What are the key regulatory requirements for Electrocatalytic CO2 reduction deployment
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Technology Background and Objectives
Electrocatalytic CO2 reduction technology has emerged as a promising approach to address the global challenge of rising carbon dioxide emissions. This technology has evolved from early theoretical concepts in the 1980s to practical laboratory demonstrations in the 2000s, and now stands at the threshold of commercial deployment. The fundamental principle involves converting CO2 into value-added chemicals and fuels using renewable electricity, effectively closing the carbon cycle while producing useful products.
The evolution of this technology has been marked by significant breakthroughs in catalyst design, from early metal-based catalysts to advanced nanomaterials and molecular catalysts with improved selectivity and efficiency. Recent years have witnessed remarkable progress in achieving higher faradaic efficiencies and current densities, particularly for high-value products such as ethylene, ethanol, and formic acid.
The primary technical objective in this field is to develop economically viable electrocatalytic systems capable of converting CO2 into targeted products with high selectivity, energy efficiency, and stability. This includes achieving faradaic efficiencies exceeding 90% for specific products, current densities above 200 mA/cm², and operational stability of several thousand hours under industrial conditions.
Secondary objectives include reducing the energy input requirements to below 6 kWh per kg of product, scaling up from laboratory to industrial scale, and integrating these systems with renewable energy sources to ensure true carbon neutrality. The technology aims to achieve cost parity with conventional petrochemical routes by 2030, requiring significant improvements in catalyst performance and system design.
From a regulatory perspective, the deployment of electrocatalytic CO2 reduction technology must navigate a complex landscape of environmental regulations, carbon pricing mechanisms, and product certification standards. The technology must comply with emissions standards, chemical manufacturing regulations, and increasingly stringent carbon accounting frameworks across different jurisdictions.
The long-term vision for this technology extends beyond mere carbon mitigation to establishing a circular carbon economy where CO2 becomes a valuable feedstock rather than a waste product. This paradigm shift aligns with global sustainability goals and offers a pathway to decarbonize hard-to-abate sectors of the chemical industry.
As climate policies worldwide increasingly favor carbon-neutral technologies, electrocatalytic CO2 reduction stands to benefit from supportive regulatory frameworks, carbon pricing mechanisms, and green product certifications. The technology's trajectory is thus shaped not only by scientific advances but also by evolving policy landscapes that increasingly recognize the value of carbon capture and utilization technologies in meeting climate targets.
The evolution of this technology has been marked by significant breakthroughs in catalyst design, from early metal-based catalysts to advanced nanomaterials and molecular catalysts with improved selectivity and efficiency. Recent years have witnessed remarkable progress in achieving higher faradaic efficiencies and current densities, particularly for high-value products such as ethylene, ethanol, and formic acid.
The primary technical objective in this field is to develop economically viable electrocatalytic systems capable of converting CO2 into targeted products with high selectivity, energy efficiency, and stability. This includes achieving faradaic efficiencies exceeding 90% for specific products, current densities above 200 mA/cm², and operational stability of several thousand hours under industrial conditions.
Secondary objectives include reducing the energy input requirements to below 6 kWh per kg of product, scaling up from laboratory to industrial scale, and integrating these systems with renewable energy sources to ensure true carbon neutrality. The technology aims to achieve cost parity with conventional petrochemical routes by 2030, requiring significant improvements in catalyst performance and system design.
From a regulatory perspective, the deployment of electrocatalytic CO2 reduction technology must navigate a complex landscape of environmental regulations, carbon pricing mechanisms, and product certification standards. The technology must comply with emissions standards, chemical manufacturing regulations, and increasingly stringent carbon accounting frameworks across different jurisdictions.
The long-term vision for this technology extends beyond mere carbon mitigation to establishing a circular carbon economy where CO2 becomes a valuable feedstock rather than a waste product. This paradigm shift aligns with global sustainability goals and offers a pathway to decarbonize hard-to-abate sectors of the chemical industry.
As climate policies worldwide increasingly favor carbon-neutral technologies, electrocatalytic CO2 reduction stands to benefit from supportive regulatory frameworks, carbon pricing mechanisms, and green product certifications. The technology's trajectory is thus shaped not only by scientific advances but also by evolving policy landscapes that increasingly recognize the value of carbon capture and utilization technologies in meeting climate targets.
Market Analysis for Electrocatalytic CO2 Reduction
The global market for electrocatalytic CO2 reduction technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. Current market valuations indicate that the carbon capture, utilization, and storage (CCUS) sector, which includes electrocatalytic CO2 reduction, is projected to reach $7 billion by 2030, with a compound annual growth rate of approximately 13.8% from 2023 to 2030.
The demand for electrocatalytic CO2 reduction technologies spans multiple industries, with the chemical manufacturing sector showing the highest adoption rate. This sector utilizes converted CO2 as feedstock for producing value-added chemicals such as methanol, ethylene, and formic acid. The energy sector follows closely, implementing these technologies for power-to-fuel applications, while the pharmaceutical and food industries are emerging as potential growth markets.
Regional analysis reveals that North America currently leads the market with approximately 35% share, attributed to favorable regulatory frameworks and substantial research funding. Europe follows at 30%, driven by stringent carbon emission regulations and ambitious climate targets. The Asia-Pacific region, particularly China and South Korea, is experiencing the fastest growth rate at 15.2% annually, fueled by government initiatives to achieve carbon neutrality.
Market segmentation by product output shows that carbon monoxide conversion technologies hold the largest market share (42%), followed by formate/formic acid (28%), methanol (18%), and ethylene (12%). This distribution reflects both the technical maturity of different conversion pathways and the market demand for these products.
Key market drivers include increasingly stringent carbon pricing mechanisms, with carbon prices in the EU Emissions Trading System reaching €80-90 per ton, creating economic incentives for CO2 utilization. Additionally, corporate sustainability commitments and ESG investment trends are directing capital toward carbon reduction technologies, with global sustainable investments in this sector exceeding $35 billion in 2022.
Market barriers remain significant, including high capital costs for electrocatalytic systems, with current installation costs ranging from $800-1,200 per ton of CO2 processing capacity. Energy efficiency challenges persist, as most systems require 3-5 kWh of electricity per kilogram of CO2 processed, necessitating access to low-cost renewable energy to maintain economic viability.
Future market growth will likely be catalyzed by technological breakthroughs in catalyst efficiency and durability, integration with renewable energy systems, and the development of standardized regulatory frameworks that provide clear pathways for technology deployment and carbon credit generation.
The demand for electrocatalytic CO2 reduction technologies spans multiple industries, with the chemical manufacturing sector showing the highest adoption rate. This sector utilizes converted CO2 as feedstock for producing value-added chemicals such as methanol, ethylene, and formic acid. The energy sector follows closely, implementing these technologies for power-to-fuel applications, while the pharmaceutical and food industries are emerging as potential growth markets.
Regional analysis reveals that North America currently leads the market with approximately 35% share, attributed to favorable regulatory frameworks and substantial research funding. Europe follows at 30%, driven by stringent carbon emission regulations and ambitious climate targets. The Asia-Pacific region, particularly China and South Korea, is experiencing the fastest growth rate at 15.2% annually, fueled by government initiatives to achieve carbon neutrality.
Market segmentation by product output shows that carbon monoxide conversion technologies hold the largest market share (42%), followed by formate/formic acid (28%), methanol (18%), and ethylene (12%). This distribution reflects both the technical maturity of different conversion pathways and the market demand for these products.
Key market drivers include increasingly stringent carbon pricing mechanisms, with carbon prices in the EU Emissions Trading System reaching €80-90 per ton, creating economic incentives for CO2 utilization. Additionally, corporate sustainability commitments and ESG investment trends are directing capital toward carbon reduction technologies, with global sustainable investments in this sector exceeding $35 billion in 2022.
Market barriers remain significant, including high capital costs for electrocatalytic systems, with current installation costs ranging from $800-1,200 per ton of CO2 processing capacity. Energy efficiency challenges persist, as most systems require 3-5 kWh of electricity per kilogram of CO2 processed, necessitating access to low-cost renewable energy to maintain economic viability.
Future market growth will likely be catalyzed by technological breakthroughs in catalyst efficiency and durability, integration with renewable energy systems, and the development of standardized regulatory frameworks that provide clear pathways for technology deployment and carbon credit generation.
Technical Challenges in Electrocatalytic CO2 Conversion
Electrocatalytic CO2 reduction technology faces several significant technical challenges that currently limit its widespread industrial deployment. The primary obstacle remains the low energy efficiency of the conversion process, with most systems operating at 30-50% efficiency, far below the 70-80% threshold needed for commercial viability. This inefficiency stems from high overpotentials required to drive the reaction and competing side reactions, particularly hydrogen evolution in aqueous systems.
Product selectivity presents another major hurdle, as CO2 reduction can yield multiple carbon products including CO, formate, methane, ethylene, and alcohols. Current catalysts often lack the specificity to target a single high-value product, resulting in complex and costly downstream separation processes. Even state-of-the-art copper-based catalysts typically achieve only 60-70% Faradaic efficiency toward a specific product.
Catalyst stability and durability remain critical concerns for industrial implementation. Many promising catalysts demonstrate significant performance degradation after only tens or hundreds of hours of operation, whereas commercial applications require thousands of hours of stable performance. Catalyst poisoning, structural changes, and leaching of active components during extended operation contribute to this degradation.
Scaling up laboratory systems to industrial proportions introduces additional challenges related to mass transport limitations. As reactor dimensions increase, CO2 diffusion becomes rate-limiting due to its low solubility in aqueous electrolytes (approximately 33 mM at ambient conditions). This creates concentration gradients that reduce overall system efficiency and alter product distribution compared to laboratory-scale demonstrations.
System integration challenges arise when attempting to couple CO2 capture with electrochemical reduction. The energy requirements for CO2 capture from dilute sources (typically 400 ppm in air) are substantial, and the captured CO2 often contains impurities that can deactivate catalysts. Creating integrated systems that efficiently manage these processes remains technically challenging.
Economic viability represents perhaps the most significant barrier to commercialization. Current production costs for electrocatalytic CO2 reduction products are estimated at 2-5 times higher than conventional fossil-based routes. This gap is driven by high electricity costs, expensive catalyst materials, limited system lifetimes, and inefficient conversion processes. Achieving cost parity will require simultaneous advances in catalyst performance, system design, and renewable electricity generation.
Product selectivity presents another major hurdle, as CO2 reduction can yield multiple carbon products including CO, formate, methane, ethylene, and alcohols. Current catalysts often lack the specificity to target a single high-value product, resulting in complex and costly downstream separation processes. Even state-of-the-art copper-based catalysts typically achieve only 60-70% Faradaic efficiency toward a specific product.
Catalyst stability and durability remain critical concerns for industrial implementation. Many promising catalysts demonstrate significant performance degradation after only tens or hundreds of hours of operation, whereas commercial applications require thousands of hours of stable performance. Catalyst poisoning, structural changes, and leaching of active components during extended operation contribute to this degradation.
Scaling up laboratory systems to industrial proportions introduces additional challenges related to mass transport limitations. As reactor dimensions increase, CO2 diffusion becomes rate-limiting due to its low solubility in aqueous electrolytes (approximately 33 mM at ambient conditions). This creates concentration gradients that reduce overall system efficiency and alter product distribution compared to laboratory-scale demonstrations.
System integration challenges arise when attempting to couple CO2 capture with electrochemical reduction. The energy requirements for CO2 capture from dilute sources (typically 400 ppm in air) are substantial, and the captured CO2 often contains impurities that can deactivate catalysts. Creating integrated systems that efficiently manage these processes remains technically challenging.
Economic viability represents perhaps the most significant barrier to commercialization. Current production costs for electrocatalytic CO2 reduction products are estimated at 2-5 times higher than conventional fossil-based routes. This gap is driven by high electricity costs, expensive catalyst materials, limited system lifetimes, and inefficient conversion processes. Achieving cost parity will require simultaneous advances in catalyst performance, system design, and renewable electricity generation.
Current Electrocatalytic CO2 Reduction Solutions
01 Metal-based catalysts for CO2 electroreduction
Various metal-based catalysts can be employed for the electrocatalytic reduction of CO2. These catalysts include transition metals, metal alloys, and metal oxides that facilitate the conversion of CO2 to valuable products such as carbon monoxide, formate, or hydrocarbons. The catalytic performance depends on the metal's electronic structure, surface morphology, and binding affinity for reaction intermediates. Optimizing these properties can enhance selectivity and efficiency in CO2 electroreduction.- Metal-based catalysts for CO2 reduction: Various metal-based catalysts can be used for electrocatalytic CO2 reduction to produce valuable chemicals. These catalysts include transition metals like copper, silver, gold, and their alloys or compounds. The catalytic performance can be enhanced by controlling the morphology, crystal structure, and surface properties of these metal catalysts. These catalysts facilitate the electron transfer process required for CO2 reduction and can be tuned to improve selectivity towards specific products.
- Carbon-based materials as electrocatalysts: Carbon-based materials such as graphene, carbon nanotubes, and doped carbon structures serve as effective electrocatalysts for CO2 reduction. These materials offer advantages including high surface area, excellent electrical conductivity, and tunable surface chemistry. Nitrogen, boron, or other heteroatom doping can create active sites on carbon materials that enhance catalytic activity. Carbon-based catalysts are particularly attractive due to their abundance, low cost, and environmental friendliness compared to precious metal catalysts.
- Electrode design and reactor configurations: The design of electrodes and reactor configurations plays a crucial role in electrocatalytic CO2 reduction efficiency. Gas diffusion electrodes can enhance CO2 mass transfer to catalytic sites, while flow cells can improve reaction kinetics. Microfluidic reactors offer precise control over reaction conditions. The electrode structure, including porosity, thickness, and hydrophobicity, significantly affects the catalytic performance. Advanced reactor designs can help overcome mass transfer limitations and improve energy efficiency of the CO2 reduction process.
- Electrolyte composition and reaction conditions: The composition of the electrolyte and reaction conditions significantly influence the efficiency and selectivity of electrocatalytic CO2 reduction. Factors such as pH, ionic strength, temperature, and the presence of specific ions can alter reaction pathways. Ionic liquids and organic electrolytes may enhance CO2 solubility and stabilize reaction intermediates. Optimizing applied potential, current density, and CO2 pressure can improve conversion rates and product selectivity. The electrolyte composition can be tailored to suppress competing hydrogen evolution reactions.
- Hybrid and composite catalyst systems: Hybrid and composite catalyst systems combine different materials to achieve synergistic effects in electrocatalytic CO2 reduction. These may include metal-organic frameworks (MOFs), metal/metal oxide composites, or catalyst-polymer combinations. Bimetallic catalysts can offer improved selectivity compared to single-metal systems. Molecular catalysts anchored on conductive supports provide homogeneous-like selectivity with heterogeneous-like stability. These hybrid systems often demonstrate enhanced catalytic activity, improved stability, and better product selectivity than their individual components.
02 Carbon-based materials as electrocatalysts
Carbon-based materials serve as effective electrocatalysts for CO2 reduction. These include carbon nanotubes, graphene, carbon quantum dots, and doped carbon structures. The high surface area, excellent conductivity, and tunable electronic properties of carbon-based materials make them promising candidates for CO2 electroreduction. Functionalization and heteroatom doping can further enhance their catalytic activity and selectivity toward specific products.Expand Specific Solutions03 Nanostructured catalysts for improved performance
Nanostructured catalysts offer enhanced performance in electrocatalytic CO2 reduction due to their high surface area, abundant active sites, and unique electronic properties. These include nanoparticles, nanowires, nanosheets, and hierarchical structures. The nanoscale architecture can be tailored to optimize mass transport, electron transfer, and intermediate binding, leading to improved catalytic activity, selectivity, and stability during the CO2 reduction process.Expand Specific Solutions04 Reactor design and system optimization
The design of electrochemical reactors and system optimization are crucial for efficient CO2 electroreduction. This includes the development of flow cells, gas diffusion electrodes, membrane electrode assemblies, and integrated systems. Factors such as electrode configuration, electrolyte composition, mass transport, and operating conditions significantly impact the performance of CO2 reduction systems. Advanced reactor designs can address challenges related to CO2 solubility, product separation, and scale-up.Expand Specific Solutions05 Bimetallic and multi-component catalysts
Bimetallic and multi-component catalysts offer synergistic effects for enhanced CO2 electroreduction. These catalysts combine two or more metals or incorporate supporting materials to create unique active sites with optimized binding energies for reaction intermediates. The composition, structure, and interface between components can be engineered to improve activity, selectivity, and stability. These catalysts often demonstrate superior performance compared to their single-component counterparts.Expand Specific Solutions
Leading Companies and Research Institutions
The electrocatalytic CO2 reduction technology market is currently in an early growth phase, characterized by significant research activity but limited commercial deployment. The global market size is projected to expand substantially as regulatory frameworks evolve to support carbon capture utilization technologies. Technical maturity varies across applications, with leading academic institutions (University of Toronto, Hong Kong Polytechnic University, Yale University) driving fundamental research while specialized companies like Dioxide Materials and RenewCO2 are advancing commercial applications. Energy giants including TotalEnergies and Repsol are strategically investing in this space, recognizing its potential for meeting emissions reduction targets. Chinese research institutions (Dalian Institute of Chemical Physics, Tianjin University) are rapidly advancing capabilities, positioning themselves as significant players in this emerging field where regulatory clarity will be crucial for widespread adoption.
The Regents of the University of California
Technical Solution: The University of California has developed pioneering research on regulatory frameworks for electrocatalytic CO2 reduction technologies. Their interdisciplinary approach combines technical expertise in catalyst development with policy analysis to address key regulatory challenges. UC researchers have created comprehensive guidelines for safety protocols specific to CO2 electroreduction processes, addressing potential hazards from hydrogen co-production and pressurized systems. Their work includes detailed life cycle assessment methodologies that quantify environmental impacts in accordance with EPA and international standards, providing crucial data for regulatory approval processes. UC has also developed standardized testing protocols for catalyst performance and durability that align with emerging certification requirements, establishing benchmarks that regulatory bodies can adopt. Additionally, their research addresses regulatory considerations for scaling laboratory technologies to industrial implementation, including materials compliance, process safety management, and emissions monitoring requirements.
Strengths: Cutting-edge research that helps define regulatory standards; strong interdisciplinary approach combining technical and policy expertise; extensive publication record that influences regulatory development. Weaknesses: As an academic institution, may have less direct experience with industrial-scale regulatory compliance processes compared to commercial entities.
Dioxide Materials, Inc.
Technical Solution: Dioxide Materials has developed proprietary electrochemical systems specifically designed to meet regulatory requirements for CO2 reduction deployment. Their technology focuses on sustainable electrolyzer designs that convert CO2 into valuable chemicals like carbon monoxide and formic acid. The company has engineered specialized membrane electrode assemblies (MEAs) with high selectivity and durability that comply with industrial safety standards. Their systems incorporate advanced monitoring capabilities to ensure emissions control during operation, meeting EPA and international environmental regulations. Dioxide Materials has also developed scalable manufacturing processes that adhere to quality control standards required by regulatory bodies, with documented performance metrics that satisfy reporting requirements for carbon utilization technologies.
Strengths: Specialized expertise in electrochemical CO2 conversion with commercially-ready systems that already meet many regulatory standards; established track record with industrial partners. Weaknesses: As a smaller company, may face challenges in navigating complex international regulatory landscapes compared to larger corporations with more extensive legal resources.
Key Patents and Scientific Breakthroughs
Electrocatalytic conversion of carbon dioxide in liquids expanded by carbon dioxide
PatentWO2020010296A1
Innovation
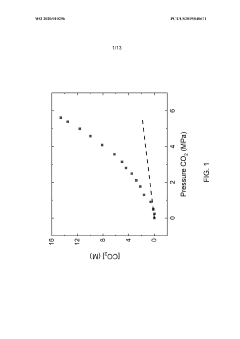
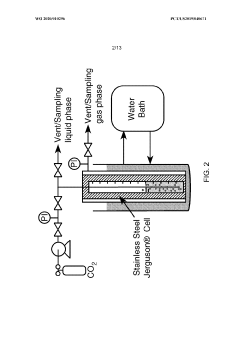
- The use of a CO2 expanded liquid medium at elevated pressures, which increases CO2 solubility and enables higher CO2 concentrations and transfer rates to catalytic surfaces, facilitating electrochemical reduction with organic liquid solvents and electrolytes that maintain a single phase and support fast electron transfer.
Acid promoted electrocatalytic reduction of carbon dioxide by square planar transition metal complexes
PatentInactiveUS4668349A
Innovation
- The use of transition metal complexes with square planar geometry in acidified aqueous or nonaqueous solutions, where the complexes are immersed, and an electrical potential of -0.8 to -1.5 volts is applied to reduce carbon dioxide to carbon monoxide, with the hydrogen ion concentration adjusted to enhance reaction rates.
Global Regulatory Framework for CO2 Utilization
The global regulatory landscape for CO2 utilization technologies is evolving rapidly as nations seek to balance climate action with economic development. Currently, regulatory frameworks vary significantly across regions, with the European Union leading through its comprehensive Carbon Capture and Utilization (CCU) policies embedded within the European Green Deal and Circular Economy Action Plan. These regulations establish clear standards for carbon accounting, product certification, and market incentives for CO2-derived products.
In North America, the United States has implemented the 45Q tax credit system, providing financial incentives of up to $60 per metric ton of CO2 utilized in commercial applications, including electrocatalytic reduction processes. Canada's Clean Fuel Standard similarly recognizes CO2 utilization as a compliance pathway, though with varying implementation requirements across provinces.
Asia-Pacific jurisdictions demonstrate diverse approaches, with China's 14th Five-Year Plan explicitly supporting CO2 utilization technologies through demonstration projects and industrial subsidies. Japan and South Korea have established regulatory sandboxes specifically for CO2 conversion technologies, allowing controlled testing of novel applications before full regulatory approval.
International standards organizations play a crucial harmonizing role, with ISO/TC 265 developing standards for CO2 capture, transportation, and utilization. These standards address critical aspects such as life cycle assessment methodologies, product quality specifications, and safety protocols specific to electrocatalytic processes.
Key regulatory considerations for electrocatalytic CO2 reduction deployment include product certification requirements, with emerging frameworks for verifying the carbon origin in chemicals and fuels. Environmental permitting processes are being adapted to accommodate these novel technologies, with particular attention to catalyst materials classification and waste handling protocols.
Market-based mechanisms represent another regulatory dimension, with emissions trading schemes in over 40 jurisdictions potentially recognizing electrocatalytic CO2 reduction as an abatement pathway. The EU's Carbon Border Adjustment Mechanism may significantly impact international deployment by potentially favoring products manufactured using carbon utilization technologies.
Cross-cutting regulatory challenges include the need for harmonized life cycle assessment methodologies to verify emissions reduction claims, intellectual property protection frameworks for novel catalysts and processes, and evolving safety standards for hydrogen co-production and handling of reaction intermediates.
In North America, the United States has implemented the 45Q tax credit system, providing financial incentives of up to $60 per metric ton of CO2 utilized in commercial applications, including electrocatalytic reduction processes. Canada's Clean Fuel Standard similarly recognizes CO2 utilization as a compliance pathway, though with varying implementation requirements across provinces.
Asia-Pacific jurisdictions demonstrate diverse approaches, with China's 14th Five-Year Plan explicitly supporting CO2 utilization technologies through demonstration projects and industrial subsidies. Japan and South Korea have established regulatory sandboxes specifically for CO2 conversion technologies, allowing controlled testing of novel applications before full regulatory approval.
International standards organizations play a crucial harmonizing role, with ISO/TC 265 developing standards for CO2 capture, transportation, and utilization. These standards address critical aspects such as life cycle assessment methodologies, product quality specifications, and safety protocols specific to electrocatalytic processes.
Key regulatory considerations for electrocatalytic CO2 reduction deployment include product certification requirements, with emerging frameworks for verifying the carbon origin in chemicals and fuels. Environmental permitting processes are being adapted to accommodate these novel technologies, with particular attention to catalyst materials classification and waste handling protocols.
Market-based mechanisms represent another regulatory dimension, with emissions trading schemes in over 40 jurisdictions potentially recognizing electrocatalytic CO2 reduction as an abatement pathway. The EU's Carbon Border Adjustment Mechanism may significantly impact international deployment by potentially favoring products manufactured using carbon utilization technologies.
Cross-cutting regulatory challenges include the need for harmonized life cycle assessment methodologies to verify emissions reduction claims, intellectual property protection frameworks for novel catalysts and processes, and evolving safety standards for hydrogen co-production and handling of reaction intermediates.
Carbon Credit Mechanisms and Economic Incentives
Carbon credit mechanisms and economic incentives play a crucial role in accelerating the deployment of electrocatalytic CO2 reduction technologies. The carbon market framework provides financial motivation for industries to invest in carbon capture and utilization technologies by monetizing CO2 emission reductions. Currently, several carbon pricing mechanisms exist globally, including cap-and-trade systems like the EU Emissions Trading System (EU ETS) and carbon taxes implemented in various jurisdictions.
For electrocatalytic CO2 reduction specifically, carbon credits can be generated through methodologies that quantify the amount of CO2 converted into valuable products. The verification process typically requires robust monitoring systems to track the source of electricity used, ensuring that the overall process results in net carbon reduction. Premium carbon credits may be awarded when renewable energy sources power the electrocatalytic processes, creating additional economic incentives.
Government subsidies and tax incentives further enhance the economic viability of these technologies. Several countries have implemented production tax credits for carbon utilization, ranging from $35-60 per ton of CO2 converted. The 45Q tax credit in the United States, recently expanded under the Inflation Reduction Act, provides significant financial support for carbon capture and utilization projects, including electrocatalytic approaches.
Green financing mechanisms are emerging as another important economic driver. Sustainable bonds and climate finance initiatives increasingly target technologies that demonstrate measurable carbon reduction impacts. The Climate Bonds Initiative has developed specific criteria for evaluating carbon utilization projects, providing a framework for investors seeking climate-positive opportunities in the electrocatalytic CO2 reduction space.
Product premiums represent an additional economic incentive pathway. Companies utilizing CO2-derived materials can often command higher market prices for their "green" products. This market-driven approach complements regulatory mechanisms and helps create demand-pull for electrocatalytic CO2 reduction technologies. Several consumer goods companies have already committed to incorporating carbon-neutral or carbon-negative materials in their supply chains.
The integration of these various economic mechanisms creates a multi-layered incentive structure that can help overcome the cost barriers associated with early-stage deployment of electrocatalytic CO2 reduction technologies. However, harmonization of carbon accounting methodologies across different jurisdictions remains a challenge that must be addressed to fully unlock the potential of these economic incentives.
For electrocatalytic CO2 reduction specifically, carbon credits can be generated through methodologies that quantify the amount of CO2 converted into valuable products. The verification process typically requires robust monitoring systems to track the source of electricity used, ensuring that the overall process results in net carbon reduction. Premium carbon credits may be awarded when renewable energy sources power the electrocatalytic processes, creating additional economic incentives.
Government subsidies and tax incentives further enhance the economic viability of these technologies. Several countries have implemented production tax credits for carbon utilization, ranging from $35-60 per ton of CO2 converted. The 45Q tax credit in the United States, recently expanded under the Inflation Reduction Act, provides significant financial support for carbon capture and utilization projects, including electrocatalytic approaches.
Green financing mechanisms are emerging as another important economic driver. Sustainable bonds and climate finance initiatives increasingly target technologies that demonstrate measurable carbon reduction impacts. The Climate Bonds Initiative has developed specific criteria for evaluating carbon utilization projects, providing a framework for investors seeking climate-positive opportunities in the electrocatalytic CO2 reduction space.
Product premiums represent an additional economic incentive pathway. Companies utilizing CO2-derived materials can often command higher market prices for their "green" products. This market-driven approach complements regulatory mechanisms and helps create demand-pull for electrocatalytic CO2 reduction technologies. Several consumer goods companies have already committed to incorporating carbon-neutral or carbon-negative materials in their supply chains.
The integration of these various economic mechanisms creates a multi-layered incentive structure that can help overcome the cost barriers associated with early-stage deployment of electrocatalytic CO2 reduction technologies. However, harmonization of carbon accounting methodologies across different jurisdictions remains a challenge that must be addressed to fully unlock the potential of these economic incentives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!