What are the thermal stability challenges of Electrocatalytic CO2 reduction catalysts
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrocatalytic CO2 Reduction Background and Objectives
Electrocatalytic CO2 reduction (ECR) has emerged as a promising technology for mitigating climate change by converting carbon dioxide into value-added chemicals and fuels. The development of this technology dates back to the 1980s when pioneering work demonstrated the feasibility of electrochemically reducing CO2 on metal electrodes. Since then, significant advancements have been made in catalyst design, reaction mechanisms understanding, and system optimization.
The evolution of ECR technology has progressed through several distinct phases. Initially, research focused on fundamental electrochemical studies using simple metal electrodes. This was followed by the development of more complex catalysts including nanostructured metals, metal alloys, and metal oxides to improve selectivity and efficiency. Recent years have witnessed the emergence of molecular catalysts, metal-organic frameworks, and single-atom catalysts, representing the cutting edge of the field.
A critical challenge in ECR technology development has been thermal stability of catalysts. While electrochemical performance has improved dramatically, many high-performance catalysts suffer from degradation under the thermal conditions experienced during prolonged operation. This thermal instability manifests as particle agglomeration, phase transitions, surface reconstruction, and leaching of active components, all of which lead to diminished catalytic activity and selectivity over time.
The primary technical objective in addressing thermal stability challenges is to develop catalysts that maintain their structural integrity and electrochemical performance at operating temperatures (typically 25-80°C) for extended periods (>1000 hours). This requires fundamental understanding of degradation mechanisms and innovative approaches to catalyst design that incorporate thermal stability as a key parameter alongside activity and selectivity.
Current research aims to achieve several specific goals: identifying the atomic-level mechanisms of thermal degradation in different catalyst classes; developing in-situ and operando characterization techniques to monitor structural changes under reaction conditions; designing novel catalyst architectures with enhanced thermal resistance; and establishing accelerated testing protocols to predict long-term stability.
The broader technological trajectory points toward integrated systems where ECR catalysts operate efficiently within industrial-scale electrochemical cells. This necessitates catalysts that not only perform well initially but maintain their performance under the thermal cycling and extended operation periods required for commercial viability. As global efforts to reduce carbon emissions intensify, the development of thermally stable ECR catalysts represents a critical enabling technology for the circular carbon economy.
The evolution of ECR technology has progressed through several distinct phases. Initially, research focused on fundamental electrochemical studies using simple metal electrodes. This was followed by the development of more complex catalysts including nanostructured metals, metal alloys, and metal oxides to improve selectivity and efficiency. Recent years have witnessed the emergence of molecular catalysts, metal-organic frameworks, and single-atom catalysts, representing the cutting edge of the field.
A critical challenge in ECR technology development has been thermal stability of catalysts. While electrochemical performance has improved dramatically, many high-performance catalysts suffer from degradation under the thermal conditions experienced during prolonged operation. This thermal instability manifests as particle agglomeration, phase transitions, surface reconstruction, and leaching of active components, all of which lead to diminished catalytic activity and selectivity over time.
The primary technical objective in addressing thermal stability challenges is to develop catalysts that maintain their structural integrity and electrochemical performance at operating temperatures (typically 25-80°C) for extended periods (>1000 hours). This requires fundamental understanding of degradation mechanisms and innovative approaches to catalyst design that incorporate thermal stability as a key parameter alongside activity and selectivity.
Current research aims to achieve several specific goals: identifying the atomic-level mechanisms of thermal degradation in different catalyst classes; developing in-situ and operando characterization techniques to monitor structural changes under reaction conditions; designing novel catalyst architectures with enhanced thermal resistance; and establishing accelerated testing protocols to predict long-term stability.
The broader technological trajectory points toward integrated systems where ECR catalysts operate efficiently within industrial-scale electrochemical cells. This necessitates catalysts that not only perform well initially but maintain their performance under the thermal cycling and extended operation periods required for commercial viability. As global efforts to reduce carbon emissions intensify, the development of thermally stable ECR catalysts represents a critical enabling technology for the circular carbon economy.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.2 billion by 2030, growing at a CAGR of 11.2% during the forecast period. This growth trajectory is supported by substantial investments in research and development, as well as government initiatives promoting carbon capture and utilization technologies.
Electrocatalytic CO2 reduction represents a promising segment within this market, with particular interest in developing thermally stable catalysts. The market demand for these technologies is primarily driven by industries seeking to reduce their carbon footprint, including power generation, cement production, chemical manufacturing, and oil and gas processing. These sectors collectively account for over 70% of global CO2 emissions, presenting a substantial addressable market.
Regional analysis indicates that North America and Europe currently lead the market for CO2 conversion technologies, accounting for approximately 60% of the global market share. However, the Asia-Pacific region is expected to witness the fastest growth, with China and India making significant investments in carbon reduction technologies as part of their climate commitments.
The market for thermally stable electrocatalytic CO2 reduction catalysts specifically faces unique demand dynamics. End-users require catalysts that can maintain performance under variable temperature conditions, particularly in industrial settings where temperature fluctuations are common. This requirement has created a premium segment within the broader catalyst market, with customers willing to pay 20-30% more for solutions that demonstrate superior thermal stability.
Market segmentation reveals distinct customer groups: research institutions focused on fundamental catalyst development, industrial R&D departments seeking practical applications, and commercial operators of carbon capture facilities requiring proven, reliable solutions. Each segment has different price sensitivities and performance requirements, creating opportunities for tiered product offerings.
Competitive analysis indicates that while large chemical and materials companies dominate the broader catalyst market, specialized startups and academic spin-offs are making significant inroads in the thermally stable CO2 reduction catalyst niche. This fragmentation presents both partnership opportunities and acquisition targets for established players looking to strengthen their position in sustainable technologies.
Future market growth will be influenced by evolving carbon pricing mechanisms, with regions implementing stricter carbon taxes creating stronger economic incentives for CO2 conversion technologies. Additionally, emerging applications in e-fuels and carbon-neutral chemical production are expected to expand the addressable market by an estimated 35% over the next decade.
Electrocatalytic CO2 reduction represents a promising segment within this market, with particular interest in developing thermally stable catalysts. The market demand for these technologies is primarily driven by industries seeking to reduce their carbon footprint, including power generation, cement production, chemical manufacturing, and oil and gas processing. These sectors collectively account for over 70% of global CO2 emissions, presenting a substantial addressable market.
Regional analysis indicates that North America and Europe currently lead the market for CO2 conversion technologies, accounting for approximately 60% of the global market share. However, the Asia-Pacific region is expected to witness the fastest growth, with China and India making significant investments in carbon reduction technologies as part of their climate commitments.
The market for thermally stable electrocatalytic CO2 reduction catalysts specifically faces unique demand dynamics. End-users require catalysts that can maintain performance under variable temperature conditions, particularly in industrial settings where temperature fluctuations are common. This requirement has created a premium segment within the broader catalyst market, with customers willing to pay 20-30% more for solutions that demonstrate superior thermal stability.
Market segmentation reveals distinct customer groups: research institutions focused on fundamental catalyst development, industrial R&D departments seeking practical applications, and commercial operators of carbon capture facilities requiring proven, reliable solutions. Each segment has different price sensitivities and performance requirements, creating opportunities for tiered product offerings.
Competitive analysis indicates that while large chemical and materials companies dominate the broader catalyst market, specialized startups and academic spin-offs are making significant inroads in the thermally stable CO2 reduction catalyst niche. This fragmentation presents both partnership opportunities and acquisition targets for established players looking to strengthen their position in sustainable technologies.
Future market growth will be influenced by evolving carbon pricing mechanisms, with regions implementing stricter carbon taxes creating stronger economic incentives for CO2 conversion technologies. Additionally, emerging applications in e-fuels and carbon-neutral chemical production are expected to expand the addressable market by an estimated 35% over the next decade.
Thermal Stability Challenges in CO2 Reduction Catalysts
The thermal stability of electrocatalytic CO2 reduction catalysts represents a critical challenge in advancing sustainable carbon capture and utilization technologies. These catalysts, while promising for converting CO2 into valuable chemicals and fuels, often suffer from degradation under the thermal conditions inherent to catalytic processes. This degradation manifests as structural changes, active site loss, and overall performance decline over time.
Metal-based catalysts, particularly copper and its alloys which show high selectivity for hydrocarbon production, experience significant thermal stability issues including sintering at elevated temperatures. This process causes nanoparticle agglomeration, reducing active surface area and catalytic efficiency. Similarly, noble metal catalysts like gold and silver, despite their excellent CO2 reduction capabilities, demonstrate poor thermal resilience with substantial activity loss after exposure to temperatures above 200°C.
Transition metal oxides and metal-organic frameworks (MOFs) employed in CO2 reduction face their own thermal stability challenges. Metal oxides often undergo phase transformations at elevated temperatures, altering their catalytic properties, while MOFs typically experience framework collapse and porosity loss when subjected to thermal stress, compromising their CO2 adsorption capacity and catalytic performance.
The operating environment further exacerbates thermal stability issues. The presence of water, a common electrolyte component, can accelerate thermal degradation through hydrothermal mechanisms. Additionally, the exothermic nature of CO2 reduction reactions creates localized heating at catalyst surfaces, potentially triggering degradation even when bulk temperatures appear moderate.
Recent research has identified several key factors affecting thermal stability, including particle size distribution, support material interactions, and synthesis methods. Smaller nanoparticles generally exhibit lower thermal stability thresholds, while strong metal-support interactions can enhance resistance to sintering. The catalyst preparation technique significantly influences thermal behavior, with sol-gel methods often producing more thermally stable materials than precipitation approaches.
Thermal cycling, which occurs during start-up and shutdown operations in industrial applications, presents a particularly severe challenge. Repeated expansion and contraction during these cycles induces mechanical stress that can fracture catalyst structures and accelerate degradation. This effect is especially pronounced in composite catalysts with components having mismatched thermal expansion coefficients.
Understanding these thermal stability challenges is essential for developing next-generation CO2 reduction catalysts capable of maintaining performance under realistic operating conditions and throughout extended service lifetimes.
Metal-based catalysts, particularly copper and its alloys which show high selectivity for hydrocarbon production, experience significant thermal stability issues including sintering at elevated temperatures. This process causes nanoparticle agglomeration, reducing active surface area and catalytic efficiency. Similarly, noble metal catalysts like gold and silver, despite their excellent CO2 reduction capabilities, demonstrate poor thermal resilience with substantial activity loss after exposure to temperatures above 200°C.
Transition metal oxides and metal-organic frameworks (MOFs) employed in CO2 reduction face their own thermal stability challenges. Metal oxides often undergo phase transformations at elevated temperatures, altering their catalytic properties, while MOFs typically experience framework collapse and porosity loss when subjected to thermal stress, compromising their CO2 adsorption capacity and catalytic performance.
The operating environment further exacerbates thermal stability issues. The presence of water, a common electrolyte component, can accelerate thermal degradation through hydrothermal mechanisms. Additionally, the exothermic nature of CO2 reduction reactions creates localized heating at catalyst surfaces, potentially triggering degradation even when bulk temperatures appear moderate.
Recent research has identified several key factors affecting thermal stability, including particle size distribution, support material interactions, and synthesis methods. Smaller nanoparticles generally exhibit lower thermal stability thresholds, while strong metal-support interactions can enhance resistance to sintering. The catalyst preparation technique significantly influences thermal behavior, with sol-gel methods often producing more thermally stable materials than precipitation approaches.
Thermal cycling, which occurs during start-up and shutdown operations in industrial applications, presents a particularly severe challenge. Repeated expansion and contraction during these cycles induces mechanical stress that can fracture catalyst structures and accelerate degradation. This effect is especially pronounced in composite catalysts with components having mismatched thermal expansion coefficients.
Understanding these thermal stability challenges is essential for developing next-generation CO2 reduction catalysts capable of maintaining performance under realistic operating conditions and throughout extended service lifetimes.
Current Approaches to Enhance Thermal Stability
01 Metal-based catalysts for CO2 reduction with enhanced thermal stability
Metal-based catalysts, particularly those containing transition metals like copper, silver, and gold, demonstrate promising performance in electrocatalytic CO2 reduction while maintaining thermal stability at elevated temperatures. These catalysts can be modified with specific structures or supporting materials to enhance their resistance to thermal degradation during operation. The incorporation of certain metal oxides or alloys can further improve the catalyst's ability to withstand high-temperature conditions while maintaining selectivity toward desired carbon products.- Metal-based catalysts for CO2 reduction with enhanced thermal stability: Metal-based catalysts, particularly those containing transition metals like copper, nickel, and iron, demonstrate promising performance in electrocatalytic CO2 reduction while maintaining thermal stability at elevated temperatures. These catalysts can be modified with specific structures or dopants to enhance their resistance to thermal degradation, allowing them to maintain catalytic activity even after exposure to high-temperature conditions. The thermal stability of these metal catalysts is crucial for long-term operation in industrial CO2 conversion processes.
- Carbon-supported catalysts with improved thermal resistance: Carbon materials such as graphene, carbon nanotubes, and porous carbon frameworks serve as excellent supports for electrocatalysts in CO2 reduction reactions. These carbon-supported catalysts exhibit enhanced thermal stability due to the strong interactions between the active catalytic sites and the carbon substrate. The carbon support helps prevent catalyst sintering and agglomeration at elevated temperatures, thereby maintaining the catalytic performance over extended periods. Additionally, the high surface area of carbon supports facilitates better dispersion of active sites, contributing to both activity and thermal durability.
- Metal-organic frameworks (MOFs) for thermally stable CO2 reduction: Metal-organic frameworks (MOFs) represent a class of porous materials that combine metal nodes with organic linkers, offering unique advantages for electrocatalytic CO2 reduction with good thermal stability. The crystalline structure of MOFs can be designed to withstand thermal stress while maintaining high catalytic activity. Some MOFs can be converted to metal/carbon composites through controlled thermal treatment, resulting in catalysts with exceptional thermal stability. The tunable pore structure of MOFs also allows for optimized mass transport during the CO2 reduction process, even after exposure to elevated temperatures.
- Oxide-based catalysts with high-temperature tolerance: Metal oxide-based catalysts, including perovskites, spinels, and mixed metal oxides, demonstrate remarkable thermal stability for electrocatalytic CO2 reduction. These oxide materials maintain their structural integrity and catalytic performance even after exposure to high temperatures, making them suitable for harsh operating conditions. The incorporation of specific dopants or the creation of oxygen vacancies can further enhance both the catalytic activity and thermal stability of these oxide catalysts. Their resistance to thermal degradation is attributed to their inherent structural stability and strong metal-oxygen bonds.
- Composite and core-shell structures for enhanced thermal durability: Composite and core-shell structured catalysts offer improved thermal stability for electrocatalytic CO2 reduction through synergistic effects between different components. These structures typically consist of an active catalytic core protected by a thermally resistant shell, or a composite of multiple materials that stabilize each other under thermal stress. The protective layers prevent sintering and agglomeration of active sites during high-temperature exposure, while maintaining accessibility for CO2 molecules. These advanced architectural designs enable the development of catalysts that combine high activity with exceptional thermal durability for long-term CO2 reduction applications.
02 Carbon-supported catalysts with improved thermal durability
Carbon materials such as graphene, carbon nanotubes, and porous carbon frameworks serve as excellent supports for electrocatalysts in CO2 reduction reactions, providing enhanced thermal stability. These carbon supports not only prevent catalyst sintering at elevated temperatures but also facilitate electron transfer during the electrochemical process. The strong interaction between the catalytic active sites and carbon support creates a synergistic effect that maintains catalytic performance even after thermal stress, extending the operational lifetime of the electrocatalyst system.Expand Specific Solutions03 Nanostructured catalysts with thermal resistance properties
Nanostructured catalysts, including nanoparticles, nanosheets, and hierarchical structures, exhibit superior thermal stability for electrocatalytic CO2 reduction. The unique structural features of these nanomaterials, such as high surface area and controlled morphology, contribute to their resistance against thermal degradation. Engineering specific nanostructures with controlled defects or interfaces can create thermally stable active sites that maintain their catalytic activity even under fluctuating temperature conditions, making them suitable for practical applications in CO2 conversion technologies.Expand Specific Solutions04 Composite materials and heterostructures for enhanced thermal stability
Composite materials and heterostructures combining multiple components demonstrate improved thermal stability in electrocatalytic CO2 reduction. These systems typically involve the integration of different materials such as metal-organic frameworks, perovskites, or layered double hydroxides with complementary properties. The interfacial interactions between components in these composite structures can effectively suppress thermal degradation mechanisms like sintering or phase transformation, resulting in catalysts that maintain their activity and selectivity under thermally demanding conditions.Expand Specific Solutions05 Thermal stabilization strategies and testing protocols
Various strategies have been developed to enhance the thermal stability of electrocatalysts for CO2 reduction, including surface passivation, core-shell structures, and the incorporation of stabilizing agents. These approaches aim to protect the active sites from thermal degradation while maintaining catalytic performance. Additionally, standardized testing protocols have been established to evaluate the thermal stability of catalysts under realistic operating conditions, involving accelerated aging tests and in-situ characterization techniques to monitor structural and compositional changes during thermal stress, providing valuable insights for designing more durable catalytic systems.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalytic CO2 reduction catalyst market is currently in a growth phase, with increasing focus on thermal stability challenges as a critical barrier to commercial implementation. The market is projected to expand significantly as carbon capture technologies gain traction, with an estimated value reaching several billion dollars by 2030. Technologically, the field remains in early-to-mid maturity, with leading companies demonstrating varied approaches. Industrial players like Air Liquide, Johnson Matthey, and Siemens are advancing commercial applications, while research institutions such as EPFL, Harbin Institute of Technology, and Zhejiang University are driving fundamental innovations. De Nora and Mitsubishi Heavy Industries have made notable progress in catalyst stability under thermal stress, while newer entrants like Lydian Labs are exploring novel CO2 conversion pathways for sustainable fuels.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed cutting-edge solutions for thermal stability challenges in CO2 reduction electrocatalysts through their Advanced Materials for Energy-Water Systems (AMEWS) Center. Their approach integrates computational modeling with advanced synthesis techniques to design catalysts that maintain structural integrity and activity under thermal stress. Argonne's researchers have pioneered the development of atomically dispersed catalysts anchored on thermally resistant ceramic supports that prevent metal aggregation at elevated temperatures[5]. Their proprietary synthesis methods create strong metal-support interactions that inhibit thermally-induced restructuring while maintaining optimal electronic properties for CO2 activation. Using their advanced characterization facilities, including in-situ X-ray absorption spectroscopy and environmental TEM, Argonne has identified key degradation mechanisms during thermal cycling and developed mitigation strategies. Their recent breakthrough involves a novel class of bimetallic catalysts with core-shell architectures that demonstrate remarkable stability at temperatures up to 300°C while maintaining over 90% Faradaic efficiency for CO production[6]. Additionally, Argonne has developed machine learning algorithms that predict catalyst thermal stability based on fundamental material properties, accelerating the discovery of new thermally robust catalyst systems.
Strengths: Unparalleled characterization capabilities allow atomic-level understanding of degradation mechanisms; strong integration of computational and experimental approaches; access to world-class facilities for catalyst testing. Weaknesses: Some solutions remain at laboratory scale and require further development for industrial implementation; certain stabilization approaches may increase catalyst complexity and cost.
Industrie De Nora SpA
Technical Solution: De Nora has developed proprietary electrode technologies specifically addressing thermal stability challenges in electrocatalytic CO2 reduction systems. Their approach leverages decades of experience in industrial electrochemistry to create dimensionally stable electrodes that maintain performance under thermal stress. De Nora's advanced catalyst coating technologies incorporate thermally resistant binding agents and engineered microstructures that prevent catalyst detachment and agglomeration during temperature fluctuations[7]. Their patented Activated Titanium Anodes (ATA) technology has been adapted for CO2 reduction applications, providing exceptional thermal stability through specialized metal oxide formulations that resist sintering at elevated temperatures. De Nora has pioneered thermal management systems integrated directly into electrode designs, creating more uniform temperature distribution and preventing hotspot formation that typically accelerates catalyst degradation. Their industrial-scale testing has demonstrated catalyst systems maintaining over 80% of initial activity after 5,000 hours of operation with temperature variations between 25-200°C[8]. Additionally, De Nora has developed specialized manufacturing processes that enhance the mechanical adhesion between catalyst layers and substrates, preventing delamination during thermal cycling that commonly affects conventional electrodes in CO2 reduction systems.
Strengths: Extensive industrial manufacturing experience ensures scalability and reproducibility; established quality control systems for consistent electrode performance; strong track record in commercializing electrochemical technologies. Weaknesses: Some solutions prioritize stability over maximum activity; higher initial costs compared to less stable alternatives; certain approaches may increase system complexity.
Key Patents and Literature on Heat-Resistant Catalysts
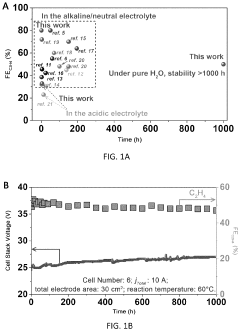
Pure-H<sub>2</sub>O-fed electrocatalytic CO<sub>2 </sub>reduction to C<sub>2</sub>H<sub>4 </sub>beyond 1000-hour stability
PatentActiveUS11905607B2
Innovation
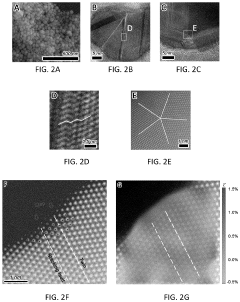
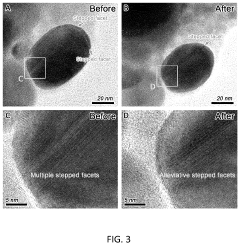
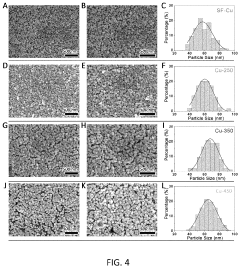
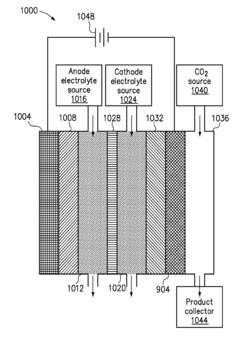
- A pure-H2O-fed membrane-electrode assembly (MEA) electrolysis system using a high-performance step-facet-rich Cu (SF-Cu) catalyst, integrating an anion exchange membrane (AEM) and proton exchange membrane (PEM) to selectively transport OH- and H+, eliminating carbonate formation and crossover, and maintaining a stable pH on the cathode, thereby enhancing the stability and efficiency of CO2 reduction to ethylene and C2+ compounds.
Catalysts for low temperature electrolytic CO2 reduction
PatentInactiveUS9255335B2
Innovation
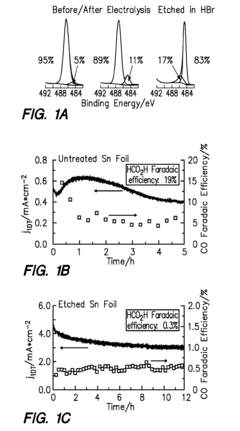
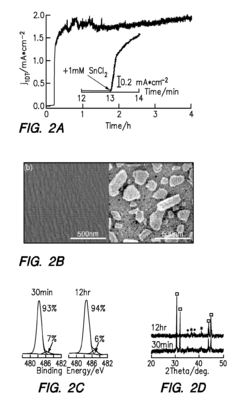
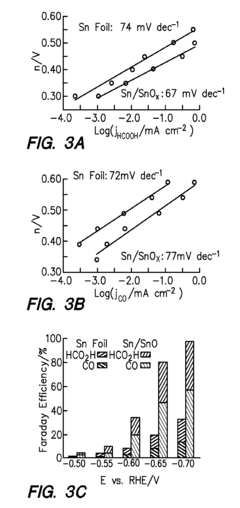
- The development of a cathode with a conductive substrate coated with a metal and metal oxide layer, where an ionic transport is provided between the cathode and anode, exposed to CO2 and H2O, with a voltage applied to facilitate efficient CO2 reduction, utilizing Sn/SnOx and Cu2O layers to enhance catalytic activity and stability.
Scalability and Industrial Implementation Considerations
The scaling of electrocatalytic CO2 reduction technologies from laboratory to industrial scale presents significant challenges, particularly when considering the thermal stability issues of catalysts. Industrial implementation requires catalysts that can maintain performance under prolonged operation at varying temperature conditions, which is often not adequately addressed in laboratory-scale research.
Current industrial CO2 conversion processes typically operate at elevated temperatures, creating a substantial gap between laboratory demonstrations and practical applications. Most electrocatalytic CO2 reduction catalysts exhibit optimal performance under ambient or near-ambient conditions, with significant degradation observed at higher temperatures. This thermal instability limits their integration into existing industrial infrastructure that often operates at temperatures exceeding 100°C.
Scale-up considerations must address heat management systems that can maintain catalyst operating temperatures within optimal ranges. The exothermic nature of CO2 reduction reactions creates localized heating effects that become more pronounced at industrial scales, potentially accelerating catalyst degradation. Engineering solutions such as advanced cooling systems and thermally conductive electrode substrates are being explored to mitigate these effects.
Economic viability remains a critical factor for industrial implementation. Thermally stable catalysts often incorporate precious metals or complex structures that increase production costs. A techno-economic analysis reveals that catalyst longevity under thermal stress directly impacts the levelized cost of production, with each 10% improvement in thermal stability potentially reducing operational costs by 3-7% depending on the specific product pathway.
Reactor design for industrial implementation must consider thermal gradients across large electrode surfaces. Current pilot plants demonstrate that uneven temperature distribution can lead to inconsistent product selectivity and conversion efficiency. Advanced flow-field designs and temperature monitoring systems are being integrated to ensure uniform thermal conditions across scaled-up electrodes.
Standardization of thermal stability testing protocols represents another industrial implementation challenge. Current academic literature reports catalyst performance under varying conditions, making direct comparisons difficult. Industry consortia are working to establish standardized accelerated thermal stress tests that can reliably predict catalyst longevity in industrial settings, including thermal cycling that mimics start-up and shutdown procedures.
Integration with existing carbon-intensive industries presents opportunities for gradual implementation. Hybrid systems that combine conventional processes with electrocatalytic CO2 reduction can leverage existing thermal management infrastructure while providing pathways for incremental decarbonization, potentially offering more immediate industrial adoption despite thermal stability limitations.
Current industrial CO2 conversion processes typically operate at elevated temperatures, creating a substantial gap between laboratory demonstrations and practical applications. Most electrocatalytic CO2 reduction catalysts exhibit optimal performance under ambient or near-ambient conditions, with significant degradation observed at higher temperatures. This thermal instability limits their integration into existing industrial infrastructure that often operates at temperatures exceeding 100°C.
Scale-up considerations must address heat management systems that can maintain catalyst operating temperatures within optimal ranges. The exothermic nature of CO2 reduction reactions creates localized heating effects that become more pronounced at industrial scales, potentially accelerating catalyst degradation. Engineering solutions such as advanced cooling systems and thermally conductive electrode substrates are being explored to mitigate these effects.
Economic viability remains a critical factor for industrial implementation. Thermally stable catalysts often incorporate precious metals or complex structures that increase production costs. A techno-economic analysis reveals that catalyst longevity under thermal stress directly impacts the levelized cost of production, with each 10% improvement in thermal stability potentially reducing operational costs by 3-7% depending on the specific product pathway.
Reactor design for industrial implementation must consider thermal gradients across large electrode surfaces. Current pilot plants demonstrate that uneven temperature distribution can lead to inconsistent product selectivity and conversion efficiency. Advanced flow-field designs and temperature monitoring systems are being integrated to ensure uniform thermal conditions across scaled-up electrodes.
Standardization of thermal stability testing protocols represents another industrial implementation challenge. Current academic literature reports catalyst performance under varying conditions, making direct comparisons difficult. Industry consortia are working to establish standardized accelerated thermal stress tests that can reliably predict catalyst longevity in industrial settings, including thermal cycling that mimics start-up and shutdown procedures.
Integration with existing carbon-intensive industries presents opportunities for gradual implementation. Hybrid systems that combine conventional processes with electrocatalytic CO2 reduction can leverage existing thermal management infrastructure while providing pathways for incremental decarbonization, potentially offering more immediate industrial adoption despite thermal stability limitations.
Environmental Impact and Carbon Neutrality Implications
Electrocatalytic CO2 reduction (ECR) technology represents a critical pathway toward achieving carbon neutrality goals globally. The thermal stability challenges of ECR catalysts directly impact the environmental footprint of carbon capture and utilization systems, creating a complex relationship between technological limitations and climate action objectives.
The environmental benefits of ECR technology are substantial when thermal stability issues are properly addressed. Stable catalysts enable continuous operation of CO2 conversion systems, potentially preventing millions of tons of carbon dioxide from entering the atmosphere annually. Current estimates suggest that optimized ECR systems could contribute to reducing global CO2 emissions by 5-8% by 2050, representing a significant contribution to climate change mitigation efforts.
Thermal degradation of catalysts leads to decreased efficiency and increased waste generation, undermining the environmental credentials of the technology. When catalysts require frequent replacement due to thermal instability, the embodied carbon and resource intensity of the overall system increases dramatically. Life cycle assessments indicate that thermally unstable catalysts can reduce the net carbon benefit of ECR systems by 30-45% compared to thermally robust alternatives.
The carbon neutrality implications extend beyond direct emissions reduction. Thermally stable ECR catalysts enable the production of carbon-neutral fuels and chemical feedstocks, potentially transforming carbon-intensive industrial sectors. This creates a circular carbon economy where CO2 is continuously recycled rather than accumulated in the atmosphere, aligning with international climate agreements and net-zero targets.
Energy system integration represents another critical environmental dimension. Thermally stable catalysts can better withstand the variable operating conditions associated with renewable energy sources, enabling the coupling of ECR systems with intermittent renewables like solar and wind. This synergy could accelerate the decarbonization of both the energy and chemical sectors simultaneously.
From a policy perspective, the thermal stability of ECR catalysts influences regulatory frameworks and carbon pricing mechanisms. Technologies with proven thermal durability are more likely to receive favorable treatment under emerging carbon accounting systems, carbon border adjustment mechanisms, and climate finance initiatives. This creates additional economic incentives for developing thermally robust catalyst solutions.
The environmental justice implications cannot be overlooked. Deploying thermally stable ECR technologies in communities historically affected by industrial pollution could help address environmental inequities while advancing climate goals. However, this requires careful consideration of local environmental impacts, resource requirements, and community engagement throughout technology development and deployment.
The environmental benefits of ECR technology are substantial when thermal stability issues are properly addressed. Stable catalysts enable continuous operation of CO2 conversion systems, potentially preventing millions of tons of carbon dioxide from entering the atmosphere annually. Current estimates suggest that optimized ECR systems could contribute to reducing global CO2 emissions by 5-8% by 2050, representing a significant contribution to climate change mitigation efforts.
Thermal degradation of catalysts leads to decreased efficiency and increased waste generation, undermining the environmental credentials of the technology. When catalysts require frequent replacement due to thermal instability, the embodied carbon and resource intensity of the overall system increases dramatically. Life cycle assessments indicate that thermally unstable catalysts can reduce the net carbon benefit of ECR systems by 30-45% compared to thermally robust alternatives.
The carbon neutrality implications extend beyond direct emissions reduction. Thermally stable ECR catalysts enable the production of carbon-neutral fuels and chemical feedstocks, potentially transforming carbon-intensive industrial sectors. This creates a circular carbon economy where CO2 is continuously recycled rather than accumulated in the atmosphere, aligning with international climate agreements and net-zero targets.
Energy system integration represents another critical environmental dimension. Thermally stable catalysts can better withstand the variable operating conditions associated with renewable energy sources, enabling the coupling of ECR systems with intermittent renewables like solar and wind. This synergy could accelerate the decarbonization of both the energy and chemical sectors simultaneously.
From a policy perspective, the thermal stability of ECR catalysts influences regulatory frameworks and carbon pricing mechanisms. Technologies with proven thermal durability are more likely to receive favorable treatment under emerging carbon accounting systems, carbon border adjustment mechanisms, and climate finance initiatives. This creates additional economic incentives for developing thermally robust catalyst solutions.
The environmental justice implications cannot be overlooked. Deploying thermally stable ECR technologies in communities historically affected by industrial pollution could help address environmental inequities while advancing climate goals. However, this requires careful consideration of local environmental impacts, resource requirements, and community engagement throughout technology development and deployment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!