maintain high performance under industrially relevant conditions. This includes addressing challenges related to mass transport limitations, CO2 solubility in aqueous electrolytes, and integration with renewable energy sources to ensure the process remains carbon-negative overall.
The ultimate goal of electrocatalytic CO2 reduction research is to establish economically viable pathways for closing the carbon cycle, effectively transforming CO2 from an environmental liability into a valuable feedstock for chemical production. Success in this endeavor could revolutionize how we approach both carbon management and chemical manufacturing, creating a more sustainable industrial ecosystem while mitigating climate change impacts.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market size for CO2 conversion was valued at approximately $1.8 billion in 2022 and is projected to reach $4.9 billion by 2030, growing at a CAGR of 13.3% during the forecast period. This growth trajectory reflects the urgent need for sustainable solutions to address climate change challenges.
Electrocatalytic CO2 reduction represents a particularly promising segment within this market, with increasing investments from both public and private sectors. Government funding for research in this area has seen a substantial increase, with the European Union allocating €300 million through its Horizon Europe program specifically for carbon capture and utilization technologies, including electrocatalytic approaches.
The industrial application landscape for CO2 conversion technologies spans multiple sectors. Chemical manufacturing leads adoption with approximately 35% market share, followed by energy production (25%), fuel synthesis (20%), and other applications including pharmaceuticals and agriculture (20%). Companies are increasingly recognizing the potential of converted CO2 as a valuable feedstock rather than merely a waste product to be sequestered.
Regional analysis reveals that North America currently dominates the market with 38% share, followed by Europe (32%), Asia-Pacific (25%), and rest of the world (5%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.7% annually, driven by China's aggressive carbon neutrality targets and significant investments in green technology infrastructure.
Key market drivers include stringent carbon emission regulations, rising carbon pricing mechanisms, and increasing corporate sustainability commitments. The European Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for CO2 conversion technologies. Additionally, consumer preference for green products is pushing companies to adopt carbon-neutral or negative production processes.
Market challenges include high capital costs for implementation, energy intensity of conversion processes, and competition from established carbon management approaches. The levelized cost of CO2 conversion through electrocatalytic methods currently ranges from $80-150 per ton, which remains higher than conventional carbon capture and storage ($40-80 per ton).
Future market growth will likely be catalyzed by technological breakthroughs in catalyst design, particularly at the electrocatalytic interface, which could significantly improve conversion efficiency and economic viability. Industry analysts predict that achieving a 30% improvement in catalytic activity could reduce conversion costs by up to 40%, potentially unlocking mass market adoption across multiple industries.
Electrocatalytic CO2 reduction represents a particularly promising segment within this market, with increasing investments from both public and private sectors. Government funding for research in this area has seen a substantial increase, with the European Union allocating €300 million through its Horizon Europe program specifically for carbon capture and utilization technologies, including electrocatalytic approaches.
The industrial application landscape for CO2 conversion technologies spans multiple sectors. Chemical manufacturing leads adoption with approximately 35% market share, followed by energy production (25%), fuel synthesis (20%), and other applications including pharmaceuticals and agriculture (20%). Companies are increasingly recognizing the potential of converted CO2 as a valuable feedstock rather than merely a waste product to be sequestered.
Regional analysis reveals that North America currently dominates the market with 38% share, followed by Europe (32%), Asia-Pacific (25%), and rest of the world (5%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.7% annually, driven by China's aggressive carbon neutrality targets and significant investments in green technology infrastructure.
Key market drivers include stringent carbon emission regulations, rising carbon pricing mechanisms, and increasing corporate sustainability commitments. The European Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for CO2 conversion technologies. Additionally, consumer preference for green products is pushing companies to adopt carbon-neutral or negative production processes.
Market challenges include high capital costs for implementation, energy intensity of conversion processes, and competition from established carbon management approaches. The levelized cost of CO2 conversion through electrocatalytic methods currently ranges from $80-150 per ton, which remains higher than conventional carbon capture and storage ($40-80 per ton).
Future market growth will likely be catalyzed by technological breakthroughs in catalyst design, particularly at the electrocatalytic interface, which could significantly improve conversion efficiency and economic viability. Industry analysts predict that achieving a 30% improvement in catalytic activity could reduce conversion costs by up to 40%, potentially unlocking mass market adoption across multiple industries.
Current Challenges in Electrocatalytic CO2 Reduction
Despite significant advancements in electrocatalytic CO2 reduction (ECR) technology, several critical challenges continue to impede its widespread implementation and commercial viability. The primary obstacle remains the low energy efficiency of the process, with current systems typically achieving only 30-50% Faradaic efficiency for valuable products like ethylene and ethanol. This inefficiency stems largely from competing hydrogen evolution reactions that consume electrons without contributing to carbon conversion.
Catalyst selectivity presents another formidable challenge. Most existing catalysts produce a mixture of products including CO, formate, methane, ethylene, and alcohols, necessitating costly downstream separation processes. The inability to precisely control reaction pathways toward specific high-value products significantly impacts economic feasibility of the technology.
Catalyst stability under industrial conditions remains problematic, with many promising materials showing performance degradation within hours or days of operation. Metal catalysts often suffer from poisoning, leaching, or structural changes during extended operation, while the harsh electrochemical environment accelerates degradation through mechanisms like oxidation and reconstruction of active sites.
Mass transport limitations at the electrode-electrolyte interface create significant concentration gradients that reduce reaction rates and selectivity. The low solubility of CO2 in aqueous electrolytes (approximately 33 mM at ambient conditions) severely restricts the availability of reactants at catalytic sites, while the formation of bicarbonate and carbonate species further complicates reaction kinetics.
Scale-up challenges persist as laboratory-scale successes often fail to translate to industrial applications. Current densities in lab demonstrations (typically 10-100 mA/cm²) fall short of the 200+ mA/cm² required for commercial viability. Additionally, the complex interplay between catalyst structure, electrolyte composition, and operating conditions makes systematic optimization difficult.
The high overpotential requirement for driving CO2 reduction reactions represents another significant barrier. Most catalysts require potentials 0.5-1.0 V beyond the thermodynamic minimum, substantially reducing energy efficiency and increasing operational costs. This challenge is particularly acute for multi-carbon product formation, which requires multiple electron transfer steps.
Finally, mechanistic understanding remains incomplete despite extensive research efforts. The complex reaction networks involving multiple intermediates and competing pathways are not fully characterized, hampering rational catalyst design. Advanced in-situ and operando characterization techniques are needed to elucidate reaction mechanisms under realistic operating conditions.
Catalyst selectivity presents another formidable challenge. Most existing catalysts produce a mixture of products including CO, formate, methane, ethylene, and alcohols, necessitating costly downstream separation processes. The inability to precisely control reaction pathways toward specific high-value products significantly impacts economic feasibility of the technology.
Catalyst stability under industrial conditions remains problematic, with many promising materials showing performance degradation within hours or days of operation. Metal catalysts often suffer from poisoning, leaching, or structural changes during extended operation, while the harsh electrochemical environment accelerates degradation through mechanisms like oxidation and reconstruction of active sites.
Mass transport limitations at the electrode-electrolyte interface create significant concentration gradients that reduce reaction rates and selectivity. The low solubility of CO2 in aqueous electrolytes (approximately 33 mM at ambient conditions) severely restricts the availability of reactants at catalytic sites, while the formation of bicarbonate and carbonate species further complicates reaction kinetics.
Scale-up challenges persist as laboratory-scale successes often fail to translate to industrial applications. Current densities in lab demonstrations (typically 10-100 mA/cm²) fall short of the 200+ mA/cm² required for commercial viability. Additionally, the complex interplay between catalyst structure, electrolyte composition, and operating conditions makes systematic optimization difficult.
The high overpotential requirement for driving CO2 reduction reactions represents another significant barrier. Most catalysts require potentials 0.5-1.0 V beyond the thermodynamic minimum, substantially reducing energy efficiency and increasing operational costs. This challenge is particularly acute for multi-carbon product formation, which requires multiple electron transfer steps.
Finally, mechanistic understanding remains incomplete despite extensive research efforts. The complex reaction networks involving multiple intermediates and competing pathways are not fully characterized, hampering rational catalyst design. Advanced in-situ and operando characterization techniques are needed to elucidate reaction mechanisms under realistic operating conditions.
State-of-the-Art Interface Engineering Approaches
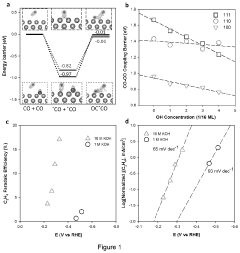
01 Metal-based catalysts for CO2 electroreduction
Various metal-based catalysts can be used for electrocatalytic CO2 reduction with enhanced catalytic activity. These include transition metals, metal alloys, and metal complexes that provide active sites for CO2 adsorption and conversion. The interface between these metal catalysts and the electrolyte plays a crucial role in determining the efficiency and selectivity of the CO2 reduction process. Modifications to the metal surface structure, composition, and electronic properties can significantly improve the catalytic performance.- Metal-based catalysts for CO2 electroreduction: Metal-based catalysts play a crucial role in electrocatalytic CO2 reduction. Various metals such as copper, silver, gold, and zinc exhibit different selectivity and activity for CO2 reduction products. The catalytic performance can be enhanced by controlling the morphology, crystal facets, and surface structure of these metal catalysts. Nanostructured metal catalysts with high surface area and abundant active sites show improved catalytic activity and selectivity for specific CO2 reduction products.
- Interface engineering for enhanced CO2 reduction: Interface engineering strategies significantly impact the catalytic activity of CO2 electroreduction systems. By modifying the catalyst-electrolyte interface, the local reaction environment can be optimized to facilitate CO2 activation and conversion. This includes controlling the hydrophobicity/hydrophilicity of the interface, creating confined reaction spaces, and designing hierarchical structures. Interface engineering helps to regulate mass transport, stabilize reaction intermediates, and improve the overall efficiency of the CO2 reduction process.
- Carbon-based materials as CO2 reduction catalysts: Carbon-based materials, including graphene, carbon nanotubes, and doped carbon structures, serve as effective catalysts or catalyst supports for CO2 electroreduction. These materials offer advantages such as high conductivity, large surface area, and tunable electronic properties. Heteroatom doping (N, S, P, B) of carbon materials creates active sites with optimized binding energies for CO2 reduction intermediates. Carbon-based catalysts often demonstrate good stability and can be produced at lower costs compared to precious metal catalysts.
- Electrolyte effects on CO2 reduction performance: The composition and properties of the electrolyte significantly influence the catalytic activity and selectivity in CO2 electroreduction. Factors such as pH, ionic strength, buffer capacity, and the presence of specific ions can alter the reaction pathways and product distribution. Ionic liquids and organic electrolytes have been explored to enhance CO2 solubility and stabilize reaction intermediates. Optimizing the electrolyte environment is crucial for suppressing competing hydrogen evolution reactions and improving faradaic efficiency toward desired CO2 reduction products.
- In-situ characterization of CO2 reduction interfaces: Advanced in-situ and operando characterization techniques provide critical insights into the dynamic processes occurring at the electrocatalytic interface during CO2 reduction. Spectroscopic methods such as Raman, infrared, and X-ray absorption spectroscopy help identify reaction intermediates and active sites under working conditions. Microscopy techniques reveal morphological changes of catalysts during operation. These characterization approaches enable understanding of structure-activity relationships and reaction mechanisms, guiding the rational design of more efficient catalytic systems for CO2 electroreduction.
02 Nanostructured interfaces for enhanced catalytic activity
Nanostructured interfaces provide increased surface area and more active sites for CO2 electroreduction. These structures include nanoporous materials, nanoparticles, nanosheets, and hierarchical architectures that optimize the interaction between the catalyst, electrolyte, and CO2 molecules. The unique properties of nanostructured interfaces, such as quantum confinement effects and high edge-to-surface ratios, contribute to improved catalytic activity and selectivity in CO2 reduction reactions.Expand Specific Solutions03 Carbon-based materials as CO2 reduction catalysts
Carbon-based materials, including graphene, carbon nanotubes, and doped carbon structures, serve as effective catalysts or catalyst supports for electrocatalytic CO2 reduction. These materials offer advantages such as high conductivity, tunable surface chemistry, and structural stability. Heteroatom doping (with N, B, S, or P) can create active sites on carbon materials, enhancing their catalytic activity for CO2 reduction by modifying the electronic structure and local charge distribution at the interface.Expand Specific Solutions04 Interface engineering for improved CO2 reduction
Interface engineering strategies focus on optimizing the catalyst-electrolyte interface to enhance CO2 reduction performance. These approaches include surface functionalization, creation of defects, introduction of heterostructures, and development of hybrid interfaces. Controlling the local environment at the interface, including pH, electric field distribution, and mass transport properties, can significantly influence the catalytic activity and product selectivity in electrocatalytic CO2 reduction reactions.Expand Specific Solutions05 Electrolyte effects on CO2 reduction interface
The composition and properties of the electrolyte significantly impact the catalytic activity at the CO2 reduction interface. Factors such as electrolyte pH, ionic strength, buffer capacity, and specific ion effects can alter the local reaction environment, affecting CO2 solubility, mass transport, and the stability of reaction intermediates. Tailoring the electrolyte composition can enhance the catalytic activity by promoting favorable reaction pathways and suppressing competing reactions like hydrogen evolution.Expand Specific Solutions
Leading Research Groups and Industrial Players
Electrocatalytic CO2 reduction technology is currently in the early commercialization phase, with a rapidly growing market driven by decarbonization initiatives. The global market is expanding at approximately 15-20% annually, reaching an estimated $500 million. Technologically, the field shows varying maturity levels across different approaches. Leading research institutions like Dalian Institute of Chemical Physics and universities (Michigan, Toronto, Utah State) are advancing fundamental catalyst design, while industrial players demonstrate different strategic approaches: energy companies (TotalEnergies, ENEOS) focus on integration with existing infrastructure; technology corporations (Siemens, 3M) develop scalable systems; and automotive manufacturers (Honda) explore synergies with hydrogen technologies. The most promising commercial developments come from specialized firms like Carbon Energy Technology (Beijing) and collaborative research between academic institutions and industrial partners.
Dalian Institute of Chemical Physics of CAS
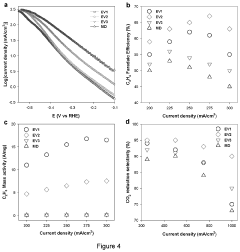
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative electrocatalytic interfaces for CO2 reduction featuring atomically dispersed metal sites on nitrogen-doped carbon supports. Their approach focuses on single-atom catalysts (SACs) where transition metals (Cu, Fe, Co, Ni) are coordinated with nitrogen in specific configurations to create M-N-C active sites. These catalysts demonstrate remarkable Faradaic efficiency (>90%) for converting CO2 to CO and other value-added products. DICP has pioneered the use of in-situ characterization techniques including X-ray absorption spectroscopy and operando infrared spectroscopy to understand reaction mechanisms at the molecular level. Their recent advances include dual-metal sites that work synergistically to lower activation barriers and improve product selectivity, achieving current densities exceeding 300 mA/cm² while maintaining stability over 100+ hours of continuous operation.
Strengths: Superior atomic-level control of active sites enabling precise tuning of product selectivity; extensive characterization capabilities for mechanistic understanding; demonstrated long-term stability under industrial-relevant conditions. Weaknesses: Some designs require complex synthesis procedures that may challenge large-scale production; potential metal leaching during extended operation could affect long-term performance.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed advanced nanostructured interfaces for electrocatalytic CO2 reduction featuring precisely engineered copper-based catalysts. Their approach focuses on controlling the morphology, facet exposure, and local electronic environment of copper surfaces to enhance C-C coupling reactions. Their researchers have pioneered plasma-treated copper catalysts with oxygen-derived subsurface species that significantly enhance ethylene and ethanol production with Faradaic efficiencies exceeding 60%. The Michigan team has developed innovative tandem catalyst systems where copper interfaces are combined with molecular co-catalysts that work synergistically to improve product selectivity. Their recent breakthroughs include the development of bimetallic interfaces with Cu-Ag and Cu-Au compositions that leverage electronic effects to tune binding energies of key intermediates. These catalysts demonstrate remarkable stability under industrially relevant conditions, maintaining high performance for over 200 hours of continuous operation at current densities above 200 mA/cm².
Strengths: Exceptional control over copper surface structure enabling unprecedented selectivity for C2+ products; sophisticated understanding of reaction mechanisms; demonstrated long-term stability under practical operating conditions. Weaknesses: Some designs require complex synthesis procedures that may limit scalability; potential surface reconstruction during operation could affect long-term selectivity; challenges in maintaining consistent performance across different batches.
Key Patents and Publications in Catalyst Interface Design
Catalysts with sharp reaction interface for electrochemical CO2 reduction with enhanced selectivity
PatentActiveUS11613819B2
Innovation
- An abrupt reaction interface electroreduction catalyst system is developed, featuring a porous gas diffusion layer and a thin catalyst layer, typically composed of copper, in contact with a high concentration potassium hydroxide electrolyte, which enhances selectivity for multi-carbon products by optimizing the reaction environment and reducing diffusion limitations.
Sustainability Impact and Carbon Neutrality Contributions
Electrocatalytic CO2 reduction technology represents a pivotal advancement in addressing global climate challenges through its direct contribution to carbon neutrality goals. This technology effectively transforms CO2 from a harmful greenhouse gas into valuable chemical feedstocks and fuels, creating a circular carbon economy that significantly reduces net emissions.
The sustainability impact of enhanced electrocatalytic CO2 reduction interfaces extends beyond mere carbon capture. By converting CO2 into commercially viable products such as carbon monoxide, formic acid, ethylene, and methanol, this technology creates economic incentives for emissions reduction while simultaneously decreasing dependence on fossil fuel-derived chemicals. This dual benefit accelerates the transition toward sustainable industrial practices.
From a carbon neutrality perspective, optimized interface designs dramatically improve conversion efficiency, enabling industrial-scale implementation that could potentially offset millions of tons of CO2 emissions annually. Current projections suggest that widespread adoption of advanced electrocatalytic systems could contribute 5-15% toward global carbon neutrality targets by 2050, representing a substantial component of climate mitigation strategies.
The life cycle assessment of these catalytic systems demonstrates favorable environmental profiles compared to traditional carbon capture technologies. Enhanced interface designs reduce energy requirements and minimize resource consumption during catalyst production, further amplifying their positive environmental impact. Studies indicate that next-generation catalysts could achieve carbon payback periods of less than six months when deployed at scale.
Integration with renewable energy sources creates particularly powerful synergies. Electrocatalytic CO2 reduction can serve as an energy storage mechanism for intermittent renewables, converting excess electricity into chemical energy while simultaneously removing atmospheric carbon. This integration pathway addresses two critical challenges in sustainable development: renewable energy storage and carbon emissions reduction.
Policy frameworks worldwide increasingly recognize electrocatalytic CO2 reduction as a key technology for meeting nationally determined contributions under the Paris Agreement. Carbon pricing mechanisms, clean energy incentives, and emissions regulations are creating favorable market conditions for accelerated development and deployment of these systems, further enhancing their potential contribution to global sustainability goals.
The sustainability impact of enhanced electrocatalytic CO2 reduction interfaces extends beyond mere carbon capture. By converting CO2 into commercially viable products such as carbon monoxide, formic acid, ethylene, and methanol, this technology creates economic incentives for emissions reduction while simultaneously decreasing dependence on fossil fuel-derived chemicals. This dual benefit accelerates the transition toward sustainable industrial practices.
From a carbon neutrality perspective, optimized interface designs dramatically improve conversion efficiency, enabling industrial-scale implementation that could potentially offset millions of tons of CO2 emissions annually. Current projections suggest that widespread adoption of advanced electrocatalytic systems could contribute 5-15% toward global carbon neutrality targets by 2050, representing a substantial component of climate mitigation strategies.
The life cycle assessment of these catalytic systems demonstrates favorable environmental profiles compared to traditional carbon capture technologies. Enhanced interface designs reduce energy requirements and minimize resource consumption during catalyst production, further amplifying their positive environmental impact. Studies indicate that next-generation catalysts could achieve carbon payback periods of less than six months when deployed at scale.
Integration with renewable energy sources creates particularly powerful synergies. Electrocatalytic CO2 reduction can serve as an energy storage mechanism for intermittent renewables, converting excess electricity into chemical energy while simultaneously removing atmospheric carbon. This integration pathway addresses two critical challenges in sustainable development: renewable energy storage and carbon emissions reduction.
Policy frameworks worldwide increasingly recognize electrocatalytic CO2 reduction as a key technology for meeting nationally determined contributions under the Paris Agreement. Carbon pricing mechanisms, clean energy incentives, and emissions regulations are creating favorable market conditions for accelerated development and deployment of these systems, further enhancing their potential contribution to global sustainability goals.
Scalability and Industrial Implementation Pathways
The scalability of electrocatalytic CO2 reduction technologies represents a critical bridge between laboratory success and commercial viability. Current lab-scale demonstrations, while promising, operate at milliampere current densities and milliliter volumes, which fall significantly short of industrial requirements. To achieve meaningful impact on carbon emissions, systems must be scaled to process tons of CO2 daily.
Engineering challenges in scaling include maintaining catalyst stability and selectivity at higher current densities. Laboratory catalysts often degrade when subjected to industrial operating conditions, with performance declining after hours rather than the months or years required for commercial applications. Heat management becomes increasingly problematic at scale, as reaction exothermicity can alter local pH and concentration gradients, potentially reducing catalytic efficiency.
Mass transport limitations present another significant hurdle. As reactor dimensions increase, ensuring uniform CO2 delivery to catalyst surfaces becomes more difficult, particularly given CO2's limited solubility in aqueous electrolytes. Industrial implementation requires innovative gas diffusion electrode designs that can maintain optimal three-phase boundaries across larger surface areas.
Several implementation pathways show promise for industrial adoption. The modular approach involves scaling through multiplication of smaller optimized units rather than increasing individual reactor size. This strategy preserves performance metrics while reducing engineering complexity and allowing for distributed implementation. Alternatively, the integrated systems approach combines CO2 capture and conversion in continuous processes, potentially reducing overall energy requirements.
Electrolyzer design evolution represents another critical pathway. Moving from traditional H-cell configurations to flow cells and then to membrane electrode assemblies mirrors the development trajectory of hydrogen fuel cells, potentially leveraging existing manufacturing infrastructure. Recent advances in zero-gap cell designs have demonstrated improved energy efficiency at higher current densities.
Economic viability remains the ultimate determinant of industrial implementation. Current techno-economic analyses suggest that electricity costs constitute 30-60% of operating expenses for electrocatalytic CO2 reduction. Integration with renewable energy sources offers both environmental benefits and potential cost advantages, particularly in regions with surplus renewable capacity.
Regulatory frameworks and carbon pricing mechanisms will significantly influence adoption timelines. Policies that value carbon utilization, rather than just carbon capture, could accelerate industrial implementation by improving economic calculations for early adopters.
Engineering challenges in scaling include maintaining catalyst stability and selectivity at higher current densities. Laboratory catalysts often degrade when subjected to industrial operating conditions, with performance declining after hours rather than the months or years required for commercial applications. Heat management becomes increasingly problematic at scale, as reaction exothermicity can alter local pH and concentration gradients, potentially reducing catalytic efficiency.
Mass transport limitations present another significant hurdle. As reactor dimensions increase, ensuring uniform CO2 delivery to catalyst surfaces becomes more difficult, particularly given CO2's limited solubility in aqueous electrolytes. Industrial implementation requires innovative gas diffusion electrode designs that can maintain optimal three-phase boundaries across larger surface areas.
Several implementation pathways show promise for industrial adoption. The modular approach involves scaling through multiplication of smaller optimized units rather than increasing individual reactor size. This strategy preserves performance metrics while reducing engineering complexity and allowing for distributed implementation. Alternatively, the integrated systems approach combines CO2 capture and conversion in continuous processes, potentially reducing overall energy requirements.
Electrolyzer design evolution represents another critical pathway. Moving from traditional H-cell configurations to flow cells and then to membrane electrode assemblies mirrors the development trajectory of hydrogen fuel cells, potentially leveraging existing manufacturing infrastructure. Recent advances in zero-gap cell designs have demonstrated improved energy efficiency at higher current densities.
Economic viability remains the ultimate determinant of industrial implementation. Current techno-economic analyses suggest that electricity costs constitute 30-60% of operating expenses for electrocatalytic CO2 reduction. Integration with renewable energy sources offers both environmental benefits and potential cost advantages, particularly in regions with surplus renewable capacity.
Regulatory frameworks and carbon pricing mechanisms will significantly influence adoption timelines. Policies that value carbon utilization, rather than just carbon capture, could accelerate industrial implementation by improving economic calculations for early adopters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!