Analysis of Electrocatalytic CO2 reduction interface and surface engineering techniques
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Electrocatalysis Background and Objectives
Electrocatalytic CO2 reduction (ECR) has emerged as a promising technology for mitigating climate change while simultaneously producing valuable chemicals and fuels. The journey of this technology began in the late 1980s with pioneering work by Hori and colleagues, who demonstrated the feasibility of converting CO2 to various hydrocarbons and alcohols using copper electrodes. Since then, the field has witnessed exponential growth in research interest, particularly over the past decade as global concerns about carbon emissions have intensified.
The evolution of ECR technology has been marked by significant breakthroughs in catalyst design, from early metal-based catalysts to advanced nanomaterials and hybrid systems. Initial catalysts suffered from poor selectivity and efficiency, but modern approaches incorporating precise surface engineering have dramatically improved performance metrics. Recent developments in single-atom catalysts and bimetallic systems represent the cutting edge of this technological progression.
Current research trends are focusing on understanding and controlling the complex interfacial phenomena that govern CO2 reduction reactions. The electrode-electrolyte interface represents a dynamic environment where multiple factors—including local pH, electric field effects, mass transport limitations, and intermediate stabilization—collectively determine reaction pathways and product distributions. Surface engineering techniques have become central to optimizing these interfacial properties.
The primary technical objectives in this field include achieving higher Faradaic efficiencies toward specific high-value products, improving energy efficiency by reducing overpotentials, enhancing catalyst stability for long-term operation, and developing scalable systems that can operate at industrially relevant current densities. Particularly challenging is the selective production of C2+ products, which requires sophisticated control of C-C coupling reactions at the catalyst surface.
From an environmental perspective, the ultimate goal is to create closed carbon cycles by utilizing renewable electricity to convert atmospheric or point-source CO2 into chemicals and fuels that would otherwise be derived from fossil resources. This approach has the dual benefit of reducing net carbon emissions while storing renewable energy in chemical bonds.
The interdisciplinary nature of ECR research necessitates collaboration across electrochemistry, materials science, surface physics, and chemical engineering. Recent advances in in-situ and operando characterization techniques have provided unprecedented insights into reaction mechanisms, while computational methods are increasingly being employed to guide rational catalyst design and predict interfacial behaviors.
As we look toward future developments, the field is poised to transition from fundamental research to practical applications, with pilot-scale demonstrations beginning to emerge. The technical trajectory suggests continued refinement of interface engineering approaches as key to unlocking the full potential of electrocatalytic CO2 reduction technology.
The evolution of ECR technology has been marked by significant breakthroughs in catalyst design, from early metal-based catalysts to advanced nanomaterials and hybrid systems. Initial catalysts suffered from poor selectivity and efficiency, but modern approaches incorporating precise surface engineering have dramatically improved performance metrics. Recent developments in single-atom catalysts and bimetallic systems represent the cutting edge of this technological progression.
Current research trends are focusing on understanding and controlling the complex interfacial phenomena that govern CO2 reduction reactions. The electrode-electrolyte interface represents a dynamic environment where multiple factors—including local pH, electric field effects, mass transport limitations, and intermediate stabilization—collectively determine reaction pathways and product distributions. Surface engineering techniques have become central to optimizing these interfacial properties.
The primary technical objectives in this field include achieving higher Faradaic efficiencies toward specific high-value products, improving energy efficiency by reducing overpotentials, enhancing catalyst stability for long-term operation, and developing scalable systems that can operate at industrially relevant current densities. Particularly challenging is the selective production of C2+ products, which requires sophisticated control of C-C coupling reactions at the catalyst surface.
From an environmental perspective, the ultimate goal is to create closed carbon cycles by utilizing renewable electricity to convert atmospheric or point-source CO2 into chemicals and fuels that would otherwise be derived from fossil resources. This approach has the dual benefit of reducing net carbon emissions while storing renewable energy in chemical bonds.
The interdisciplinary nature of ECR research necessitates collaboration across electrochemistry, materials science, surface physics, and chemical engineering. Recent advances in in-situ and operando characterization techniques have provided unprecedented insights into reaction mechanisms, while computational methods are increasingly being employed to guide rational catalyst design and predict interfacial behaviors.
As we look toward future developments, the field is poised to transition from fundamental research to practical applications, with pilot-scale demonstrations beginning to emerge. The technical trajectory suggests continued refinement of interface engineering approaches as key to unlocking the full potential of electrocatalytic CO2 reduction technology.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market size for CO2 conversion was valued at approximately $1.8 billion in 2022 and is projected to reach $4.2 billion by 2030, growing at a CAGR of 11.2% during the forecast period. This growth trajectory is supported by substantial investments from both public and private sectors, with government funding for carbon capture and utilization projects exceeding $10 billion globally in recent years.
Electrocatalytic CO2 reduction represents one of the most promising segments within this market, accounting for roughly 24% of the total CO2 conversion technologies market. This segment is expected to grow faster than the overall market at a CAGR of 13.5% through 2030, reflecting the increasing commercial viability of these technologies and their potential for integration with renewable energy systems.
Geographically, North America currently leads the market with approximately 35% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, particularly driven by China's aggressive carbon neutrality targets and substantial investments in green technology infrastructure.
By application sector, the chemical industry represents the largest end-user segment (40%), followed by fuel production (30%) and mineralization processes (15%). The remaining 15% is distributed across various niche applications. The chemical industry's dominance is attributed to the economic value of converting CO2 into high-value chemical feedstocks and the relative maturity of these conversion pathways.
Key market drivers include increasingly stringent carbon emission regulations, rising carbon pricing mechanisms, growing corporate sustainability commitments, and technological advancements that are steadily reducing the cost of CO2 conversion. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating strong economic incentives for CO2 utilization technologies.
Market challenges remain significant, including high capital costs, energy intensity of conversion processes, and competition from conventional production methods. The levelized cost of converted CO2-based products typically remains 20-40% higher than fossil-based alternatives, though this gap is narrowing as technologies mature and economies of scale are realized.
The competitive landscape features a mix of established industrial gas companies, specialized technology startups, and chemical industry incumbents. Recent market consolidation has been observed, with several major acquisitions indicating the strategic importance of this technology space to traditional industrial players.
Electrocatalytic CO2 reduction represents one of the most promising segments within this market, accounting for roughly 24% of the total CO2 conversion technologies market. This segment is expected to grow faster than the overall market at a CAGR of 13.5% through 2030, reflecting the increasing commercial viability of these technologies and their potential for integration with renewable energy systems.
Geographically, North America currently leads the market with approximately 35% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, particularly driven by China's aggressive carbon neutrality targets and substantial investments in green technology infrastructure.
By application sector, the chemical industry represents the largest end-user segment (40%), followed by fuel production (30%) and mineralization processes (15%). The remaining 15% is distributed across various niche applications. The chemical industry's dominance is attributed to the economic value of converting CO2 into high-value chemical feedstocks and the relative maturity of these conversion pathways.
Key market drivers include increasingly stringent carbon emission regulations, rising carbon pricing mechanisms, growing corporate sustainability commitments, and technological advancements that are steadily reducing the cost of CO2 conversion. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating strong economic incentives for CO2 utilization technologies.
Market challenges remain significant, including high capital costs, energy intensity of conversion processes, and competition from conventional production methods. The levelized cost of converted CO2-based products typically remains 20-40% higher than fossil-based alternatives, though this gap is narrowing as technologies mature and economies of scale are realized.
The competitive landscape features a mix of established industrial gas companies, specialized technology startups, and chemical industry incumbents. Recent market consolidation has been observed, with several major acquisitions indicating the strategic importance of this technology space to traditional industrial players.
Current Challenges in Electrocatalytic CO2 Reduction
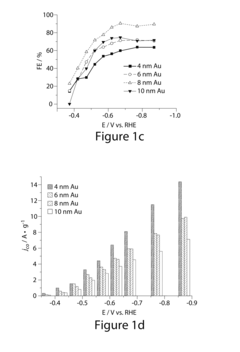
Despite significant advancements in electrocatalytic CO2 reduction (ECR) technology, several critical challenges continue to impede its widespread implementation and commercial viability. The primary obstacle remains the low energy efficiency of the process, with current systems typically achieving only 30-50% Faradaic efficiency for valuable products like ethylene and ethanol. This inefficiency stems largely from competing hydrogen evolution reactions that consume electrons without contributing to carbon conversion.
Catalyst selectivity presents another major hurdle, as most existing catalysts produce a mixture of products rather than targeting specific high-value chemicals. The formation of C-C bonds necessary for producing multi-carbon products remains particularly challenging, with most systems favoring simpler products like carbon monoxide or formate. Even state-of-the-art copper-based catalysts struggle to maintain selectivity above 60% for any single C2+ product.
Catalyst stability under reaction conditions represents a significant concern, with many promising materials suffering from deactivation through mechanisms such as poisoning, restructuring, or leaching. Studies indicate that most advanced catalysts show performance degradation within 100 hours of operation, falling short of the thousands of hours required for industrial viability.
Mass transport limitations at the electrode-electrolyte interface create concentration gradients that reduce reaction rates and selectivity. The low solubility of CO2 in aqueous electrolytes (approximately 33 mM at ambient conditions) further restricts reaction kinetics, while the formation of a pH gradient near the electrode surface alters local reaction conditions and product distribution.
Scale-up challenges persist as laboratory successes often fail to translate to larger systems. Current densities in lab-scale devices typically range from 10-200 mA/cm², whereas industrial applications would require at least 500 mA/cm² for economic viability. The gap between controlled laboratory environments and practical industrial conditions remains substantial.
System integration issues complicate matters further, as ECR technology must be effectively coupled with renewable energy sources to achieve true carbon neutrality. The intermittent nature of renewable electricity creates operational challenges for maintaining optimal reaction conditions and catalyst performance.
Economic barriers also remain significant, with current cost estimates for electrochemical CO2 conversion ranging from $200-600 per ton of CO2 processed, substantially higher than the $50-100 per ton threshold generally considered necessary for commercial adoption. The capital costs of specialized electrodes, membranes, and system components contribute significantly to this economic challenge.
Catalyst selectivity presents another major hurdle, as most existing catalysts produce a mixture of products rather than targeting specific high-value chemicals. The formation of C-C bonds necessary for producing multi-carbon products remains particularly challenging, with most systems favoring simpler products like carbon monoxide or formate. Even state-of-the-art copper-based catalysts struggle to maintain selectivity above 60% for any single C2+ product.
Catalyst stability under reaction conditions represents a significant concern, with many promising materials suffering from deactivation through mechanisms such as poisoning, restructuring, or leaching. Studies indicate that most advanced catalysts show performance degradation within 100 hours of operation, falling short of the thousands of hours required for industrial viability.
Mass transport limitations at the electrode-electrolyte interface create concentration gradients that reduce reaction rates and selectivity. The low solubility of CO2 in aqueous electrolytes (approximately 33 mM at ambient conditions) further restricts reaction kinetics, while the formation of a pH gradient near the electrode surface alters local reaction conditions and product distribution.
Scale-up challenges persist as laboratory successes often fail to translate to larger systems. Current densities in lab-scale devices typically range from 10-200 mA/cm², whereas industrial applications would require at least 500 mA/cm² for economic viability. The gap between controlled laboratory environments and practical industrial conditions remains substantial.
System integration issues complicate matters further, as ECR technology must be effectively coupled with renewable energy sources to achieve true carbon neutrality. The intermittent nature of renewable electricity creates operational challenges for maintaining optimal reaction conditions and catalyst performance.
Economic barriers also remain significant, with current cost estimates for electrochemical CO2 conversion ranging from $200-600 per ton of CO2 processed, substantially higher than the $50-100 per ton threshold generally considered necessary for commercial adoption. The capital costs of specialized electrodes, membranes, and system components contribute significantly to this economic challenge.
State-of-the-Art Surface Engineering Approaches
01 Surface modification of electrocatalysts for CO2 reduction
Surface modification techniques can enhance the performance of electrocatalysts for CO2 reduction. These modifications include creating defects, introducing dopants, or applying coatings to alter the electronic structure and binding properties of the catalyst surface. Such modifications can improve selectivity, activity, and stability of the catalysts by optimizing the adsorption energies of reaction intermediates and facilitating electron transfer at the interface.- Metal-based catalysts for CO2 electroreduction: Metal-based catalysts play a crucial role in electrocatalytic CO2 reduction. Various metals and their alloys can be engineered at the surface level to enhance catalytic activity and selectivity. Surface modifications such as creating defects, controlling crystal facets, and tuning electronic properties can significantly improve CO2 conversion efficiency. These catalysts can be designed to target specific products like CO, formate, or hydrocarbons by optimizing the binding energies of intermediates.
- Nanostructured interfaces for enhanced catalytic performance: Nanostructuring of catalyst surfaces creates high-performance interfaces for CO2 electroreduction. By engineering nanoporous structures, nanosheets, or nanoparticles, the active surface area can be dramatically increased, providing more catalytic sites. These nanostructured interfaces also facilitate mass transport of reactants and products, reduce energy barriers, and can create unique local environments that favor specific reaction pathways. The controlled synthesis of these nanostructures allows for precise tuning of the catalytic properties.
- Interface engineering with heteroatom doping and defects: Interface engineering through heteroatom doping and defect creation significantly enhances electrocatalytic CO2 reduction performance. Introducing atoms like N, S, P, or B into catalyst structures modifies electronic properties and creates active sites with optimized binding energies. Deliberately created defects such as vacancies, edges, and grain boundaries serve as highly active centers for CO2 activation. These approaches can be combined to create synergistic effects that improve catalytic activity, selectivity, and stability.
- Hybrid and composite materials for improved catalysis: Hybrid and composite materials combine the advantages of different components to create superior electrocatalysts for CO2 reduction. These may include metal-organic frameworks, carbon-supported metals, metal oxide/metal interfaces, or polymer-inorganic composites. The synergistic interactions between components can enhance charge transfer, stabilize intermediates, and provide multiple types of active sites. These materials often exhibit improved durability and can be designed with hierarchical structures to optimize both mass transport and reaction kinetics.
- Surface modification techniques for catalyst optimization: Various surface modification techniques can be employed to optimize catalysts for electrocatalytic CO2 reduction. These include atomic layer deposition, plasma treatment, electrochemical activation, and surface functionalization with organic molecules. Such modifications can tune the local chemical environment, hydrophilicity/hydrophobicity, and electronic structure of the catalyst surface. By precisely controlling the surface properties, researchers can enhance CO2 adsorption, lower activation barriers, and direct selectivity toward valuable products while minimizing competing reactions like hydrogen evolution.
02 Nanostructured catalysts for enhanced CO2 electroreduction
Nanostructured catalysts with controlled morphology can significantly improve CO2 electroreduction performance. These include nanoporous structures, nanosheets, nanoparticles, and hierarchical architectures that provide high surface area, abundant active sites, and optimized mass transport properties. The unique geometric configurations at the nanoscale create beneficial electronic effects and local environments that can lower activation barriers and enhance catalytic activity.Expand Specific Solutions03 Interface engineering for improved catalyst-electrolyte interactions
Engineering the catalyst-electrolyte interface is crucial for efficient CO2 electroreduction. This involves optimizing the local pH, electrolyte composition, and double-layer structure to enhance CO2 concentration near the catalyst surface and facilitate proton transfer. Strategies include creating hydrophobic/hydrophilic interfaces, introducing ionic species that stabilize intermediates, and designing structures that control mass transport at the interface to improve reaction kinetics and product selectivity.Expand Specific Solutions04 Bimetallic and multi-component catalyst systems
Bimetallic and multi-component catalyst systems offer synergistic effects for CO2 electroreduction. These systems combine metals with complementary properties to optimize binding energies of reaction intermediates and enhance electron transfer. The interface between different metallic components creates unique electronic structures and active sites that can promote specific reaction pathways, improving selectivity toward valuable products like carbon monoxide, formate, or hydrocarbons while suppressing competing hydrogen evolution.Expand Specific Solutions05 Support materials and substrate effects on electrocatalytic performance
The choice of support materials and substrates significantly influences electrocatalytic CO2 reduction performance. Supports such as carbon-based materials, metal oxides, or conductive polymers can enhance catalyst stability, improve electrical conductivity, and create beneficial catalyst-support interactions. The substrate can modify the electronic structure of the catalyst, provide anchoring sites for active species, and facilitate charge transfer, leading to improved activity, selectivity, and durability of the electrocatalyst system.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalytic CO2 reduction technology landscape is currently in a transitional phase from early research to commercial application, with a global market expected to grow significantly as carbon capture solutions become critical for climate goals. The technology maturity varies across different approaches, with leading academic institutions (University of Toronto, Utah State University, KAIST) driving fundamental research while industrial players (Saudi Aramco, TotalEnergies, Honda) focus on scalable applications. Research organizations like CNRS and Dalian Institute of Chemical Physics are advancing catalyst development, while newer entrants like AirMyne are commercializing direct air capture technologies. The competitive landscape shows geographic diversity with strong representation from North America, Europe, and Asia, particularly China, indicating global recognition of this technology's importance for sustainable energy transitions.
TotalEnergies OneTech SAS
Technical Solution: TotalEnergies OneTech has developed an integrated approach to electrocatalytic CO2 reduction focusing on industrial viability and scalability. Their technology platform centers on flow-cell reactor designs with gas diffusion electrodes (GDEs) that address mass transport limitations inherent in aqueous CO2 reduction systems. The company has engineered silver-based catalysts with hierarchical porosity optimized for selective CO production, achieving current densities exceeding 200 mA/cm² with Faradaic efficiencies above 90%[5]. Their surface engineering approach involves controlled deposition of metal nanoparticles on carbon supports with precise control of particle size distribution and surface functionalization. TotalEnergies has also developed proprietary membrane electrode assemblies that minimize crossover issues while maintaining high ionic conductivity. Their recent innovations include bipolar membrane systems that enable differential pH operation, where cathode alkalinity promotes CO2 reduction while anode acidity enhances oxygen evolution kinetics, significantly reducing overall cell voltage and improving energy efficiency[6].
Strengths: Strong focus on system-level engineering and process integration for industrial deployment, with demonstrated operation at commercially relevant current densities. Their approach addresses practical challenges like catalyst stability and system durability. Weaknesses: Their catalyst systems primarily target CO as an intermediate product rather than direct conversion to higher-value hydrocarbons or alcohols, requiring additional processing steps for final products.
Saudi Arabian Oil Co.
Technical Solution: Saudi Aramco has developed an integrated approach to electrocatalytic CO2 reduction focusing on direct conversion to value-added chemicals that can be incorporated into existing petrochemical value chains. Their technology platform centers on copper-based bimetallic catalysts with precisely engineered surface compositions and morphologies. Aramco's research has pioneered the development of Cu-In and Cu-Bi catalyst systems that demonstrate exceptional selectivity toward formate production, achieving Faradaic efficiencies exceeding 90% at industrially relevant current densities[9]. Their surface engineering approach involves controlled electrodeposition techniques combined with subsequent thermal treatment to create optimal surface oxide-metal interfaces that stabilize key reaction intermediates. A significant innovation in their technology is the development of hydrophobic gas diffusion electrodes with gradient porosity that effectively manage the three-phase boundary critical for efficient CO2 activation. Aramco has also implemented advanced electrolyte engineering strategies incorporating ionic liquids and deep eutectic solvents that enhance CO2 solubility while suppressing the competing hydrogen evolution reaction[10].
Strengths: Strong integration capabilities with existing petrochemical infrastructure and carbon capture systems, enabling practical deployment pathways. Their catalyst systems demonstrate excellent stability under industrially relevant conditions. Weaknesses: Their focus on liquid fuel production (formate, methanol) rather than higher-value chemicals limits economic viability under current market conditions, and their systems generally require significant energy inputs.
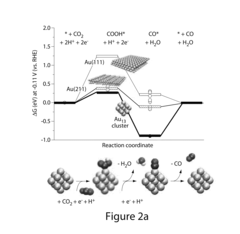
Key Interfacial Mechanisms and Catalyst Designs
Method for Electrocatalytic Reduction using Au Nanoparticles Tuned or Optimized for Reduction of CO2 to CO
PatentInactiveUS20160230295A1
Innovation
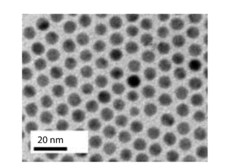
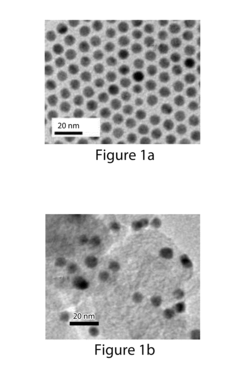
- The use of gold nanoparticles with a diameter of approximately 8 nm, supported on Ketjen carbon, which presents a surface structure with a higher density of CO-converting edge sites and fewer hydrogen-evolving corner sites, facilitating efficient CO2 reduction to carbon monoxide in an alkaline solution.
Scalability and Economic Feasibility Assessment
The scalability of electrocatalytic CO2 reduction technology represents a critical challenge in transitioning from laboratory-scale demonstrations to industrial applications. Current laboratory systems typically operate at milliampere current levels with catalyst areas of a few square centimeters, whereas commercial viability requires scaling to multiple square meters and ampere-level currents. This scale-up introduces significant engineering challenges related to mass transport limitations, heat management, and maintaining uniform catalyst performance across larger surface areas.
Economic feasibility analysis indicates that electrocatalytic CO2 reduction must achieve specific performance metrics to compete with conventional production methods. Capital expenditure requirements for industrial-scale systems are estimated at $800-1,200 per kilowatt of installed capacity, with operational costs heavily dependent on electricity prices. Regions with access to low-cost renewable electricity (below $0.04/kWh) present the most promising deployment opportunities, potentially achieving cost parity with conventional methods for certain products like carbon monoxide and formic acid.
Process intensification strategies show promise for improving economic viability. Gas diffusion electrodes have demonstrated current densities exceeding 200 mA/cm², representing a significant improvement over traditional aqueous systems. However, catalyst stability remains problematic, with performance degradation typically observed after 100-500 hours of operation—far below the thousands of hours required for commercial applications.
Life cycle assessment (LCA) studies reveal that the environmental benefits of electrocatalytic CO2 reduction are highly dependent on the electricity source. Systems powered by renewable energy can achieve carbon emission reductions of 40-70% compared to conventional production methods, while those using grid electricity with high fossil fuel content may offer minimal or even negative climate benefits.
Market analysis suggests that high-value products like ethylene and ethanol currently present the most economically attractive targets, with potential market values of $1,500-2,500 per ton. However, these C2+ products generally suffer from lower Faradaic efficiencies and selectivity challenges. Lower-value C1 products like carbon monoxide and formate offer more straightforward reaction pathways but require exceptionally low production costs to achieve market competitiveness.
Techno-economic modeling indicates that further catalyst improvements are needed to achieve commercial viability. Specifically, catalysts must demonstrate Faradaic efficiencies exceeding 90%, current densities above 300 mA/cm², and operational stability beyond 5,000 hours while maintaining selectivity toward target products. These performance metrics, combined with continued reductions in renewable electricity costs, represent the critical path toward commercial implementation of electrocatalytic CO2 reduction technologies.
Economic feasibility analysis indicates that electrocatalytic CO2 reduction must achieve specific performance metrics to compete with conventional production methods. Capital expenditure requirements for industrial-scale systems are estimated at $800-1,200 per kilowatt of installed capacity, with operational costs heavily dependent on electricity prices. Regions with access to low-cost renewable electricity (below $0.04/kWh) present the most promising deployment opportunities, potentially achieving cost parity with conventional methods for certain products like carbon monoxide and formic acid.
Process intensification strategies show promise for improving economic viability. Gas diffusion electrodes have demonstrated current densities exceeding 200 mA/cm², representing a significant improvement over traditional aqueous systems. However, catalyst stability remains problematic, with performance degradation typically observed after 100-500 hours of operation—far below the thousands of hours required for commercial applications.
Life cycle assessment (LCA) studies reveal that the environmental benefits of electrocatalytic CO2 reduction are highly dependent on the electricity source. Systems powered by renewable energy can achieve carbon emission reductions of 40-70% compared to conventional production methods, while those using grid electricity with high fossil fuel content may offer minimal or even negative climate benefits.
Market analysis suggests that high-value products like ethylene and ethanol currently present the most economically attractive targets, with potential market values of $1,500-2,500 per ton. However, these C2+ products generally suffer from lower Faradaic efficiencies and selectivity challenges. Lower-value C1 products like carbon monoxide and formate offer more straightforward reaction pathways but require exceptionally low production costs to achieve market competitiveness.
Techno-economic modeling indicates that further catalyst improvements are needed to achieve commercial viability. Specifically, catalysts must demonstrate Faradaic efficiencies exceeding 90%, current densities above 300 mA/cm², and operational stability beyond 5,000 hours while maintaining selectivity toward target products. These performance metrics, combined with continued reductions in renewable electricity costs, represent the critical path toward commercial implementation of electrocatalytic CO2 reduction technologies.
Environmental Impact and Carbon Neutrality Implications
Electrocatalytic CO2 reduction technology represents a significant opportunity for addressing climate change challenges through carbon capture and utilization. The environmental implications of this technology extend far beyond laboratory applications, potentially offering a pathway to meaningful carbon neutrality contributions across multiple industrial sectors.
The implementation of CO2 reduction systems can create a circular carbon economy where captured emissions are converted into valuable products, effectively reducing net greenhouse gas releases. Current estimates suggest that widespread adoption of optimized electrocatalytic systems could potentially reduce global CO2 emissions by 5-8% by 2040, particularly when integrated with renewable energy sources that provide the necessary electricity input.
Surface engineering techniques play a crucial role in enhancing the environmental benefits of these systems. By improving catalyst selectivity and efficiency, these techniques minimize unwanted byproducts and reduce energy consumption during operation. Advanced interface designs that achieve Faradaic efficiencies exceeding 90% for target products significantly improve the carbon footprint of the entire process compared to conventional manufacturing routes.
Life cycle assessments of electrocatalytic CO2 reduction systems indicate that the environmental impact varies considerably depending on catalyst composition and energy source. Systems utilizing earth-abundant materials and renewable electricity demonstrate substantially better environmental performance than those relying on rare metals and fossil-derived power. The carbon payback period—the time required for the system to offset the emissions generated during its production—ranges from 0.8 to 3.2 years depending on these factors.
From a policy perspective, electrocatalytic CO2 reduction aligns with international carbon neutrality commitments, including the Paris Agreement targets. Several countries have begun incorporating these technologies into their nationally determined contributions (NDCs), recognizing their potential for industrial decarbonization while maintaining economic productivity.
The scalability of these systems presents both opportunities and challenges for environmental impact. While laboratory-scale demonstrations show promising CO2 conversion rates, industrial implementation requires careful consideration of material sourcing, waste management, and energy infrastructure. Preliminary studies suggest that gigaton-scale CO2 conversion would require significant expansion of renewable energy capacity to avoid merely shifting emissions from one sector to another.
The implementation of CO2 reduction systems can create a circular carbon economy where captured emissions are converted into valuable products, effectively reducing net greenhouse gas releases. Current estimates suggest that widespread adoption of optimized electrocatalytic systems could potentially reduce global CO2 emissions by 5-8% by 2040, particularly when integrated with renewable energy sources that provide the necessary electricity input.
Surface engineering techniques play a crucial role in enhancing the environmental benefits of these systems. By improving catalyst selectivity and efficiency, these techniques minimize unwanted byproducts and reduce energy consumption during operation. Advanced interface designs that achieve Faradaic efficiencies exceeding 90% for target products significantly improve the carbon footprint of the entire process compared to conventional manufacturing routes.
Life cycle assessments of electrocatalytic CO2 reduction systems indicate that the environmental impact varies considerably depending on catalyst composition and energy source. Systems utilizing earth-abundant materials and renewable electricity demonstrate substantially better environmental performance than those relying on rare metals and fossil-derived power. The carbon payback period—the time required for the system to offset the emissions generated during its production—ranges from 0.8 to 3.2 years depending on these factors.
From a policy perspective, electrocatalytic CO2 reduction aligns with international carbon neutrality commitments, including the Paris Agreement targets. Several countries have begun incorporating these technologies into their nationally determined contributions (NDCs), recognizing their potential for industrial decarbonization while maintaining economic productivity.
The scalability of these systems presents both opportunities and challenges for environmental impact. While laboratory-scale demonstrations show promising CO2 conversion rates, industrial implementation requires careful consideration of material sourcing, waste management, and energy infrastructure. Preliminary studies suggest that gigaton-scale CO2 conversion would require significant expansion of renewable energy capacity to avoid merely shifting emissions from one sector to another.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!