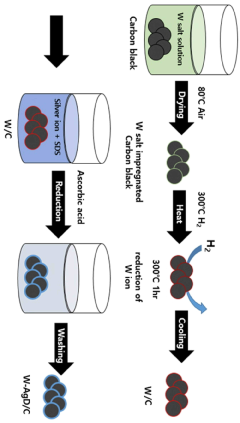
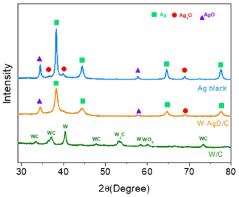
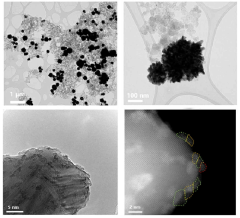
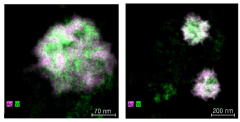
Comparative analysis of Electrocatalytic CO2 reduction electrode and catalyst strategies
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Technology Background and Objectives
Electrocatalytic CO2 reduction (ECR) has emerged as a promising technology for mitigating climate change while simultaneously producing valuable chemicals and fuels. The development of this technology dates back to the 1980s when initial studies demonstrated the feasibility of converting CO2 into various carbon-based products through electrochemical processes. Over the past four decades, significant advancements have been made in understanding reaction mechanisms, designing catalysts, and engineering electrodes to enhance efficiency and selectivity.
The evolution of ECR technology has been characterized by three distinct phases. The first phase (1980s-2000s) focused on fundamental electrochemistry and proof-of-concept demonstrations. The second phase (2000s-2015) saw increased attention to catalyst design and reaction selectivity. The current phase (2015-present) has witnessed exponential growth in research activity, driven by urgent climate concerns and advances in nanomaterials and in-situ characterization techniques.
The primary technical objective in ECR research is to develop electrode and catalyst systems capable of converting CO2 to target products with high energy efficiency, selectivity, and stability. Specifically, researchers aim to achieve Faradaic efficiencies exceeding 90% for valuable C1-C3 products, current densities above 200 mA/cm² at industrially relevant potentials, and operational stability of thousands of hours.
Current trends in ECR technology include the development of bimetallic and multimetallic catalysts to leverage synergistic effects, the exploration of single-atom catalysts for maximized atom efficiency, and the integration of molecular catalysts with heterogeneous supports. Additionally, there is growing interest in tandem catalytic systems that can overcome thermodynamic limitations through coupled reactions.
The field is also witnessing a shift toward practical considerations for industrial implementation, including the design of gas diffusion electrodes to overcome CO2 solubility limitations, the development of membrane electrode assemblies for efficient ion transport, and the engineering of flow cell configurations for continuous operation.
Looking forward, ECR technology is expected to evolve toward integrated systems that combine renewable electricity generation with CO2 capture and conversion. The ultimate goal is to establish a circular carbon economy where CO2 is viewed as a valuable feedstock rather than a waste product, enabling the production of carbon-neutral fuels and chemicals while contributing to climate change mitigation efforts.
The evolution of ECR technology has been characterized by three distinct phases. The first phase (1980s-2000s) focused on fundamental electrochemistry and proof-of-concept demonstrations. The second phase (2000s-2015) saw increased attention to catalyst design and reaction selectivity. The current phase (2015-present) has witnessed exponential growth in research activity, driven by urgent climate concerns and advances in nanomaterials and in-situ characterization techniques.
The primary technical objective in ECR research is to develop electrode and catalyst systems capable of converting CO2 to target products with high energy efficiency, selectivity, and stability. Specifically, researchers aim to achieve Faradaic efficiencies exceeding 90% for valuable C1-C3 products, current densities above 200 mA/cm² at industrially relevant potentials, and operational stability of thousands of hours.
Current trends in ECR technology include the development of bimetallic and multimetallic catalysts to leverage synergistic effects, the exploration of single-atom catalysts for maximized atom efficiency, and the integration of molecular catalysts with heterogeneous supports. Additionally, there is growing interest in tandem catalytic systems that can overcome thermodynamic limitations through coupled reactions.
The field is also witnessing a shift toward practical considerations for industrial implementation, including the design of gas diffusion electrodes to overcome CO2 solubility limitations, the development of membrane electrode assemblies for efficient ion transport, and the engineering of flow cell configurations for continuous operation.
Looking forward, ECR technology is expected to evolve toward integrated systems that combine renewable electricity generation with CO2 capture and conversion. The ultimate goal is to establish a circular carbon economy where CO2 is viewed as a valuable feedstock rather than a waste product, enabling the production of carbon-neutral fuels and chemicals while contributing to climate change mitigation efforts.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.2 billion by 2030, representing a compound annual growth rate (CAGR) of 11.2%. This growth trajectory reflects the urgent need for sustainable solutions to address climate change challenges.
Electrocatalytic CO2 reduction represents a promising segment within this market, with particular interest in electrode and catalyst technologies. The demand for these technologies is primarily driven by industries seeking to achieve carbon neutrality goals, including chemical manufacturing, energy production, and transportation sectors. Government initiatives and carbon pricing mechanisms in regions such as the European Union, North America, and parts of Asia have created favorable market conditions for CO2 conversion technologies.
The market segmentation reveals distinct application areas for electrocatalytic CO2 reduction products. Conversion to carbon monoxide represents approximately 28% of the market share, followed by formic acid (23%), methanol (19%), ethylene (15%), and other products (15%). This distribution highlights the versatility of CO2 conversion technologies in producing various value-added chemicals and fuels.
Regional analysis indicates that North America currently leads the market with a 35% share, followed by Europe (30%), Asia-Pacific (25%), and rest of the world (10%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 13.5% during the forecast period, primarily due to increasing industrial activities and governmental support for green technologies in countries like China, Japan, and South Korea.
Key market drivers include stringent carbon emission regulations, increasing corporate sustainability commitments, and the growing demand for renewable fuels and chemicals. The declining cost of renewable electricity is particularly significant for electrocatalytic approaches, as it directly impacts the economic viability of these technologies. Recent technological advancements in catalyst design and electrode materials have also contributed to improved efficiency and selectivity, making these solutions more commercially attractive.
Market challenges include high capital costs, scalability issues, and competition from alternative carbon capture and utilization technologies. The economic feasibility of electrocatalytic CO2 reduction remains dependent on factors such as electricity prices, carbon pricing mechanisms, and product market values. Despite these challenges, the increasing focus on circular carbon economy concepts and the potential for integration with renewable energy systems present significant opportunities for market expansion in the coming years.
Electrocatalytic CO2 reduction represents a promising segment within this market, with particular interest in electrode and catalyst technologies. The demand for these technologies is primarily driven by industries seeking to achieve carbon neutrality goals, including chemical manufacturing, energy production, and transportation sectors. Government initiatives and carbon pricing mechanisms in regions such as the European Union, North America, and parts of Asia have created favorable market conditions for CO2 conversion technologies.
The market segmentation reveals distinct application areas for electrocatalytic CO2 reduction products. Conversion to carbon monoxide represents approximately 28% of the market share, followed by formic acid (23%), methanol (19%), ethylene (15%), and other products (15%). This distribution highlights the versatility of CO2 conversion technologies in producing various value-added chemicals and fuels.
Regional analysis indicates that North America currently leads the market with a 35% share, followed by Europe (30%), Asia-Pacific (25%), and rest of the world (10%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 13.5% during the forecast period, primarily due to increasing industrial activities and governmental support for green technologies in countries like China, Japan, and South Korea.
Key market drivers include stringent carbon emission regulations, increasing corporate sustainability commitments, and the growing demand for renewable fuels and chemicals. The declining cost of renewable electricity is particularly significant for electrocatalytic approaches, as it directly impacts the economic viability of these technologies. Recent technological advancements in catalyst design and electrode materials have also contributed to improved efficiency and selectivity, making these solutions more commercially attractive.
Market challenges include high capital costs, scalability issues, and competition from alternative carbon capture and utilization technologies. The economic feasibility of electrocatalytic CO2 reduction remains dependent on factors such as electricity prices, carbon pricing mechanisms, and product market values. Despite these challenges, the increasing focus on circular carbon economy concepts and the potential for integration with renewable energy systems present significant opportunities for market expansion in the coming years.
Current Electrocatalytic CO2 Reduction Challenges
Despite significant advancements in electrocatalytic CO2 reduction (ECR) technology, several critical challenges continue to impede its widespread commercial implementation. The primary obstacle remains the low energy efficiency of the overall process, with current systems typically achieving only 30-50% energy conversion efficiency. This inefficiency stems largely from high overpotentials required to drive the reaction, resulting in substantial energy losses during the conversion process.
Selectivity presents another major challenge, as the CO2 reduction reaction competes with the hydrogen evolution reaction in aqueous electrolytes. Most catalysts struggle to achieve high Faradaic efficiency toward specific value-added products, often producing a mixture of C1, C2, and C3 compounds that necessitates costly separation processes. Even state-of-the-art copper-based catalysts rarely exceed 60% selectivity for any single C2+ product.
Catalyst stability remains problematic under the harsh electrochemical conditions required for CO2 reduction. Many promising materials suffer from deactivation mechanisms including poisoning, leaching, structural reorganization, and carbon deposition. Most laboratory studies demonstrate stability for only tens of hours, whereas industrial viability requires thousands of hours of consistent performance.
Mass transport limitations significantly constrain reaction rates in current systems. The low solubility of CO2 in aqueous electrolytes (approximately 33 mM at ambient conditions) creates concentration gradients near electrode surfaces, while the formation of pH gradients further complicates reaction kinetics and product selectivity.
Scale-up challenges persist as most high-performing catalysts have been demonstrated only at laboratory scales with low current densities (typically <200 mA/cm²). Industrial implementation requires current densities exceeding 500 mA/cm² while maintaining selectivity and stability. The gap between lab-scale demonstrations and industrial requirements remains substantial.
The economic viability of ECR technologies is further challenged by high catalyst costs, particularly when utilizing precious metals or complex nanostructured materials. Additionally, the lack of standardized testing protocols and performance metrics makes meaningful comparisons between different catalyst systems difficult, hampering systematic progress in the field.
Geographically, research efforts are concentrated in North America, Europe, and East Asia, with significant disparities in technological capabilities. While fundamental research is globally distributed, advanced catalyst development and system integration expertise remain concentrated in a few technological hubs, creating barriers to widespread implementation.
Selectivity presents another major challenge, as the CO2 reduction reaction competes with the hydrogen evolution reaction in aqueous electrolytes. Most catalysts struggle to achieve high Faradaic efficiency toward specific value-added products, often producing a mixture of C1, C2, and C3 compounds that necessitates costly separation processes. Even state-of-the-art copper-based catalysts rarely exceed 60% selectivity for any single C2+ product.
Catalyst stability remains problematic under the harsh electrochemical conditions required for CO2 reduction. Many promising materials suffer from deactivation mechanisms including poisoning, leaching, structural reorganization, and carbon deposition. Most laboratory studies demonstrate stability for only tens of hours, whereas industrial viability requires thousands of hours of consistent performance.
Mass transport limitations significantly constrain reaction rates in current systems. The low solubility of CO2 in aqueous electrolytes (approximately 33 mM at ambient conditions) creates concentration gradients near electrode surfaces, while the formation of pH gradients further complicates reaction kinetics and product selectivity.
Scale-up challenges persist as most high-performing catalysts have been demonstrated only at laboratory scales with low current densities (typically <200 mA/cm²). Industrial implementation requires current densities exceeding 500 mA/cm² while maintaining selectivity and stability. The gap between lab-scale demonstrations and industrial requirements remains substantial.
The economic viability of ECR technologies is further challenged by high catalyst costs, particularly when utilizing precious metals or complex nanostructured materials. Additionally, the lack of standardized testing protocols and performance metrics makes meaningful comparisons between different catalyst systems difficult, hampering systematic progress in the field.
Geographically, research efforts are concentrated in North America, Europe, and East Asia, with significant disparities in technological capabilities. While fundamental research is globally distributed, advanced catalyst development and system integration expertise remain concentrated in a few technological hubs, creating barriers to widespread implementation.
Electrode Materials and Catalyst Design Approaches
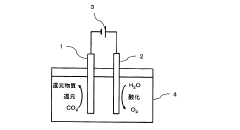
01 Metal-based catalysts for CO2 electroreduction
Metal-based catalysts, particularly transition metals like copper, silver, gold, and zinc, are widely used for electrocatalytic CO2 reduction due to their ability to facilitate electron transfer. These catalysts can be modified through alloying, surface structuring, or nanostructuring to enhance their catalytic activity, selectivity towards specific products (such as CO, formate, or hydrocarbons), and stability during long-term operation. The binding energy of reaction intermediates on these metal surfaces plays a crucial role in determining product selectivity.- Metal-based catalysts for CO2 electroreduction: Various metal-based catalysts have been developed for electrocatalytic CO2 reduction with improved efficiency and selectivity. These include single metal catalysts, bimetallic catalysts, and metal alloys. Metals such as copper, silver, gold, zinc, and their combinations show different product selectivity and Faradaic efficiency. The structure and composition of these metal catalysts significantly influence their catalytic performance, stability, and the distribution of reduction products.
- Carbon-based materials as supports and catalysts: Carbon-based materials serve as both supports for metal catalysts and as catalysts themselves in CO2 electroreduction. These include graphene, carbon nanotubes, carbon fibers, and doped carbon materials. The high surface area, excellent conductivity, and tunable surface properties of carbon materials enhance catalyst dispersion, electron transfer, and CO2 adsorption. Nitrogen, boron, or sulfur doping of carbon materials can create active sites that improve catalytic performance and stability during long-term operation.
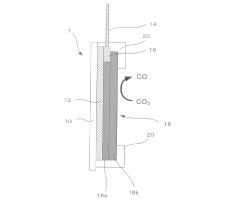
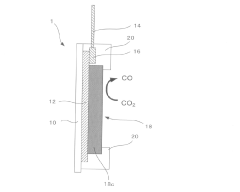
- Electrode design and engineering for enhanced performance: Advanced electrode designs significantly impact the efficiency, selectivity, and stability of CO2 electroreduction systems. Gas diffusion electrodes (GDEs) facilitate mass transport of CO2 to catalyst active sites, addressing CO2 solubility limitations. Three-dimensional porous structures increase the density of active sites and improve reactant diffusion. Surface modification techniques and hierarchical structures optimize the local reaction environment, enhancing catalytic performance and long-term operational stability.
- Novel catalyst synthesis methods for improved stability: Innovative synthesis methods have been developed to create catalysts with enhanced stability for CO2 electroreduction. These include atomic layer deposition, electrodeposition, hydrothermal synthesis, and sol-gel methods. These techniques allow precise control over catalyst morphology, particle size, and composition. Encapsulation strategies and core-shell structures protect catalysts from degradation, leaching, and poisoning during extended operation, maintaining high activity and selectivity over time.
- Electrolyte composition and reaction conditions optimization: The composition of the electrolyte and reaction conditions significantly influence the efficiency, selectivity, and stability of CO2 electroreduction catalysts. Parameters such as pH, temperature, pressure, and electrolyte ions affect the reaction pathways and product distribution. Ionic liquids and buffered solutions can enhance CO2 solubility and suppress competing hydrogen evolution reactions. Optimizing applied potential, current density, and flow rate improves energy efficiency and maintains catalyst stability during continuous operation.
02 Carbon-based and composite electrode materials
Carbon-based materials such as graphene, carbon nanotubes, and porous carbon structures serve as excellent supports for catalysts in CO2 electroreduction. These materials provide high surface area, good electrical conductivity, and mechanical stability. Composite electrodes combining carbon materials with metal nanoparticles or metal-organic frameworks show enhanced catalytic performance due to synergistic effects. The porous structure of these electrodes facilitates mass transport of CO2 to active sites while maintaining high electrical conductivity for efficient electron transfer.Expand Specific Solutions03 Single-atom catalysts and atomically dispersed active sites
Single-atom catalysts (SACs) represent an emerging class of materials for CO2 electroreduction, where individual metal atoms are anchored to supports like nitrogen-doped carbon or metal oxides. These catalysts maximize atom efficiency and often exhibit unique selectivity patterns compared to their bulk counterparts. The isolated nature of active sites in SACs allows for precise tuning of the local electronic environment, leading to enhanced activity and selectivity. Coordination environment engineering of the metal centers is crucial for optimizing catalytic performance.Expand Specific Solutions04 Electrolyte composition and reaction conditions optimization
The composition of the electrolyte significantly impacts the efficiency, selectivity, and stability of CO2 electroreduction catalysts. Parameters such as pH, ionic strength, buffer capacity, and the presence of specific ions can alter reaction pathways and product distributions. Advanced electrolyte engineering approaches include the use of ionic liquids, organic additives, and local pH control strategies. Operating conditions such as temperature, pressure, and potential also play critical roles in determining catalyst performance and need to be optimized for specific catalyst systems.Expand Specific Solutions05 Stability enhancement strategies and degradation mechanisms
Maintaining catalyst stability during long-term CO2 electroreduction remains a significant challenge. Common degradation mechanisms include metal leaching, surface poisoning by reaction intermediates, structural collapse, and agglomeration of nanoparticles. Strategies to enhance stability include encapsulation of active sites, core-shell structures, surface passivation layers, and the development of self-healing catalysts. Understanding the relationship between catalyst structure and stability is essential for designing durable electrodes for practical applications. In-situ and operando characterization techniques provide valuable insights into degradation processes under reaction conditions.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalytic CO2 reduction technology market is currently in a growth phase, with increasing momentum driven by global decarbonization efforts. The competitive landscape features established industrial players like Siemens Energy, TotalEnergies, Panasonic, and Repsol alongside emerging specialized companies such as Carbon Energy Technology and Liquid Sun Oy. Academic institutions, particularly in China (Dalian Institute of Chemical Physics, Fudan University), North America (University of California, University of Toronto), and Europe (College de France), are driving fundamental research advancements. The technology remains in early commercial maturity, with most players focusing on catalyst development and electrode optimization to improve efficiency, selectivity, and durability. Industry-academic collaborations are accelerating development, with companies like Honda, ENEOS, and Idemitsu investing in scalable solutions to address the growing carbon utilization market.
Dalian Institute of Chemical Physics of CAS
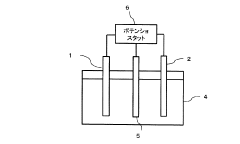
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced copper-based catalysts for CO2 electroreduction with precise control of morphology and composition. Their approach focuses on nanostructured Cu catalysts with high-index facets and engineered defects to enhance C-C coupling for multi-carbon product formation. DICP has pioneered the development of Cu-based bimetallic catalysts (Cu-Au, Cu-Ag) that demonstrate synergistic effects for improved Faradaic efficiency toward ethylene and ethanol. Their research includes in-situ characterization techniques to understand reaction mechanisms and catalyst degradation pathways during CO2 reduction. DICP has also developed novel flow-cell reactor designs that address mass transport limitations and enable higher current densities (>200 mA/cm²) while maintaining product selectivity[1][2].
Strengths: Superior catalyst design with atomic-level precision, strong expertise in operando characterization techniques, and integrated reactor engineering approach. Weaknesses: Potential challenges in scaling up laboratory catalysts to industrial applications and relatively high costs associated with some of their advanced materials.
The Governing Council of the University of Toronto
Technical Solution: The University of Toronto has developed a comprehensive electrocatalytic CO2 reduction strategy centered on molecularly-tuned interfaces. Their approach involves molecular engineering of catalyst-electrolyte interfaces to control the local reaction environment and enhance product selectivity. They've pioneered the use of ionic liquids and organic modifiers as co-catalysts that stabilize key intermediates during CO2 reduction. Their electrode designs incorporate hierarchical porosity to optimize mass transport while maintaining high active site density. The Toronto team has developed novel M-N-C (metal-nitrogen-carbon) catalysts derived from metal-organic frameworks that show exceptional selectivity toward CO formation at low overpotentials. Their recent work includes the development of tandem catalytic systems that can convert CO2 directly to multi-carbon products like ethanol and acetate with Faradaic efficiencies exceeding 60% through careful tuning of local pH and buffer effects[3][4].
Strengths: Innovative interface engineering approaches, strong fundamental understanding of reaction mechanisms, and development of practical catalyst systems with high selectivity. Weaknesses: Some of their most selective catalysts still require relatively high overpotentials, and long-term stability remains a challenge in practical applications.
Key Patents and Breakthroughs in CO2 Electrocatalysis
Electrode for carbon dioxide reduction and carbon dioxide reduction device
PatentActiveJP2017171963A
Innovation
- An electrode substrate with an Mn complex catalyst having an electron-withdrawing substituent on the diimine ligand, combined with a carbon material like nanocarbon, carbon cloth, or carbon paper, facilitates carbon dioxide reduction in water with an overvoltage of 400 mV or less.
Electrocatalyst for CO2 reduction and method for manufacturing the same
PatentActiveKR1020210141132A
Innovation
- A carbon-based electrode catalyst is developed with metal particles and metal nanoparticles in the form of dendrites on the surface, enhancing current density and energy efficiency through a method involving a carbon-based carrier, metal precursor dispersion, and dendrite growth on metal particles.
Techno-economic Assessment of CO2 Reduction Systems
The techno-economic assessment of CO2 reduction systems requires comprehensive evaluation of both technical performance and economic viability. Current electrocatalytic CO2 reduction technologies demonstrate promising conversion efficiencies but face significant challenges in scaling to industrial applications. Capital expenditure for these systems ranges from $500-2,000/kW depending on system configuration, with noble metal catalyst systems representing the higher end of this spectrum.
Operating costs are dominated by electricity consumption, typically accounting for 60-75% of total operational expenses. At current electricity prices of $0.05-0.12/kWh in most industrial markets, the levelized cost of products from CO2 reduction systems ranges from $1.20-3.50/kg for C1 products like carbon monoxide and formate, and $2.50-6.00/kg for C2+ products such as ethylene and ethanol.
System lifetime represents a critical economic factor, with catalyst degradation often limiting continuous operation to 2,000-5,000 hours before replacement or regeneration becomes necessary. This translates to maintenance costs averaging 15-25% of initial capital investment annually. Copper-based catalysts, while effective for C2+ product formation, typically demonstrate faster degradation rates compared to silver or gold catalysts used for CO production.
Energy efficiency metrics reveal that current systems operate at 30-45% Faradaic efficiency for target products, with energy conversion efficiencies of 15-30% when accounting for all system losses. These efficiencies must improve to 50-60% to achieve cost parity with conventional production methods for most chemical products.
Market analysis indicates that CO2-derived products currently command a 20-40% premium in certain green chemistry markets, partially offsetting higher production costs. However, this premium is expected to decrease as production scales and regulatory frameworks evolve. Carbon pricing mechanisms, currently ranging from $25-85/ton CO2 in various jurisdictions, significantly impact economic viability.
Sensitivity analysis demonstrates that electricity cost reductions of 30% or efficiency improvements of 20% could reduce production costs by approximately 25%, potentially enabling market competitiveness without subsidies for certain product streams. The integration with renewable energy sources presents opportunities for further cost optimization, particularly in regions with high renewable penetration and favorable regulatory environments.
Operating costs are dominated by electricity consumption, typically accounting for 60-75% of total operational expenses. At current electricity prices of $0.05-0.12/kWh in most industrial markets, the levelized cost of products from CO2 reduction systems ranges from $1.20-3.50/kg for C1 products like carbon monoxide and formate, and $2.50-6.00/kg for C2+ products such as ethylene and ethanol.
System lifetime represents a critical economic factor, with catalyst degradation often limiting continuous operation to 2,000-5,000 hours before replacement or regeneration becomes necessary. This translates to maintenance costs averaging 15-25% of initial capital investment annually. Copper-based catalysts, while effective for C2+ product formation, typically demonstrate faster degradation rates compared to silver or gold catalysts used for CO production.
Energy efficiency metrics reveal that current systems operate at 30-45% Faradaic efficiency for target products, with energy conversion efficiencies of 15-30% when accounting for all system losses. These efficiencies must improve to 50-60% to achieve cost parity with conventional production methods for most chemical products.
Market analysis indicates that CO2-derived products currently command a 20-40% premium in certain green chemistry markets, partially offsetting higher production costs. However, this premium is expected to decrease as production scales and regulatory frameworks evolve. Carbon pricing mechanisms, currently ranging from $25-85/ton CO2 in various jurisdictions, significantly impact economic viability.
Sensitivity analysis demonstrates that electricity cost reductions of 30% or efficiency improvements of 20% could reduce production costs by approximately 25%, potentially enabling market competitiveness without subsidies for certain product streams. The integration with renewable energy sources presents opportunities for further cost optimization, particularly in regions with high renewable penetration and favorable regulatory environments.
Environmental Impact and Carbon Neutrality Implications
Electrocatalytic CO2 reduction technology represents a significant opportunity for addressing climate change challenges through carbon capture and utilization. The environmental impact of this technology extends far beyond laboratory efficiency metrics, potentially transforming how industries approach carbon management and neutrality goals.
The implementation of CO2 reduction systems offers substantial environmental benefits through direct atmospheric carbon dioxide removal. When powered by renewable energy sources, these systems create a carbon-negative cycle that actively reduces greenhouse gas concentrations while producing valuable chemical feedstocks. This dual functionality positions electrocatalytic CO2 reduction as a cornerstone technology for meeting increasingly stringent carbon neutrality targets across industrial sectors.
Life cycle assessments of various electrode and catalyst strategies reveal important sustainability considerations. Precious metal catalysts like gold and silver demonstrate high selectivity but present environmental concerns related to mining impacts and resource scarcity. In contrast, earth-abundant catalysts based on copper, zinc, or carbon materials offer reduced environmental footprints despite sometimes lower conversion efficiencies. The environmental trade-offs between catalyst performance and ecological impact must be carefully balanced when scaling these technologies.
Energy source integration significantly influences the carbon neutrality potential of electrocatalytic systems. Analysis shows that CO2 reduction processes powered by fossil fuel-derived electricity may actually increase net carbon emissions due to inefficiencies in the energy conversion chain. However, when coupled with renewable energy sources like solar or wind power, these systems can achieve true carbon-negative operation, with studies indicating potential carbon offsets of 0.5-2.0 tons CO2 per ton of product depending on the specific catalyst and electrode configuration.
Industrial implementation pathways present varying environmental implications. Point-source capture systems integrated directly with industrial emissions offer immediate carbon intensity reductions for existing facilities. Direct air capture coupled with electrocatalytic reduction provides broader atmospheric remediation but at higher energy costs. The optimal deployment strategy depends on regional energy infrastructure, industrial composition, and environmental policy frameworks.
Regulatory frameworks increasingly recognize electrocatalytic CO2 reduction as a viable carbon management strategy. Carbon pricing mechanisms, clean energy incentives, and emissions trading systems are evolving to incorporate these technologies, potentially accelerating their adoption through economic incentives aligned with environmental goals. The integration of these systems into formal carbon accounting methodologies represents a critical step toward mainstream industrial adoption.
The implementation of CO2 reduction systems offers substantial environmental benefits through direct atmospheric carbon dioxide removal. When powered by renewable energy sources, these systems create a carbon-negative cycle that actively reduces greenhouse gas concentrations while producing valuable chemical feedstocks. This dual functionality positions electrocatalytic CO2 reduction as a cornerstone technology for meeting increasingly stringent carbon neutrality targets across industrial sectors.
Life cycle assessments of various electrode and catalyst strategies reveal important sustainability considerations. Precious metal catalysts like gold and silver demonstrate high selectivity but present environmental concerns related to mining impacts and resource scarcity. In contrast, earth-abundant catalysts based on copper, zinc, or carbon materials offer reduced environmental footprints despite sometimes lower conversion efficiencies. The environmental trade-offs between catalyst performance and ecological impact must be carefully balanced when scaling these technologies.
Energy source integration significantly influences the carbon neutrality potential of electrocatalytic systems. Analysis shows that CO2 reduction processes powered by fossil fuel-derived electricity may actually increase net carbon emissions due to inefficiencies in the energy conversion chain. However, when coupled with renewable energy sources like solar or wind power, these systems can achieve true carbon-negative operation, with studies indicating potential carbon offsets of 0.5-2.0 tons CO2 per ton of product depending on the specific catalyst and electrode configuration.
Industrial implementation pathways present varying environmental implications. Point-source capture systems integrated directly with industrial emissions offer immediate carbon intensity reductions for existing facilities. Direct air capture coupled with electrocatalytic reduction provides broader atmospheric remediation but at higher energy costs. The optimal deployment strategy depends on regional energy infrastructure, industrial composition, and environmental policy frameworks.
Regulatory frameworks increasingly recognize electrocatalytic CO2 reduction as a viable carbon management strategy. Carbon pricing mechanisms, clean energy incentives, and emissions trading systems are evolving to incorporate these technologies, potentially accelerating their adoption through economic incentives aligned with environmental goals. The integration of these systems into formal carbon accounting methodologies represents a critical step toward mainstream industrial adoption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!