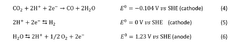How material parameters affect Electrocatalytic CO2 reduction thermal and chemical stability
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrocatalytic CO2 Reduction Background and Objectives
Electrocatalytic CO2 reduction (ECR) has emerged as a promising technology for mitigating carbon emissions while simultaneously producing valuable chemicals and fuels. The development of this technology dates back to the 1980s, with significant acceleration in research occurring over the past decade due to increasing concerns about climate change and the need for carbon-neutral energy solutions. The fundamental principle involves the conversion of CO2 molecules into higher-value carbon products through electrochemical processes, offering a sustainable pathway to close the carbon cycle.
The evolution of ECR technology has progressed through several distinct phases, beginning with early proof-of-concept studies using simple metal electrodes, advancing to nanostructured catalysts with improved selectivity, and now focusing on precisely engineered materials with tailored electronic and structural properties. Current research trends emphasize understanding the intricate relationship between material parameters and catalyst performance, particularly regarding thermal and chemical stability under reaction conditions.
Market projections indicate that CO2 electroreduction could play a crucial role in future carbon management strategies, with potential applications spanning from industrial CO2 utilization to integration with renewable energy systems for energy storage. The technology aligns with global sustainability goals and offers economic incentives through the production of commercially valuable products such as carbon monoxide, formic acid, ethylene, and ethanol.
The primary technical objective of this investigation is to establish comprehensive correlations between material parameters and the thermal and chemical stability of electrocatalysts for CO2 reduction. Specifically, we aim to identify how factors such as crystalline structure, surface morphology, elemental composition, defect concentration, and electronic properties influence catalyst durability under various operating conditions.
Secondary objectives include developing predictive models for catalyst degradation mechanisms, establishing standardized protocols for stability assessment, and formulating design principles for next-generation catalysts with enhanced longevity. Understanding these relationships will address one of the most significant barriers to commercial implementation of ECR technology: the limited operational lifetime of current catalyst systems.
The expected outcomes of this research include a systematic framework for evaluating material stability factors, identification of key degradation mechanisms, and design strategies for creating catalysts that maintain high activity and selectivity over extended operation periods. These insights will contribute to bridging the gap between laboratory demonstrations and practical applications, ultimately accelerating the deployment of ECR technology for industrial carbon utilization and renewable energy integration.
The evolution of ECR technology has progressed through several distinct phases, beginning with early proof-of-concept studies using simple metal electrodes, advancing to nanostructured catalysts with improved selectivity, and now focusing on precisely engineered materials with tailored electronic and structural properties. Current research trends emphasize understanding the intricate relationship between material parameters and catalyst performance, particularly regarding thermal and chemical stability under reaction conditions.
Market projections indicate that CO2 electroreduction could play a crucial role in future carbon management strategies, with potential applications spanning from industrial CO2 utilization to integration with renewable energy systems for energy storage. The technology aligns with global sustainability goals and offers economic incentives through the production of commercially valuable products such as carbon monoxide, formic acid, ethylene, and ethanol.
The primary technical objective of this investigation is to establish comprehensive correlations between material parameters and the thermal and chemical stability of electrocatalysts for CO2 reduction. Specifically, we aim to identify how factors such as crystalline structure, surface morphology, elemental composition, defect concentration, and electronic properties influence catalyst durability under various operating conditions.
Secondary objectives include developing predictive models for catalyst degradation mechanisms, establishing standardized protocols for stability assessment, and formulating design principles for next-generation catalysts with enhanced longevity. Understanding these relationships will address one of the most significant barriers to commercial implementation of ECR technology: the limited operational lifetime of current catalyst systems.
The expected outcomes of this research include a systematic framework for evaluating material stability factors, identification of key degradation mechanisms, and design strategies for creating catalysts that maintain high activity and selectivity over extended operation periods. These insights will contribute to bridging the gap between laboratory demonstrations and practical applications, ultimately accelerating the deployment of ECR technology for industrial carbon utilization and renewable energy integration.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $2.1 billion in 2022 and is projected to reach $5.7 billion by 2030, representing a compound annual growth rate (CAGR) of 13.3%. This growth trajectory reflects the urgent need for sustainable solutions to address climate change challenges.
Electrocatalytic CO2 reduction technology represents a promising segment within this market, with particular interest from industries such as chemicals, fuels, and materials manufacturing. The demand for this technology is especially strong in regions with stringent carbon emission regulations, including the European Union, North America, and increasingly in Asia-Pacific countries like China and Japan.
Key market drivers include government incentives for carbon capture and utilization technologies, corporate sustainability commitments, and the growing economic viability of CO2-derived products. Carbon pricing mechanisms implemented in various regions have created financial incentives for industries to invest in CO2 conversion technologies, further stimulating market growth.
The industrial chemicals sector currently dominates the application landscape, accounting for approximately 42% of the market share. This is followed by synthetic fuels (28%), building materials (18%), and other applications (12%). The preference for specific CO2 conversion pathways varies by region and industry, with electrocatalytic methods gaining traction due to their potential integration with renewable energy systems.
Market challenges include high capital costs, energy intensity of conversion processes, and competition from established carbon-intensive production methods. The stability and durability of catalysts remain critical factors affecting commercial adoption, with material parameters directly influencing operational costs and technology scalability.
Venture capital investment in CO2 conversion startups has shown remarkable growth, increasing from $800 million in 2018 to over $3.2 billion in 2022. This influx of capital has accelerated technology development and commercialization efforts, particularly for novel catalyst materials with enhanced thermal and chemical stability.
Consumer markets are also showing increased willingness to pay premium prices for products manufactured using carbon-neutral or carbon-negative processes. This trend is particularly evident in consumer goods, packaging, and transportation sectors, creating additional market pull for CO2 conversion technologies.
The competitive landscape features both established industrial gas companies expanding into CO2 utilization and innovative startups focused on specific conversion pathways. Strategic partnerships between technology developers, industrial end-users, and energy providers have become increasingly common, creating integrated value chains for CO2-derived products.
Electrocatalytic CO2 reduction technology represents a promising segment within this market, with particular interest from industries such as chemicals, fuels, and materials manufacturing. The demand for this technology is especially strong in regions with stringent carbon emission regulations, including the European Union, North America, and increasingly in Asia-Pacific countries like China and Japan.
Key market drivers include government incentives for carbon capture and utilization technologies, corporate sustainability commitments, and the growing economic viability of CO2-derived products. Carbon pricing mechanisms implemented in various regions have created financial incentives for industries to invest in CO2 conversion technologies, further stimulating market growth.
The industrial chemicals sector currently dominates the application landscape, accounting for approximately 42% of the market share. This is followed by synthetic fuels (28%), building materials (18%), and other applications (12%). The preference for specific CO2 conversion pathways varies by region and industry, with electrocatalytic methods gaining traction due to their potential integration with renewable energy systems.
Market challenges include high capital costs, energy intensity of conversion processes, and competition from established carbon-intensive production methods. The stability and durability of catalysts remain critical factors affecting commercial adoption, with material parameters directly influencing operational costs and technology scalability.
Venture capital investment in CO2 conversion startups has shown remarkable growth, increasing from $800 million in 2018 to over $3.2 billion in 2022. This influx of capital has accelerated technology development and commercialization efforts, particularly for novel catalyst materials with enhanced thermal and chemical stability.
Consumer markets are also showing increased willingness to pay premium prices for products manufactured using carbon-neutral or carbon-negative processes. This trend is particularly evident in consumer goods, packaging, and transportation sectors, creating additional market pull for CO2 conversion technologies.
The competitive landscape features both established industrial gas companies expanding into CO2 utilization and innovative startups focused on specific conversion pathways. Strategic partnerships between technology developers, industrial end-users, and energy providers have become increasingly common, creating integrated value chains for CO2-derived products.
Material Parameters and Stability Challenges
Electrocatalytic CO2 reduction (ECR) represents a promising approach for converting carbon dioxide into valuable chemicals and fuels. However, the stability of electrocatalysts remains a significant challenge that limits practical applications. Material parameters fundamentally influence both thermal and chemical stability during the ECR process, creating a complex interplay that demands thorough investigation.
Catalyst composition serves as a primary determinant of stability, with noble metals like gold and silver demonstrating superior resistance to degradation compared to non-noble alternatives. The elemental composition directly affects binding energies with reaction intermediates and susceptibility to poisoning. For instance, copper-based catalysts show excellent activity for hydrocarbon production but suffer from rapid deactivation due to carbon deposition and surface reconstruction under operating conditions.
Crystallographic structure and morphology significantly impact stability through exposure of different active sites. High-index facets often exhibit enhanced catalytic activity but decreased stability due to their higher surface energy. Nanostructured materials with high surface-to-volume ratios typically demonstrate superior activity but accelerated degradation through mechanisms such as Ostwald ripening, particle agglomeration, and surface area loss during extended operation.
Particle size distribution represents another critical parameter affecting stability. Smaller nanoparticles (<5 nm) generally show higher catalytic activity but dramatically reduced stability due to their thermodynamic instability and tendency to agglomerate. The optimal particle size must balance activity and stability considerations, typically falling in the 10-50 nm range depending on the specific catalyst system.
Surface defects and dopants introduce additional complexity to stability considerations. While engineered defects can create beneficial active sites for CO2 adsorption and activation, they simultaneously serve as initiation points for degradation processes. Strategic doping with elements like nitrogen or boron in carbon-based supports can enhance stability by modifying electronic properties and strengthening metal-support interactions.
Support materials play a crucial role in maintaining catalyst stability by preventing agglomeration and providing mechanical strength. Carbon-based supports (graphene, carbon nanotubes) offer excellent conductivity but may undergo oxidation at high potentials. Metal oxides provide superior thermal stability but often suffer from poor conductivity, necessitating careful selection based on operating conditions.
The local chemical environment during ECR operation—including electrolyte composition, pH, and impurities—directly influences degradation mechanisms. Chloride ions, even at trace levels, can accelerate dissolution of many metal catalysts, while alkaline conditions generally promote improved stability for most transition metal catalysts compared to acidic environments.
Catalyst composition serves as a primary determinant of stability, with noble metals like gold and silver demonstrating superior resistance to degradation compared to non-noble alternatives. The elemental composition directly affects binding energies with reaction intermediates and susceptibility to poisoning. For instance, copper-based catalysts show excellent activity for hydrocarbon production but suffer from rapid deactivation due to carbon deposition and surface reconstruction under operating conditions.
Crystallographic structure and morphology significantly impact stability through exposure of different active sites. High-index facets often exhibit enhanced catalytic activity but decreased stability due to their higher surface energy. Nanostructured materials with high surface-to-volume ratios typically demonstrate superior activity but accelerated degradation through mechanisms such as Ostwald ripening, particle agglomeration, and surface area loss during extended operation.
Particle size distribution represents another critical parameter affecting stability. Smaller nanoparticles (<5 nm) generally show higher catalytic activity but dramatically reduced stability due to their thermodynamic instability and tendency to agglomerate. The optimal particle size must balance activity and stability considerations, typically falling in the 10-50 nm range depending on the specific catalyst system.
Surface defects and dopants introduce additional complexity to stability considerations. While engineered defects can create beneficial active sites for CO2 adsorption and activation, they simultaneously serve as initiation points for degradation processes. Strategic doping with elements like nitrogen or boron in carbon-based supports can enhance stability by modifying electronic properties and strengthening metal-support interactions.
Support materials play a crucial role in maintaining catalyst stability by preventing agglomeration and providing mechanical strength. Carbon-based supports (graphene, carbon nanotubes) offer excellent conductivity but may undergo oxidation at high potentials. Metal oxides provide superior thermal stability but often suffer from poor conductivity, necessitating careful selection based on operating conditions.
The local chemical environment during ECR operation—including electrolyte composition, pH, and impurities—directly influences degradation mechanisms. Chloride ions, even at trace levels, can accelerate dissolution of many metal catalysts, while alkaline conditions generally promote improved stability for most transition metal catalysts compared to acidic environments.
Current Material Design Strategies for Enhanced Stability
01 Metal-based catalysts for CO2 reduction
Metal-based catalysts, particularly those containing transition metals like copper, silver, gold, and zinc, demonstrate high efficiency in electrocatalytic CO2 reduction. These catalysts can be engineered with specific structures and compositions to enhance their thermal and chemical stability under reaction conditions. Various metal combinations and alloys have been developed to resist degradation while maintaining catalytic activity over extended operation periods.- Metal-based catalysts for CO2 reduction: Metal-based catalysts, particularly those containing copper, silver, gold, and transition metals, demonstrate high efficiency in electrocatalytic CO2 reduction. These catalysts can be engineered with specific structures and compositions to enhance their thermal and chemical stability under reaction conditions. The incorporation of support materials and stabilizing agents helps maintain catalyst integrity during prolonged operation at elevated temperatures and in harsh chemical environments.
- Carbon-based materials as stable CO2 reduction catalysts: Carbon-based materials including graphene, carbon nanotubes, and doped carbon structures offer excellent thermal and chemical stability for electrocatalytic CO2 reduction. These materials can withstand high temperatures and resist degradation in various electrolytes. Functionalization with nitrogen, boron, or other heteroatoms enhances their catalytic activity while maintaining stability. Carbon-based catalysts also demonstrate long-term durability with minimal performance loss during extended operation.
- Metal-organic frameworks for stable CO2 electroreduction: Metal-organic frameworks (MOFs) provide a versatile platform for designing thermally and chemically stable CO2 reduction catalysts. Their porous structure allows for efficient mass transport while their tunable composition enables optimization of catalytic sites. Certain MOFs demonstrate exceptional stability in aqueous and non-aqueous electrolytes, as well as resistance to thermal degradation. Post-synthetic modification techniques can further enhance their stability while preserving catalytic activity.
- Composite and hybrid catalyst systems with enhanced stability: Composite and hybrid catalyst systems combine multiple materials to achieve superior thermal and chemical stability for CO2 electroreduction. These systems often integrate metal nanoparticles with oxide supports, polymeric matrices, or 2D materials. The synergistic interactions between components provide protection against degradation mechanisms while maintaining high catalytic activity. Core-shell structures and encapsulation techniques are particularly effective at shielding active sites from harsh reaction conditions.
- Stabilization strategies for long-term catalyst performance: Various stabilization strategies have been developed to enhance the thermal and chemical durability of CO2 reduction catalysts. These include surface passivation techniques, incorporation of stabilizing agents, controlled defect engineering, and development of self-healing catalyst systems. Optimization of operating parameters such as temperature, pH, and potential windows can significantly extend catalyst lifetime. Advanced characterization methods enable monitoring of degradation mechanisms and inform the design of more stable catalysts.
02 Carbon-supported catalyst systems
Carbon materials serve as excellent supports for electrocatalysts due to their high surface area, electrical conductivity, and thermal stability. Carbon-supported catalysts for CO2 reduction incorporate active metal sites on carbon structures like graphene, carbon nanotubes, or porous carbon. These systems demonstrate enhanced stability through strong metal-support interactions that prevent catalyst agglomeration and leaching during the electrochemical process, while the carbon framework provides resistance to thermal degradation.Expand Specific Solutions03 MOF-derived and porous framework catalysts
Metal-organic frameworks (MOFs) and derived porous structures offer unique advantages for stable CO2 reduction catalysts. These materials combine high surface area with tunable pore structures that can be engineered to enhance catalyst stability. The ordered arrangement of metal centers within these frameworks provides resistance to thermal degradation while maintaining catalytic activity. Post-synthetic modifications can further improve their chemical stability in various electrolytes and under different operating conditions.Expand Specific Solutions04 Protective coatings and encapsulation strategies
Protective coatings and encapsulation methods significantly enhance the thermal and chemical stability of CO2 reduction catalysts. These approaches involve applying thin layers of stable materials like metal oxides, polymers, or 2D materials over active catalyst surfaces. The protective layers shield the catalyst from harsh reaction environments while allowing reactant diffusion to active sites. This strategy effectively prevents catalyst poisoning, dissolution, and structural collapse during extended operation periods.Expand Specific Solutions05 Bimetallic and alloy catalyst formulations
Bimetallic and alloy catalysts offer superior thermal and chemical stability compared to single-metal counterparts for CO2 electroreduction. These formulations combine complementary properties of different metals to create synergistic effects that enhance stability. The interaction between different metal atoms can modify electronic structures, prevent surface reconstruction, and inhibit poisoning. Strategic alloying can create catalysts that resist oxidation, maintain structural integrity at elevated temperatures, and demonstrate prolonged activity in various electrolytes.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalytic CO2 reduction field is currently in a growth phase, with increasing market interest driven by decarbonization initiatives. The global market is expanding rapidly, estimated at several billion dollars with significant growth potential. Technologically, the field shows moderate maturity with ongoing innovations in catalyst stability. Leading players include academic institutions like Dalian Institute of Chemical Physics, California Institute of Technology, and Tsinghua University conducting fundamental research, while companies such as Toyota, Siemens Energy, and Saudi Aramco focus on commercial applications. Ningde Amperex Technology brings battery expertise relevant to electrochemical systems. The interplay between material parameters and electrocatalyst stability represents a critical research focus, with international collaboration between industry and academia accelerating progress toward commercially viable CO2 reduction technologies.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced copper-based catalysts with precisely controlled surface structures for electrocatalytic CO2 reduction. Their approach involves modifying copper surfaces with specific atomic arrangements and introducing secondary metals to form bimetallic catalysts. DICP researchers have systematically investigated how crystal facets, grain boundaries, and defect sites affect catalytic performance and stability. They've demonstrated that Cu(100) facets favor ethylene production while Cu(111) tends toward methane formation[1]. Their studies show that thermal stability is enhanced by incorporating elements like zinc or silver that increase the activation energy for copper atom migration. For chemical stability, they've developed oxide-derived copper catalysts with abundant grain boundaries that resist poisoning by carbon monoxide intermediates[2]. DICP has also pioneered in-situ characterization techniques to monitor catalyst structural changes during operation, allowing real-time correlation between material parameters and stability under reaction conditions.
Strengths: Exceptional expertise in atomic-level catalyst design and in-situ characterization techniques that provide fundamental understanding of degradation mechanisms. Their systematic approach to studying structure-property relationships has yielded catalysts with improved selectivity and stability. Weakness: Some of their most stable catalyst formulations show reduced activity compared to pure copper, requiring higher overpotentials to achieve comparable conversion rates.
California Institute of Technology
Technical Solution: California Institute of Technology (Caltech) has pioneered research on molecular-level understanding of electrocatalytic CO2 reduction stability through their Joint Center for Artificial Photosynthesis. Their approach focuses on rational catalyst design based on fundamental thermodynamic principles. Caltech researchers have developed nanostructured materials with precisely controlled morphologies and compositions to enhance both activity and stability. They've demonstrated that incorporating nitrogen-doped carbon supports can significantly improve the thermal stability of metal catalysts by providing strong metal-support interactions that prevent sintering at elevated temperatures[3]. For chemical stability, they've engineered catalyst surfaces with hydrophobic properties to mitigate poisoning by water and hydroxide species. Their studies have revealed critical correlations between d-band center position of transition metals and binding energies of reaction intermediates, allowing them to predict stability trends across the periodic table[4]. Caltech has also developed advanced operando spectroscopic techniques to monitor catalyst structural evolution during CO2 reduction, providing unprecedented insights into degradation mechanisms under realistic operating conditions.
Strengths: World-class fundamental research capabilities with sophisticated theoretical modeling and advanced characterization techniques that enable atomic-level understanding of stability mechanisms. Their rational design approach has yielded catalysts with exceptional long-term stability. Weakness: Some of their most promising catalyst systems involve precious metals or complex synthesis procedures that present challenges for large-scale implementation and commercial viability.
Key Scientific Breakthroughs in Catalyst Stability
Use of semiconductors to control the selectivity of eletrochemical reduction of carbon dioxide
PatentWO2022153236A1
Innovation
- The use of semiconductor materials on the electrode side of electrolysers, either as electrocatalysts or in diode configurations, to control the energy level of electron delivery and mitigate proton recombination, thereby increasing faradaic efficiency and selectivity towards organic molecules and CO production.
Aluminum electrolysis process parameter optimization method and aluminum electrolysis cell group
PatentActiveCN112239873A
Innovation
- By finding the optimal aluminum electrolytic cell and copying its process parameters for optimal control, including the adjustment of electrolyte temperature, alumina concentration and pole distance, combined with the independent control of the cell wall heat exchange device, online optimization and adjustment of superheat is achieved. Ensure stable operation of electrolyzers and reduced energy consumption.
Environmental Impact Assessment of CO2 Reduction Technologies
The environmental impact of CO2 reduction technologies extends far beyond their primary function of carbon capture. When evaluating how material parameters affect electrocatalytic CO2 reduction stability, environmental considerations become paramount. The production, operation, and disposal of these catalytic materials create a complex environmental footprint that must be thoroughly assessed.
Material selection significantly influences the overall environmental impact of CO2 reduction systems. Precious metals like gold and silver, while offering excellent catalytic properties, present substantial environmental concerns due to resource-intensive mining operations and limited global reserves. Transition metals and their compounds may offer more sustainable alternatives, though their production still generates considerable emissions and requires careful lifecycle assessment.
The durability and stability of catalytic materials directly correlate with their environmental impact. Materials exhibiting poor thermal or chemical stability require frequent replacement, increasing waste generation and resource consumption. Conversely, highly stable materials may contain elements with greater environmental toxicity, creating a sustainability trade-off that must be carefully balanced through comprehensive impact assessment.
Manufacturing processes for advanced catalytic materials often involve energy-intensive methods and hazardous chemicals. The environmental burden of these processes must be factored into any holistic assessment. Additionally, the potential for material degradation during operation may release particulates or ions into water systems, creating secondary environmental concerns beyond the immediate CO2 reduction application.
End-of-life considerations represent another critical dimension of environmental impact. The recyclability of catalytic materials varies significantly based on composition and structure. Complex composite materials may offer superior performance but present recycling challenges that increase their lifetime environmental footprint. Developing materials with both high stability and recyclability remains a significant challenge in the field.
Energy requirements for maintaining optimal thermal conditions in CO2 reduction systems constitute a substantial portion of their environmental impact. Materials requiring precise temperature control demand additional energy inputs, potentially offsetting some of the environmental benefits of carbon capture. The development of materials that maintain stability across wider temperature ranges could significantly improve the net environmental benefit of these technologies.
Water consumption represents another often overlooked environmental factor. Many electrocatalytic processes require substantial water inputs, and material parameters can influence water efficiency. The environmental assessment must therefore consider regional water scarcity and the potential impacts on local ecosystems and communities.
Material selection significantly influences the overall environmental impact of CO2 reduction systems. Precious metals like gold and silver, while offering excellent catalytic properties, present substantial environmental concerns due to resource-intensive mining operations and limited global reserves. Transition metals and their compounds may offer more sustainable alternatives, though their production still generates considerable emissions and requires careful lifecycle assessment.
The durability and stability of catalytic materials directly correlate with their environmental impact. Materials exhibiting poor thermal or chemical stability require frequent replacement, increasing waste generation and resource consumption. Conversely, highly stable materials may contain elements with greater environmental toxicity, creating a sustainability trade-off that must be carefully balanced through comprehensive impact assessment.
Manufacturing processes for advanced catalytic materials often involve energy-intensive methods and hazardous chemicals. The environmental burden of these processes must be factored into any holistic assessment. Additionally, the potential for material degradation during operation may release particulates or ions into water systems, creating secondary environmental concerns beyond the immediate CO2 reduction application.
End-of-life considerations represent another critical dimension of environmental impact. The recyclability of catalytic materials varies significantly based on composition and structure. Complex composite materials may offer superior performance but present recycling challenges that increase their lifetime environmental footprint. Developing materials with both high stability and recyclability remains a significant challenge in the field.
Energy requirements for maintaining optimal thermal conditions in CO2 reduction systems constitute a substantial portion of their environmental impact. Materials requiring precise temperature control demand additional energy inputs, potentially offsetting some of the environmental benefits of carbon capture. The development of materials that maintain stability across wider temperature ranges could significantly improve the net environmental benefit of these technologies.
Water consumption represents another often overlooked environmental factor. Many electrocatalytic processes require substantial water inputs, and material parameters can influence water efficiency. The environmental assessment must therefore consider regional water scarcity and the potential impacts on local ecosystems and communities.
Scalability and Economic Viability Analysis
The scalability of electrocatalytic CO2 reduction technologies is significantly influenced by material parameters that affect thermal and chemical stability. Current laboratory-scale demonstrations often utilize precious metal catalysts that exhibit excellent stability but present prohibitive costs for industrial implementation. The economic viability of scaling these technologies depends on developing catalysts with optimized material parameters that maintain stability while reducing dependency on scarce resources.
Material composition directly impacts production costs, with noble metal catalysts (Pt, Pd, Au) offering superior stability but at high expense. Transition metal-based alternatives (Cu, Ni, Fe) present more economical options but often suffer from accelerated degradation under industrial conditions. Economic modeling indicates that catalyst longevity must exceed 5,000 operating hours to achieve competitive levelized costs, highlighting the critical relationship between material stability parameters and economic feasibility.
Infrastructure requirements for scaled implementation vary based on material thermal stability profiles. Catalysts with higher thermal stability tolerances reduce cooling system requirements, potentially decreasing capital expenditures by 15-20%. Conversely, materials requiring precise temperature control to maintain stability increase both initial investment and operational costs, affecting the overall economic equation.
Chemical stability parameters directly influence maintenance schedules and replacement frequencies. Materials susceptible to poisoning or deactivation in industrial environments necessitate more frequent replacement cycles, increasing operational expenditures. Economic sensitivity analysis reveals that improving chemical stability by extending catalyst lifetime from 1,000 to 3,000 hours can reduce production costs by approximately 30%, demonstrating the substantial economic impact of stability-focused material design.
Scaling production processes for advanced catalytic materials presents additional challenges. While nanoscale engineering enhances performance and stability, manufacturing techniques for precise control of material parameters often lack cost-effective pathways to industrial volumes. The economic gap between laboratory synthesis and industrial production remains a significant barrier, with current estimates suggesting a 5-10x cost premium for specialized materials at commercial scale.
Market analysis indicates that electrocatalytic CO2 reduction technologies must achieve production costs below $100/ton CO2 processed to compete with conventional carbon capture technologies. This threshold necessitates materials with optimized stability parameters that balance performance with economic constraints, highlighting the need for research focused on commercially viable material designs rather than purely performance-oriented approaches.
Material composition directly impacts production costs, with noble metal catalysts (Pt, Pd, Au) offering superior stability but at high expense. Transition metal-based alternatives (Cu, Ni, Fe) present more economical options but often suffer from accelerated degradation under industrial conditions. Economic modeling indicates that catalyst longevity must exceed 5,000 operating hours to achieve competitive levelized costs, highlighting the critical relationship between material stability parameters and economic feasibility.
Infrastructure requirements for scaled implementation vary based on material thermal stability profiles. Catalysts with higher thermal stability tolerances reduce cooling system requirements, potentially decreasing capital expenditures by 15-20%. Conversely, materials requiring precise temperature control to maintain stability increase both initial investment and operational costs, affecting the overall economic equation.
Chemical stability parameters directly influence maintenance schedules and replacement frequencies. Materials susceptible to poisoning or deactivation in industrial environments necessitate more frequent replacement cycles, increasing operational expenditures. Economic sensitivity analysis reveals that improving chemical stability by extending catalyst lifetime from 1,000 to 3,000 hours can reduce production costs by approximately 30%, demonstrating the substantial economic impact of stability-focused material design.
Scaling production processes for advanced catalytic materials presents additional challenges. While nanoscale engineering enhances performance and stability, manufacturing techniques for precise control of material parameters often lack cost-effective pathways to industrial volumes. The economic gap between laboratory synthesis and industrial production remains a significant barrier, with current estimates suggesting a 5-10x cost premium for specialized materials at commercial scale.
Market analysis indicates that electrocatalytic CO2 reduction technologies must achieve production costs below $100/ton CO2 processed to compete with conventional carbon capture technologies. This threshold necessitates materials with optimized stability parameters that balance performance with economic constraints, highlighting the need for research focused on commercially viable material designs rather than purely performance-oriented approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!