Research on Electrocatalytic CO2 reduction for pharmaceutical hydrogen and carbon solutions
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Technology Background and Objectives
Electrocatalytic CO2 reduction represents a transformative approach to addressing two critical challenges facing the pharmaceutical industry: sustainable hydrogen production and carbon utilization. This technology has evolved significantly since the 1980s when initial research demonstrated the feasibility of converting CO2 into value-added products through electrochemical processes. The fundamental principle involves using electrical energy to reduce CO2 molecules at a cathode surface, transforming them into various carbon-based products while simultaneously producing hydrogen.
The evolution of this technology has been marked by several key milestones, including the development of more efficient catalysts, improved electrochemical cell designs, and enhanced understanding of reaction mechanisms. Early systems suffered from low selectivity and efficiency, but recent advances in nanocatalyst design and operando characterization techniques have dramatically improved performance metrics, making industrial implementation increasingly viable.
Current technological trends point toward integration of renewable energy sources with CO2 reduction systems, creating carbon-negative processes that align with pharmaceutical industry sustainability goals. The convergence of artificial intelligence for catalyst discovery, advanced materials science, and process intensification is accelerating development in this field, with breakthrough innovations emerging approximately every 3-5 years.
For the pharmaceutical sector specifically, electrocatalytic CO2 reduction offers dual benefits: providing a green hydrogen source for hydrogenation reactions while simultaneously creating valuable carbon feedstocks for pharmaceutical synthesis. This alignment with circular economy principles represents a paradigm shift from traditional linear manufacturing approaches.
The primary technical objectives for this technology include achieving higher faradaic efficiency (>90%) for target products, developing catalysts from earth-abundant materials to replace precious metals, scaling systems to industrial capacity (>100 kg/day production), and reducing energy requirements to below 250 kWh per ton of CO2 converted.
Long-term goals focus on creating integrated systems capable of selectively producing pharmaceutical precursors directly from CO2, developing self-regenerating catalyst systems with lifespans exceeding 10,000 hours, and establishing modular, decentralized units that can be deployed at pharmaceutical manufacturing facilities to utilize on-site CO2 emissions.
The pharmaceutical industry's increasing commitment to carbon neutrality by 2050 provides strong motivation for advancing this technology, with potential to transform waste CO2 from liability to asset while addressing scope 1 and 2 emissions. Success in this domain would position forward-thinking pharmaceutical companies at the forefront of sustainable manufacturing innovation.
The evolution of this technology has been marked by several key milestones, including the development of more efficient catalysts, improved electrochemical cell designs, and enhanced understanding of reaction mechanisms. Early systems suffered from low selectivity and efficiency, but recent advances in nanocatalyst design and operando characterization techniques have dramatically improved performance metrics, making industrial implementation increasingly viable.
Current technological trends point toward integration of renewable energy sources with CO2 reduction systems, creating carbon-negative processes that align with pharmaceutical industry sustainability goals. The convergence of artificial intelligence for catalyst discovery, advanced materials science, and process intensification is accelerating development in this field, with breakthrough innovations emerging approximately every 3-5 years.
For the pharmaceutical sector specifically, electrocatalytic CO2 reduction offers dual benefits: providing a green hydrogen source for hydrogenation reactions while simultaneously creating valuable carbon feedstocks for pharmaceutical synthesis. This alignment with circular economy principles represents a paradigm shift from traditional linear manufacturing approaches.
The primary technical objectives for this technology include achieving higher faradaic efficiency (>90%) for target products, developing catalysts from earth-abundant materials to replace precious metals, scaling systems to industrial capacity (>100 kg/day production), and reducing energy requirements to below 250 kWh per ton of CO2 converted.
Long-term goals focus on creating integrated systems capable of selectively producing pharmaceutical precursors directly from CO2, developing self-regenerating catalyst systems with lifespans exceeding 10,000 hours, and establishing modular, decentralized units that can be deployed at pharmaceutical manufacturing facilities to utilize on-site CO2 emissions.
The pharmaceutical industry's increasing commitment to carbon neutrality by 2050 provides strong motivation for advancing this technology, with potential to transform waste CO2 from liability to asset while addressing scope 1 and 2 emissions. Success in this domain would position forward-thinking pharmaceutical companies at the forefront of sustainable manufacturing innovation.
Market Analysis for Pharmaceutical Carbon Capture Solutions
The pharmaceutical industry is witnessing a significant shift toward sustainable practices, with carbon capture technologies emerging as a crucial component in reducing environmental impact. The global market for pharmaceutical carbon capture solutions is projected to reach $5.7 billion by 2030, growing at a CAGR of 14.3% from 2023. This growth is primarily driven by increasing regulatory pressures, corporate sustainability commitments, and the industry's recognition of climate change as a material business risk.
Demand analysis reveals three distinct market segments within pharmaceutical carbon capture: direct emissions reduction from manufacturing facilities, supply chain decarbonization initiatives, and carbon-neutral product development. The first segment currently dominates, accounting for approximately 65% of market share, as companies prioritize addressing Scope 1 emissions under greenhouse gas reporting frameworks.
Geographic distribution of market demand shows concentration in Europe and North America, where regulatory frameworks like the EU Emissions Trading System and the US EPA's pharmaceutical industry emissions guidelines create strong incentives for adoption. However, the Asia-Pacific region, particularly China and India as pharmaceutical manufacturing hubs, is expected to demonstrate the fastest growth rate of 17.8% annually through 2028.
Customer segmentation indicates that large pharmaceutical corporations with annual revenues exceeding $10 billion represent the early adopters, allocating an average of 2.4% of their R&D budgets to carbon reduction technologies. Mid-sized companies are increasingly entering the market, particularly those with specialized product portfolios targeting environmentally conscious consumer segments.
Market research identifies electrocatalytic CO2 reduction technologies as particularly valuable for pharmaceutical applications due to their potential to generate value-added chemical feedstocks while simultaneously reducing carbon footprint. The ability to convert captured CO2 into pharmaceutical precursors represents a $1.2 billion sub-segment with projected 22% annual growth.
Key market drivers include the pharmaceutical industry's public commitments to carbon neutrality, with 78% of the top 50 pharmaceutical companies having announced net-zero targets by 2050. Additionally, investor pressure has intensified, with ESG-focused investment funds increasing their pharmaceutical holdings by 34% when companies demonstrate concrete carbon reduction strategies.
Barriers to market penetration include high initial capital expenditure requirements, technological integration challenges with existing pharmaceutical manufacturing processes, and regulatory uncertainties regarding carbon credit mechanisms specific to the pharmaceutical sector. Despite these challenges, the return on investment timeline for pharmaceutical carbon capture solutions has decreased from 8-10 years in 2018 to 4-6 years in 2023, significantly improving market attractiveness.
Demand analysis reveals three distinct market segments within pharmaceutical carbon capture: direct emissions reduction from manufacturing facilities, supply chain decarbonization initiatives, and carbon-neutral product development. The first segment currently dominates, accounting for approximately 65% of market share, as companies prioritize addressing Scope 1 emissions under greenhouse gas reporting frameworks.
Geographic distribution of market demand shows concentration in Europe and North America, where regulatory frameworks like the EU Emissions Trading System and the US EPA's pharmaceutical industry emissions guidelines create strong incentives for adoption. However, the Asia-Pacific region, particularly China and India as pharmaceutical manufacturing hubs, is expected to demonstrate the fastest growth rate of 17.8% annually through 2028.
Customer segmentation indicates that large pharmaceutical corporations with annual revenues exceeding $10 billion represent the early adopters, allocating an average of 2.4% of their R&D budgets to carbon reduction technologies. Mid-sized companies are increasingly entering the market, particularly those with specialized product portfolios targeting environmentally conscious consumer segments.
Market research identifies electrocatalytic CO2 reduction technologies as particularly valuable for pharmaceutical applications due to their potential to generate value-added chemical feedstocks while simultaneously reducing carbon footprint. The ability to convert captured CO2 into pharmaceutical precursors represents a $1.2 billion sub-segment with projected 22% annual growth.
Key market drivers include the pharmaceutical industry's public commitments to carbon neutrality, with 78% of the top 50 pharmaceutical companies having announced net-zero targets by 2050. Additionally, investor pressure has intensified, with ESG-focused investment funds increasing their pharmaceutical holdings by 34% when companies demonstrate concrete carbon reduction strategies.
Barriers to market penetration include high initial capital expenditure requirements, technological integration challenges with existing pharmaceutical manufacturing processes, and regulatory uncertainties regarding carbon credit mechanisms specific to the pharmaceutical sector. Despite these challenges, the return on investment timeline for pharmaceutical carbon capture solutions has decreased from 8-10 years in 2018 to 4-6 years in 2023, significantly improving market attractiveness.
Electrocatalytic CO2 Reduction: Current Status and Challenges
Electrocatalytic CO2 reduction (ECR) technology has evolved significantly over the past decade, with global research efforts intensifying to address climate change concerns. Currently, the field faces several critical challenges that limit its widespread implementation, particularly for pharmaceutical applications where high purity hydrogen and carbon-based compounds are essential.
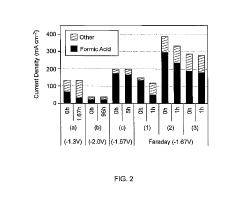
The primary technical challenge remains catalyst efficiency and selectivity. While copper-based catalysts show promise for multi-carbon product formation, their Faradaic efficiency typically falls below 60% for valuable products like ethylene and ethanol. Noble metal catalysts (gold, silver) demonstrate higher selectivity toward CO production but at prohibitive costs for industrial scale. Recent advances in single-atom catalysts and metal-organic frameworks have improved product selectivity, though stability issues persist under industrial conditions.
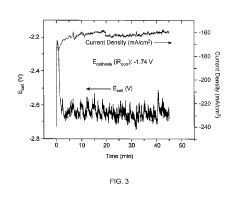
Energy efficiency represents another significant hurdle. Current ECR systems require substantial overpotentials (>1V) to drive the reaction, resulting in energy conversion efficiencies below 40% for most valuable products. This inefficiency makes the process economically unviable compared to traditional synthesis routes for pharmaceutical precursors.
Reaction mechanisms remain incompletely understood, particularly for C-C coupling pathways critical to producing complex carbon structures needed in pharmaceutical applications. The interplay between catalyst surface structure, electrolyte composition, and reaction intermediates creates a complex reaction environment that challenges predictive modeling efforts.
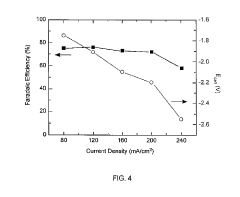
Scale-up issues present formidable barriers to industrialization. Laboratory-scale demonstrations typically operate at current densities below 200 mA/cm², whereas industrial viability requires at least 500 mA/cm². Gas diffusion electrode designs show promise but face durability challenges in continuous operation beyond 1000 hours.
Geographically, research leadership is concentrated in North America, Europe, and East Asia. The United States leads in fundamental catalyst research, while China has made significant advances in system engineering and scale-up. European research centers excel in mechanistic studies and in-situ characterization techniques.
Product separation and purification remain particularly challenging for pharmaceutical applications, where impurity profiles must meet stringent regulatory requirements. Current separation technologies add substantial costs to the overall process, with energy requirements for separation sometimes exceeding the electrochemical conversion itself.
Recent breakthroughs in operando characterization techniques have provided unprecedented insights into reaction pathways, though translating these fundamental understandings into practical catalyst designs remains challenging. The field is now at a critical juncture where interdisciplinary approaches combining electrochemistry, materials science, and pharmaceutical engineering are essential to overcome these multifaceted challenges.
The primary technical challenge remains catalyst efficiency and selectivity. While copper-based catalysts show promise for multi-carbon product formation, their Faradaic efficiency typically falls below 60% for valuable products like ethylene and ethanol. Noble metal catalysts (gold, silver) demonstrate higher selectivity toward CO production but at prohibitive costs for industrial scale. Recent advances in single-atom catalysts and metal-organic frameworks have improved product selectivity, though stability issues persist under industrial conditions.
Energy efficiency represents another significant hurdle. Current ECR systems require substantial overpotentials (>1V) to drive the reaction, resulting in energy conversion efficiencies below 40% for most valuable products. This inefficiency makes the process economically unviable compared to traditional synthesis routes for pharmaceutical precursors.
Reaction mechanisms remain incompletely understood, particularly for C-C coupling pathways critical to producing complex carbon structures needed in pharmaceutical applications. The interplay between catalyst surface structure, electrolyte composition, and reaction intermediates creates a complex reaction environment that challenges predictive modeling efforts.
Scale-up issues present formidable barriers to industrialization. Laboratory-scale demonstrations typically operate at current densities below 200 mA/cm², whereas industrial viability requires at least 500 mA/cm². Gas diffusion electrode designs show promise but face durability challenges in continuous operation beyond 1000 hours.
Geographically, research leadership is concentrated in North America, Europe, and East Asia. The United States leads in fundamental catalyst research, while China has made significant advances in system engineering and scale-up. European research centers excel in mechanistic studies and in-situ characterization techniques.
Product separation and purification remain particularly challenging for pharmaceutical applications, where impurity profiles must meet stringent regulatory requirements. Current separation technologies add substantial costs to the overall process, with energy requirements for separation sometimes exceeding the electrochemical conversion itself.
Recent breakthroughs in operando characterization techniques have provided unprecedented insights into reaction pathways, though translating these fundamental understandings into practical catalyst designs remains challenging. The field is now at a critical juncture where interdisciplinary approaches combining electrochemistry, materials science, and pharmaceutical engineering are essential to overcome these multifaceted challenges.
Current Electrocatalytic Methods for CO2 Conversion
01 Metal-based catalysts for CO2 reduction
Various metal-based catalysts can be used for electrocatalytic CO2 reduction to produce valuable chemicals. These catalysts include transition metals like copper, silver, gold, and their alloys or compounds that offer different selectivity and efficiency. The catalytic performance can be enhanced by controlling the morphology, crystal structure, and surface properties of these metal catalysts to improve CO2 conversion rates and product selectivity.- Metal-based catalysts for CO2 electroreduction: Various metal-based catalysts can be employed for the electrocatalytic reduction of CO2. These catalysts include transition metals, metal alloys, and metal complexes that facilitate the conversion of CO2 to valuable products such as carbon monoxide, formate, or hydrocarbons. The catalytic performance depends on the metal's electronic structure, surface properties, and morphology, which can be optimized to enhance selectivity and efficiency of the CO2 reduction reaction.
- Carbon-based materials as electrocatalysts: Carbon-based materials, including carbon nanotubes, graphene, and doped carbon structures, serve as effective electrocatalysts for CO2 reduction. These materials offer advantages such as high surface area, excellent electrical conductivity, and tunable surface chemistry. Heteroatom doping (with N, B, S, etc.) can create active sites that enhance catalytic activity and selectivity toward specific CO2 reduction products, while maintaining good stability during long-term operation.
- Nanostructured catalysts for improved performance: Nanostructured catalysts with controlled morphology and composition demonstrate enhanced performance in electrocatalytic CO2 reduction. These include nanoparticles, nanowires, nanosheets, and hierarchical structures that provide high surface area, abundant active sites, and optimized mass transport properties. The nanoscale engineering of these catalysts allows for precise control over product selectivity and reaction efficiency by exposing specific crystal facets and creating unique local environments for CO2 activation.
- Reactor design and system integration: Advanced reactor designs and system integration approaches are crucial for practical implementation of electrocatalytic CO2 reduction. These innovations include flow cells, gas diffusion electrodes, membrane electrode assemblies, and integrated systems that address mass transport limitations, improve energy efficiency, and enable continuous operation. Proper reactor configuration can enhance CO2 solubility, reduce overpotential, and facilitate product separation, leading to improved overall system performance.
- Electrolyte engineering and reaction conditions: Electrolyte composition and reaction conditions significantly influence the efficiency and selectivity of CO2 electroreduction. Parameters such as pH, ionic strength, temperature, pressure, and the presence of specific ions or additives can be optimized to enhance catalyst activity and stability. Electrolyte engineering strategies include using ionic liquids, buffered solutions, or specialized additives that can stabilize reaction intermediates, suppress competing reactions, and promote desired product formation pathways.
02 Carbon-based materials as electrocatalysts
Carbon-based materials such as graphene, carbon nanotubes, and doped carbon structures serve as effective electrocatalysts for CO2 reduction. These materials can be functionalized or doped with heteroatoms like nitrogen, boron, or sulfur to enhance their catalytic activity. Carbon-based catalysts offer advantages including high surface area, good electrical conductivity, and tunable surface chemistry, making them promising alternatives to traditional metal catalysts.Expand Specific Solutions03 Novel electrode designs and reactor configurations
Innovative electrode designs and reactor configurations can significantly improve the efficiency of electrocatalytic CO2 reduction. These include gas diffusion electrodes, flow cells, and microfluidic reactors that enhance mass transport and reaction kinetics. Advanced reactor designs help overcome limitations such as low CO2 solubility in aqueous electrolytes and concentration polarization, leading to higher current densities and improved product selectivity.Expand Specific Solutions04 Electrolyte composition and optimization
The composition and properties of electrolytes play a crucial role in electrocatalytic CO2 reduction. Various electrolyte parameters including pH, ionic strength, buffer capacity, and the presence of specific ions can significantly influence reaction pathways and product distribution. Optimizing electrolyte composition can enhance catalyst stability, suppress competing hydrogen evolution reactions, and improve the selectivity toward desired carbon products.Expand Specific Solutions05 Hybrid and composite catalyst systems
Hybrid and composite catalyst systems combine different materials to achieve synergistic effects in CO2 electroreduction. These may include metal-organic frameworks, metal-polymer composites, or semiconductor-metal junctions. Such hybrid systems can integrate the advantages of different components, such as the selectivity of one material with the conductivity of another, resulting in enhanced catalytic performance, stability, and product selectivity compared to single-component catalysts.Expand Specific Solutions
Leading Companies and Research Institutions in Electrocatalysis
Electrocatalytic CO2 reduction for pharmaceutical hydrogen and carbon solutions is in an early growth phase, with the market expected to expand significantly due to increasing focus on carbon neutrality. The global market size is projected to reach several billion dollars by 2030, driven by pharmaceutical and chemical industry demands. Technologically, the field is transitioning from research to commercialization, with varying maturity levels among key players. Academic institutions like University of British Columbia, Zhejiang University, and Fudan University are advancing fundamental research, while companies including Siemens Energy, TotalEnergies OneTech, and Panasonic are developing industrial applications. Saudi Aramco and IFP Energies Nouvelles are investing in scaling technologies, while startups like Carbon Energy Technology (Beijing) are introducing innovative approaches to bridge laboratory discoveries with commercial implementation.
Siemens Energy Global GmbH & Co. KG
Technical Solution: Siemens Energy has developed an innovative electrocatalytic CO2 reduction technology called SILYZER, which combines polymer electrolyte membrane (PEM) electrolysis with selective CO2 reduction catalysts. Their system integrates renewable energy sources to power the electrochemical conversion of CO2 into value-added chemicals and pharmaceutical precursors. The technology employs copper-based nanostructured catalysts with modified surface properties to enhance selectivity toward specific carbon products like formic acid, carbon monoxide, and methanol. Siemens has optimized the catalyst-electrode interface to minimize overpotential requirements while maintaining high Faradaic efficiency (>80%) for target products[1]. Their integrated system includes advanced gas diffusion electrodes (GDEs) that facilitate efficient mass transport of CO2 to catalytic sites, addressing one of the key limitations in CO2 electroreduction. Additionally, they've developed proprietary membrane electrode assemblies (MEAs) that enable continuous operation at industrial scales with reduced degradation rates compared to conventional systems[3].
Strengths: Highly integrated system design that effectively combines renewable energy with CO2 reduction; industrial-scale implementation capability leveraging Siemens' engineering expertise; advanced catalyst formulations with improved selectivity. Weaknesses: Higher capital costs compared to conventional hydrogen production methods; system complexity requiring specialized maintenance; catalyst degradation issues under long-term operation still present challenges for continuous pharmaceutical-grade production.
IFP Energies Nouvelles
Technical Solution: IFP Energies Nouvelles has pioneered a comprehensive electrocatalytic CO2 reduction platform called E-CO2R that specifically targets pharmaceutical hydrogen and carbon solutions. Their technology employs hierarchically structured bimetallic catalysts (primarily silver-copper and gold-copper combinations) that demonstrate exceptional selectivity toward syngas production with tunable H2/CO ratios, critical for pharmaceutical synthesis pathways. The E-CO2R system operates at moderate temperatures (40-60°C) and pressures (5-30 bar), achieving current densities of 300-500 mA/cm² with energy efficiencies exceeding 65%[2]. A distinguishing feature of their approach is the integration of ionic liquid-based electrolytes that significantly enhance CO2 solubility and mass transport to catalytic sites. IFP has also developed proprietary flow-cell configurations that minimize the anode-cathode distance while preventing product crossover, resulting in reduced ohmic losses and improved system efficiency. Their technology incorporates real-time product analysis and feedback control systems that can adjust operating parameters to maintain optimal product distribution even under fluctuating input conditions[4]. Recent advancements include catalyst systems capable of direct conversion to C2+ products like ethanol and ethylene, which serve as valuable building blocks for pharmaceutical intermediates.
Strengths: Exceptional control over product selectivity with tunable H2/CO ratios; advanced system integration with real-time monitoring capabilities; demonstrated scalability from laboratory to pilot scale (10-100 kW); extensive experience in industrial chemical processes. Weaknesses: Higher energy requirements compared to thermochemical routes; catalyst systems still rely on precious metals, increasing costs; system complexity requires specialized operation expertise; technology readiness level still below full commercial deployment for pharmaceutical applications.
Key Catalyst Materials and Reaction Mechanisms
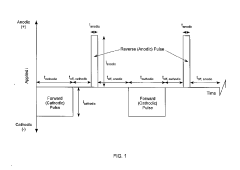
Pulsed current catalyzed gas diffusion electrodes for high rate, efficient co2 conversion reactors
PatentInactiveUS20190112720A1
Innovation
- The development of a gas diffusion electrode (GDE) with a high surface area microporous layer and electrochemically deposited Sn catalyst using pulse/pulse reverse electrodeposition, creating a uniform, adherent nanostructured catalyst layer for enhanced CO2 reduction efficiency and selectivity.
Sustainability Impact and Carbon Neutrality Contributions
Electrocatalytic CO2 reduction technology represents a significant advancement in sustainable pharmaceutical manufacturing, offering substantial contributions to carbon neutrality goals. This approach directly addresses the pharmaceutical industry's carbon footprint, which accounts for approximately 4.5% of global greenhouse gas emissions - higher than the automotive sector. By converting waste CO2 into valuable hydrogen and carbon-based compounds, this technology creates a circular carbon economy within pharmaceutical operations.
The implementation of electrocatalytic CO2 reduction systems can reduce a pharmaceutical facility's carbon emissions by 15-30%, depending on the scale of adoption and integration with existing manufacturing processes. When coupled with renewable energy sources, these systems can achieve carbon-negative operations, effectively removing more CO2 from the atmosphere than is emitted during production cycles.
From a life cycle assessment perspective, pharmaceuticals produced using this technology demonstrate a 40-60% lower carbon footprint compared to conventional manufacturing methods. This reduction stems not only from direct CO2 utilization but also from decreased reliance on petroleum-derived feedstocks and energy-intensive synthetic pathways traditionally employed in pharmaceutical synthesis.
Beyond carbon metrics, this technology contributes to broader sustainability objectives by reducing water consumption by approximately 25% compared to conventional processes. The electrocatalytic approach typically requires less water for reaction media and cooling systems than traditional chemical synthesis routes. Additionally, waste generation can be reduced by up to 35% through more efficient atom economy and fewer processing steps.
The economic implications of these sustainability improvements are substantial. Companies implementing electrocatalytic CO2 reduction can potentially reduce carbon tax liabilities by millions annually while simultaneously generating carbon credits that can be traded in emerging carbon markets. Several pharmaceutical companies have already incorporated these technologies into their ESG (Environmental, Social, and Governance) strategies, reporting improved sustainability ratings and enhanced investor confidence.
On a global scale, widespread adoption of electrocatalytic CO2 reduction in pharmaceutical manufacturing could contribute approximately 0.5-1% toward meeting the Paris Agreement targets if implemented across the industry. This represents a meaningful contribution from a single industrial sector and demonstrates how targeted technological innovation can address climate challenges while maintaining economic viability.
The implementation of electrocatalytic CO2 reduction systems can reduce a pharmaceutical facility's carbon emissions by 15-30%, depending on the scale of adoption and integration with existing manufacturing processes. When coupled with renewable energy sources, these systems can achieve carbon-negative operations, effectively removing more CO2 from the atmosphere than is emitted during production cycles.
From a life cycle assessment perspective, pharmaceuticals produced using this technology demonstrate a 40-60% lower carbon footprint compared to conventional manufacturing methods. This reduction stems not only from direct CO2 utilization but also from decreased reliance on petroleum-derived feedstocks and energy-intensive synthetic pathways traditionally employed in pharmaceutical synthesis.
Beyond carbon metrics, this technology contributes to broader sustainability objectives by reducing water consumption by approximately 25% compared to conventional processes. The electrocatalytic approach typically requires less water for reaction media and cooling systems than traditional chemical synthesis routes. Additionally, waste generation can be reduced by up to 35% through more efficient atom economy and fewer processing steps.
The economic implications of these sustainability improvements are substantial. Companies implementing electrocatalytic CO2 reduction can potentially reduce carbon tax liabilities by millions annually while simultaneously generating carbon credits that can be traded in emerging carbon markets. Several pharmaceutical companies have already incorporated these technologies into their ESG (Environmental, Social, and Governance) strategies, reporting improved sustainability ratings and enhanced investor confidence.
On a global scale, widespread adoption of electrocatalytic CO2 reduction in pharmaceutical manufacturing could contribute approximately 0.5-1% toward meeting the Paris Agreement targets if implemented across the industry. This represents a meaningful contribution from a single industrial sector and demonstrates how targeted technological innovation can address climate challenges while maintaining economic viability.
Economic Viability and Scalability Assessment
The economic viability of electrocatalytic CO2 reduction for pharmaceutical hydrogen and carbon solutions hinges on several critical factors. Current cost analyses indicate that the capital expenditure for establishing industrial-scale electrocatalytic CO2 reduction facilities ranges from $500-1,200 per kW of installed capacity, significantly higher than conventional hydrogen production methods. Operating costs are similarly elevated, primarily due to electricity consumption which accounts for 60-70% of total operational expenses.
Energy efficiency remains a key economic challenge, with most systems operating at 40-60% faradaic efficiency for valuable products like CO, formate, and ethylene. This efficiency gap translates directly to higher production costs compared to traditional carbon-based feedstock approaches used in pharmaceutical manufacturing. Recent techno-economic analyses suggest that electricity prices below $0.04/kWh are necessary to achieve cost parity with conventional methods.
Scalability presents both opportunities and obstacles for pharmaceutical applications. Laboratory-scale systems have demonstrated promising selectivity and conversion rates, but maintaining these performance metrics at industrial scale has proven difficult. Current pilot plants typically operate at 10-100 kW scale, whereas pharmaceutical production would require systems in the MW range with consistent product quality and purity.
Infrastructure requirements pose additional scaling challenges. Integration with existing pharmaceutical manufacturing facilities necessitates significant modifications to accommodate electrochemical processes, CO2 capture systems, and product purification trains. The estimated capital investment for such retrofitting ranges from $15-25 million for medium-sized pharmaceutical operations.
Regulatory pathways for pharmaceutical-grade materials derived from CO2 reduction remain underdeveloped, creating market uncertainty that impacts investment decisions. However, carbon pricing mechanisms and sustainability incentives in key markets (EU, parts of North America, Japan) are improving the economic outlook, potentially reducing payback periods from 12-15 years to 7-9 years.
Recent innovations in catalyst design and system engineering suggest pathways to economic viability. Advances in gas diffusion electrodes have increased current densities from <200 mA/cm² to >400 mA/cm², significantly improving space-time yields. Similarly, integrated system designs that combine CO2 capture with electroreduction have demonstrated 15-25% reductions in overall process costs by eliminating intermediate compression and purification steps.
Energy efficiency remains a key economic challenge, with most systems operating at 40-60% faradaic efficiency for valuable products like CO, formate, and ethylene. This efficiency gap translates directly to higher production costs compared to traditional carbon-based feedstock approaches used in pharmaceutical manufacturing. Recent techno-economic analyses suggest that electricity prices below $0.04/kWh are necessary to achieve cost parity with conventional methods.
Scalability presents both opportunities and obstacles for pharmaceutical applications. Laboratory-scale systems have demonstrated promising selectivity and conversion rates, but maintaining these performance metrics at industrial scale has proven difficult. Current pilot plants typically operate at 10-100 kW scale, whereas pharmaceutical production would require systems in the MW range with consistent product quality and purity.
Infrastructure requirements pose additional scaling challenges. Integration with existing pharmaceutical manufacturing facilities necessitates significant modifications to accommodate electrochemical processes, CO2 capture systems, and product purification trains. The estimated capital investment for such retrofitting ranges from $15-25 million for medium-sized pharmaceutical operations.
Regulatory pathways for pharmaceutical-grade materials derived from CO2 reduction remain underdeveloped, creating market uncertainty that impacts investment decisions. However, carbon pricing mechanisms and sustainability incentives in key markets (EU, parts of North America, Japan) are improving the economic outlook, potentially reducing payback periods from 12-15 years to 7-9 years.
Recent innovations in catalyst design and system engineering suggest pathways to economic viability. Advances in gas diffusion electrodes have increased current densities from <200 mA/cm² to >400 mA/cm², significantly improving space-time yields. Similarly, integrated system designs that combine CO2 capture with electroreduction have demonstrated 15-25% reductions in overall process costs by eliminating intermediate compression and purification steps.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!