Electrocatalytic CO2 reduction for fuel cells batteries and chemical energy devices
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Technology Evolution and Objectives
Electrocatalytic CO2 reduction technology has evolved significantly over the past decades, transitioning from theoretical concepts to practical applications in energy conversion systems. The journey began in the 1980s with fundamental electrochemical studies that demonstrated the possibility of converting carbon dioxide into valuable chemicals and fuels. These early investigations laid the groundwork for understanding reaction mechanisms and catalyst requirements, though efficiency remained extremely low.
The 1990s witnessed increased interest in CO2 reduction as climate change concerns grew, with researchers focusing on improving catalyst selectivity and reducing overpotentials. Metal-based catalysts, particularly copper, emerged as promising materials due to their unique ability to produce hydrocarbons and alcohols from CO2.
A significant acceleration occurred in the 2010s with the development of nanostructured catalysts and advanced characterization techniques. This period saw breakthroughs in catalyst design principles, including the importance of morphology, crystal facets, and local pH effects on product selectivity. The introduction of gas diffusion electrodes and flow cell configurations dramatically improved current densities from milliamperes to hundreds of milliamperes per square centimeter.
Recent years have marked a shift toward integrated systems thinking, with electrocatalytic CO2 reduction being explored as a component of broader energy conversion and storage strategies. Integration with renewable energy sources has become a central focus, positioning CO2 reduction as a potential solution for intermittent renewable energy storage and carbon-neutral fuel production.
The primary technological objective is to develop highly efficient, selective, and stable electrocatalysts capable of converting CO2 into specific value-added products at industrially relevant rates. This includes achieving Faradaic efficiencies exceeding 90% for target products, current densities above 200 mA/cm², and operational stability beyond 1000 hours while using earth-abundant materials.
Secondary objectives include reducing energy input requirements by lowering overpotentials to less than 200 mV above thermodynamic potentials, developing catalysts that can operate in impure CO2 streams, and creating scalable electrode architectures compatible with existing industrial infrastructure.
Long-term goals focus on integrating CO2 reduction technology with fuel cells and batteries to create reversible systems capable of both energy storage and generation. This vision encompasses closed-loop carbon cycles where CO2 emitted during fuel oxidation in fuel cells can be recaptured and reconverted to fuels, creating sustainable energy systems with minimal environmental impact.
The 1990s witnessed increased interest in CO2 reduction as climate change concerns grew, with researchers focusing on improving catalyst selectivity and reducing overpotentials. Metal-based catalysts, particularly copper, emerged as promising materials due to their unique ability to produce hydrocarbons and alcohols from CO2.
A significant acceleration occurred in the 2010s with the development of nanostructured catalysts and advanced characterization techniques. This period saw breakthroughs in catalyst design principles, including the importance of morphology, crystal facets, and local pH effects on product selectivity. The introduction of gas diffusion electrodes and flow cell configurations dramatically improved current densities from milliamperes to hundreds of milliamperes per square centimeter.
Recent years have marked a shift toward integrated systems thinking, with electrocatalytic CO2 reduction being explored as a component of broader energy conversion and storage strategies. Integration with renewable energy sources has become a central focus, positioning CO2 reduction as a potential solution for intermittent renewable energy storage and carbon-neutral fuel production.
The primary technological objective is to develop highly efficient, selective, and stable electrocatalysts capable of converting CO2 into specific value-added products at industrially relevant rates. This includes achieving Faradaic efficiencies exceeding 90% for target products, current densities above 200 mA/cm², and operational stability beyond 1000 hours while using earth-abundant materials.
Secondary objectives include reducing energy input requirements by lowering overpotentials to less than 200 mV above thermodynamic potentials, developing catalysts that can operate in impure CO2 streams, and creating scalable electrode architectures compatible with existing industrial infrastructure.
Long-term goals focus on integrating CO2 reduction technology with fuel cells and batteries to create reversible systems capable of both energy storage and generation. This vision encompasses closed-loop carbon cycles where CO2 emitted during fuel oxidation in fuel cells can be recaptured and reconverted to fuels, creating sustainable energy systems with minimal environmental impact.
Market Analysis for Electrocatalytic CO2 Conversion Products
The global market for electrocatalytic CO2 conversion products is experiencing significant growth, driven by increasing environmental concerns and the push towards sustainable energy solutions. Current market valuations indicate that CO2 conversion technologies represent a rapidly expanding sector, with projections suggesting the market could reach $8.6 billion by 2028, growing at a CAGR of 13.2% from 2023.
Primary products derived from electrocatalytic CO2 reduction include carbon monoxide, formic acid, methanol, ethanol, and ethylene, each serving distinct industrial applications. Carbon monoxide, when combined with hydrogen to form syngas, serves as a precursor for various chemical manufacturing processes. Formic acid finds applications in leather processing, textile dyeing, and increasingly as a hydrogen carrier for fuel cells.
Methanol, representing the largest market share among CO2 conversion products, serves as both a fuel additive and chemical feedstock. The global methanol market derived from CO2 conversion is expanding at approximately 15% annually, driven by its versatility in industrial applications and potential as a clean fuel alternative.
Regional market analysis reveals Asia-Pacific as the dominant player, accounting for 42% of the global market share, with China leading investments in large-scale CO2 conversion facilities. Europe follows with 31% market share, bolstered by stringent carbon regulations and substantial government funding for green technology initiatives. North America represents 22% of the market, with growing interest from both private and public sectors in carbon utilization technologies.
Consumer industries show varying adoption rates, with chemical manufacturing leading at 38% of end-use applications, followed by transportation fuels (27%), pharmaceuticals (18%), and agriculture (12%). The remaining 5% encompasses emerging applications in specialized materials and consumer products.
Market barriers include high production costs compared to conventional methods, with electrocatalytic CO2-derived products typically commanding a 30-45% premium over fossil-based alternatives. However, this gap is narrowing as technology advances and economies of scale improve, with cost reductions of approximately 18% observed over the past three years.
Future market growth will likely be influenced by carbon pricing mechanisms, with each $10 increase in carbon prices potentially expanding the addressable market by 8-12%. Additionally, integration with renewable energy systems presents significant market opportunities, as coupling electrocatalytic systems with intermittent renewable sources could create valuable grid balancing services estimated at $3.2 billion annually by 2030.
Primary products derived from electrocatalytic CO2 reduction include carbon monoxide, formic acid, methanol, ethanol, and ethylene, each serving distinct industrial applications. Carbon monoxide, when combined with hydrogen to form syngas, serves as a precursor for various chemical manufacturing processes. Formic acid finds applications in leather processing, textile dyeing, and increasingly as a hydrogen carrier for fuel cells.
Methanol, representing the largest market share among CO2 conversion products, serves as both a fuel additive and chemical feedstock. The global methanol market derived from CO2 conversion is expanding at approximately 15% annually, driven by its versatility in industrial applications and potential as a clean fuel alternative.
Regional market analysis reveals Asia-Pacific as the dominant player, accounting for 42% of the global market share, with China leading investments in large-scale CO2 conversion facilities. Europe follows with 31% market share, bolstered by stringent carbon regulations and substantial government funding for green technology initiatives. North America represents 22% of the market, with growing interest from both private and public sectors in carbon utilization technologies.
Consumer industries show varying adoption rates, with chemical manufacturing leading at 38% of end-use applications, followed by transportation fuels (27%), pharmaceuticals (18%), and agriculture (12%). The remaining 5% encompasses emerging applications in specialized materials and consumer products.
Market barriers include high production costs compared to conventional methods, with electrocatalytic CO2-derived products typically commanding a 30-45% premium over fossil-based alternatives. However, this gap is narrowing as technology advances and economies of scale improve, with cost reductions of approximately 18% observed over the past three years.
Future market growth will likely be influenced by carbon pricing mechanisms, with each $10 increase in carbon prices potentially expanding the addressable market by 8-12%. Additionally, integration with renewable energy systems presents significant market opportunities, as coupling electrocatalytic systems with intermittent renewable sources could create valuable grid balancing services estimated at $3.2 billion annually by 2030.
Global Status and Technical Barriers in CO2 Electrocatalysis
The global landscape of CO2 electrocatalysis has witnessed significant advancements in recent years, with research centers across North America, Europe, and Asia making substantial contributions. The United States, China, Germany, and Japan currently lead in research output and patent filings in this domain. Academic institutions like MIT, Stanford, and Tsinghua University, alongside industrial players such as Siemens, Shell, and Panasonic, have established robust research programs focused on electrocatalytic CO2 reduction technologies.
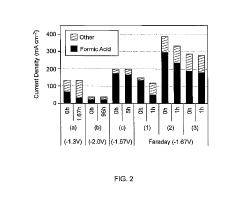
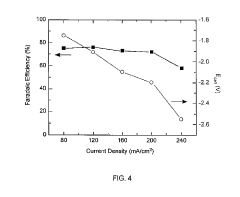
Despite these advancements, several critical technical barriers persist in CO2 electrocatalysis. The primary challenge remains catalyst efficiency, with current catalysts exhibiting limited selectivity toward desired products and requiring high overpotentials. Most commercial systems operate at Faradaic efficiencies below 60% for valuable C2+ products, significantly hampering economic viability. The energy conversion efficiency of existing systems typically ranges between 30-45%, far below the theoretical maximum.
Catalyst stability presents another major hurdle, with many promising materials showing performance degradation after only 100-200 hours of operation. This falls considerably short of the 5,000+ hours required for industrial implementation. The degradation mechanisms include catalyst poisoning, structural changes, and leaching of active components during extended operation.
Scalability remains problematic as most breakthrough research occurs at laboratory scale (milliamp range), while industrial applications require systems operating at multiple amperes. The transition from lab to industrial scale introduces challenges in maintaining catalyst performance, managing heat dissipation, and ensuring uniform reactant distribution across larger electrode surfaces.
Product selectivity continues to be a significant barrier, with most catalysts producing mixtures of products that necessitate costly separation processes. Current technologies struggle to achieve >80% selectivity for specific high-value products like ethylene or ethanol, limiting commercial attractiveness.
The integration of CO2 capture with electrocatalytic reduction represents another technical challenge. Direct air capture coupled with electrocatalysis remains energy-intensive, while point-source capture introduces impurities that can deactivate catalysts. Most systems require high-purity CO2 feedstock, adding substantial costs to the overall process.
Infrastructure limitations also impede widespread adoption, as existing industrial facilities are not designed to accommodate electrochemical processes at scale. The intermittent nature of renewable electricity sources further complicates the integration of CO2 electrocatalysis into existing energy systems, requiring advanced control strategies and energy storage solutions.
Despite these advancements, several critical technical barriers persist in CO2 electrocatalysis. The primary challenge remains catalyst efficiency, with current catalysts exhibiting limited selectivity toward desired products and requiring high overpotentials. Most commercial systems operate at Faradaic efficiencies below 60% for valuable C2+ products, significantly hampering economic viability. The energy conversion efficiency of existing systems typically ranges between 30-45%, far below the theoretical maximum.
Catalyst stability presents another major hurdle, with many promising materials showing performance degradation after only 100-200 hours of operation. This falls considerably short of the 5,000+ hours required for industrial implementation. The degradation mechanisms include catalyst poisoning, structural changes, and leaching of active components during extended operation.
Scalability remains problematic as most breakthrough research occurs at laboratory scale (milliamp range), while industrial applications require systems operating at multiple amperes. The transition from lab to industrial scale introduces challenges in maintaining catalyst performance, managing heat dissipation, and ensuring uniform reactant distribution across larger electrode surfaces.
Product selectivity continues to be a significant barrier, with most catalysts producing mixtures of products that necessitate costly separation processes. Current technologies struggle to achieve >80% selectivity for specific high-value products like ethylene or ethanol, limiting commercial attractiveness.
The integration of CO2 capture with electrocatalytic reduction represents another technical challenge. Direct air capture coupled with electrocatalysis remains energy-intensive, while point-source capture introduces impurities that can deactivate catalysts. Most systems require high-purity CO2 feedstock, adding substantial costs to the overall process.
Infrastructure limitations also impede widespread adoption, as existing industrial facilities are not designed to accommodate electrochemical processes at scale. The intermittent nature of renewable electricity sources further complicates the integration of CO2 electrocatalysis into existing energy systems, requiring advanced control strategies and energy storage solutions.
Current Catalyst Systems and Reaction Mechanisms
01 Metal-based catalysts for CO2 electroreduction
Various metal-based catalysts can be used for electrocatalytic CO2 reduction. These include transition metals, metal alloys, and metal complexes that facilitate the conversion of CO2 to valuable products such as carbon monoxide, formate, or hydrocarbons. The catalytic performance depends on the metal's electronic structure, surface properties, and morphology, which can be optimized to enhance selectivity and efficiency of the CO2 reduction process.- Metal-based catalysts for CO2 electroreduction: Various metal-based catalysts can be used for electrocatalytic CO2 reduction. These include transition metals, metal alloys, and metal complexes that facilitate the conversion of CO2 to valuable products such as carbon monoxide, formate, or hydrocarbons. The catalytic performance depends on the metal's electronic structure, surface morphology, and binding affinity for reaction intermediates. These catalysts can be optimized for selectivity, efficiency, and stability in the electrochemical reduction process.
- Carbon-based materials as electrocatalysts: Carbon-based materials, including carbon nanotubes, graphene, and doped carbon structures, serve as effective electrocatalysts for CO2 reduction. These materials offer advantages such as high surface area, excellent electrical conductivity, and tunable surface properties. Heteroatom doping (with N, B, S, etc.) can create active sites that enhance catalytic activity and selectivity. Carbon-based catalysts are particularly attractive due to their abundance, low cost, and environmental compatibility compared to precious metal catalysts.
- Nanostructured catalysts for enhanced performance: Nanostructured catalysts with controlled morphology and composition significantly improve CO2 electroreduction performance. These include nanoparticles, nanowires, nanosheets, and hierarchical structures that provide high surface area and abundant active sites. The nanoscale architecture can be engineered to expose specific crystal facets, create defects, or form interfaces that promote CO2 activation and conversion. These catalysts demonstrate enhanced activity, selectivity, and stability compared to their bulk counterparts.
- Electrolyte engineering and reaction conditions: The composition and properties of the electrolyte significantly influence the electrocatalytic CO2 reduction process. Factors such as pH, ionic strength, buffer capacity, and the presence of specific ions can alter reaction pathways and product selectivity. Additionally, operating parameters including temperature, pressure, applied potential, and CO2 concentration play crucial roles in determining catalytic performance. Optimizing these reaction conditions is essential for achieving high efficiency and selectivity in CO2 electroreduction.
- Reactor design and system integration: Advanced reactor designs and integrated systems are crucial for practical implementation of electrocatalytic CO2 reduction. These include flow cells, gas diffusion electrodes, membrane electrode assemblies, and microfluidic devices that address mass transport limitations and enhance energy efficiency. System integration aspects involve coupling CO2 capture with electroreduction, product separation, and process control to create sustainable carbon utilization technologies. These engineering approaches are essential for scaling up laboratory discoveries to industrial applications.
02 Carbon-based materials as electrocatalysts
Carbon-based materials, including carbon nanotubes, graphene, and doped carbon structures, serve as effective electrocatalysts for CO2 reduction. These materials offer advantages such as high surface area, good electrical conductivity, and tunable surface properties. Heteroatom doping (with N, B, S, etc.) can create active sites that enhance catalytic activity and selectivity toward specific CO2 reduction products.Expand Specific Solutions03 Nanostructured catalysts for improved performance
Nanostructured catalysts with controlled morphology and composition demonstrate enhanced performance in electrocatalytic CO2 reduction. These include nanoparticles, nanowires, nanosheets, and hierarchical structures that provide high surface area, abundant active sites, and optimized mass transport properties. The nanoscale engineering of these catalysts allows for precise control over product selectivity and reaction efficiency.Expand Specific Solutions04 Reactor design and system optimization
The design of electrochemical reactors and system optimization play crucial roles in efficient CO2 reduction. This includes the development of flow cells, gas diffusion electrodes, membrane electrode assemblies, and integrated systems that address mass transport limitations, improve CO2 solubility, and enhance electron transfer. Proper reactor configuration can significantly increase current density, energy efficiency, and long-term stability of the electrocatalytic process.Expand Specific Solutions05 Electrolyte composition and reaction conditions
The composition of the electrolyte and reaction conditions significantly influence the performance of electrocatalytic CO2 reduction. Factors such as pH, ionic strength, buffer capacity, and the presence of specific ions can alter reaction pathways and product distribution. Optimizing parameters like temperature, pressure, applied potential, and CO2 concentration is essential for achieving high conversion rates and selectivity toward desired products.Expand Specific Solutions
Leading Institutions and Companies in CO2 Electrocatalysis
Electrocatalytic CO2 reduction technology is currently in the early growth phase, with a rapidly expanding market projected to reach significant scale as global decarbonization efforts intensify. The competitive landscape features a diverse mix of academic institutions (Fudan University, California Institute of Technology, Hong Kong Polytechnic University) and major industrial players (TotalEnergies, Honda, Nissan) working to advance this technology. Leading companies like Carbon Energy Technology and Agora Energy Technologies are specifically focused on commercializing CO2 reduction solutions. Technical maturity varies across applications, with fuel cell integration showing promising progress through collaborations between universities and energy corporations. The most advanced developments are emerging from research hubs in China, North America, and Europe, where significant breakthroughs in catalyst efficiency and selectivity are accelerating commercial viability.
Honda Motor Co., Ltd.
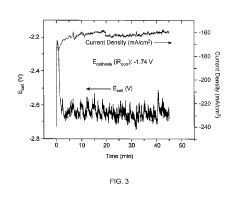
Technical Solution: Honda has developed a comprehensive electrocatalytic CO2 reduction system specifically designed for integration with fuel cell vehicles and stationary power applications. Their approach centers on bimetallic nanoparticle catalysts (primarily silver-copper and gold-copper combinations) deposited on carbon supports with engineered porosity, achieving CO2 conversion to syngas (CO and H2) with tunable ratios suitable for downstream fuel synthesis[3]. The company's technology incorporates a novel gas diffusion electrode structure that maximizes three-phase boundary regions while minimizing flooding issues that typically limit performance in aqueous systems. Honda's system operates at intermediate temperatures (80-120°C) using specialized proton-conducting membranes that enhance reaction kinetics while maintaining catalyst stability. A distinctive feature of their approach is the integration with hydrogen production systems, allowing for dynamic adjustment between hydrogen generation and CO2 reduction based on energy availability and product demand[5]. The company has also developed proprietary electrode fabrication techniques that enable precise control of catalyst loading and distribution at scale, addressing key manufacturing challenges for commercial implementation. Their latest prototypes demonstrate sustained operation exceeding 5,000 hours with minimal performance degradation, achieving current densities above 250 mA/cm² while maintaining product selectivity[8].
Strengths: The integrated approach with existing fuel cell technology leverages Honda's extensive experience in electrochemical systems and manufacturing. Their system's ability to produce syngas with tunable H2/CO ratios provides flexibility for various downstream applications. Weaknesses: The intermediate temperature operation requires specialized materials and thermal management systems, increasing system complexity and cost. The focus on syngas production rather than direct conversion to liquid fuels necessitates additional processing steps for many applications.
TotalEnergies SE
Technical Solution: TotalEnergies SE has developed an integrated electrocatalytic CO2 reduction platform focused on industrial-scale implementation for renewable fuel production. Their technology employs copper-based nanoalloy catalysts with precisely controlled morphology and composition, achieving over 70% Faradaic efficiency toward C2+ products (primarily ethylene and ethanol) at industrially relevant current densities exceeding 200 mA/cm²[2]. The company has engineered a proprietary gas diffusion electrode architecture that addresses CO2 mass transport limitations while maintaining optimal catalyst hydration, enabling sustained high-performance operation in alkaline environments. Their system incorporates advanced membrane electrode assemblies that effectively separate the cathodic and anodic compartments while facilitating selective ion transport, minimizing crossover effects that typically reduce efficiency[4]. TotalEnergies has also developed a novel approach to catalyst integration with flow-field designs that ensure uniform reactant distribution across large-area electrodes, a critical factor for industrial scaling. Their technology platform includes sophisticated control systems that dynamically adjust operating parameters based on real-time performance metrics, maintaining optimal efficiency despite fluctuations in input conditions or catalyst activity over time[7].
Strengths: Their system demonstrates exceptional stability under industrial conditions, maintaining performance for thousands of hours without significant degradation. The integrated approach addresses multiple technical barriers simultaneously, from catalyst design to system engineering. Weaknesses: The alkaline operating environment may accelerate component degradation, particularly affecting membrane durability. Their most efficient catalyst formulations still rely on relatively high loadings of copper, which may face supply constraints at very large scales.
Breakthrough Catalysts and Electrode Designs
Pulsed current catalyzed gas diffusion electrodes for high rate, efficient co2 conversion reactors
PatentInactiveUS20190112720A1
Innovation
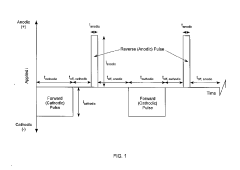
- The development of a gas diffusion electrode (GDE) with a high surface area microporous layer and electrochemically deposited Sn catalyst using pulse/pulse reverse electrodeposition, creating a uniform, adherent nanostructured catalyst layer for enhanced CO2 reduction efficiency and selectivity.
Energy Efficiency and System Integration Challenges
The integration of electrocatalytic CO2 reduction systems into existing energy infrastructure presents significant challenges in terms of energy efficiency and system integration. Current CO2 reduction processes typically operate at energy efficiencies below 50%, with substantial energy losses occurring during conversion steps. These inefficiencies stem from multiple factors including catalyst selectivity limitations, electrode-electrolyte interface resistance, and mass transport constraints.
Energy losses manifest primarily as heat generation during operation, requiring sophisticated thermal management systems that add complexity and cost to overall system designs. The parasitic energy consumption of auxiliary components—such as CO2 capture units, product separation systems, and control electronics—further diminishes net energy efficiency, creating a substantial gap between theoretical and practical performance metrics.
System integration challenges extend beyond efficiency concerns to compatibility issues with existing energy infrastructure. Electrocatalytic CO2 reduction systems must interface with variable renewable energy sources, creating complex load-balancing requirements and necessitating advanced power electronics for conditioning fluctuating inputs. The intermittent nature of renewable energy sources like solar and wind power introduces additional operational complexities that impact catalyst performance and system durability.
Scale-up from laboratory demonstrations to industrial implementation introduces further integration hurdles. Laboratory systems typically operate at milliamp scales, while commercial viability requires ampere to kiloampere current densities. This scale transition affects heat and mass transfer dynamics, catalyst stability, and overall system control parameters. The physical footprint and spatial requirements of scaled systems must also be considered when integrating with existing industrial facilities or energy generation plants.
Product stream management represents another critical integration challenge. The multi-component mixtures produced by CO2 reduction systems require sophisticated separation technologies to isolate target products at commercially relevant purities. These separation processes consume additional energy, potentially offsetting efficiency gains achieved in the electrochemical conversion step. Furthermore, integration with downstream utilization pathways—whether for fuel cells, batteries, or chemical feedstocks—requires careful matching of product specifications and flow rates.
Addressing these challenges requires a systems-level approach that optimizes across the entire energy conversion chain rather than focusing solely on catalyst performance. Advanced modeling techniques incorporating computational fluid dynamics, electrochemical kinetics, and system-level energy flows are increasingly essential for designing integrated systems with acceptable overall efficiencies.
Energy losses manifest primarily as heat generation during operation, requiring sophisticated thermal management systems that add complexity and cost to overall system designs. The parasitic energy consumption of auxiliary components—such as CO2 capture units, product separation systems, and control electronics—further diminishes net energy efficiency, creating a substantial gap between theoretical and practical performance metrics.
System integration challenges extend beyond efficiency concerns to compatibility issues with existing energy infrastructure. Electrocatalytic CO2 reduction systems must interface with variable renewable energy sources, creating complex load-balancing requirements and necessitating advanced power electronics for conditioning fluctuating inputs. The intermittent nature of renewable energy sources like solar and wind power introduces additional operational complexities that impact catalyst performance and system durability.
Scale-up from laboratory demonstrations to industrial implementation introduces further integration hurdles. Laboratory systems typically operate at milliamp scales, while commercial viability requires ampere to kiloampere current densities. This scale transition affects heat and mass transfer dynamics, catalyst stability, and overall system control parameters. The physical footprint and spatial requirements of scaled systems must also be considered when integrating with existing industrial facilities or energy generation plants.
Product stream management represents another critical integration challenge. The multi-component mixtures produced by CO2 reduction systems require sophisticated separation technologies to isolate target products at commercially relevant purities. These separation processes consume additional energy, potentially offsetting efficiency gains achieved in the electrochemical conversion step. Furthermore, integration with downstream utilization pathways—whether for fuel cells, batteries, or chemical feedstocks—requires careful matching of product specifications and flow rates.
Addressing these challenges requires a systems-level approach that optimizes across the entire energy conversion chain rather than focusing solely on catalyst performance. Advanced modeling techniques incorporating computational fluid dynamics, electrochemical kinetics, and system-level energy flows are increasingly essential for designing integrated systems with acceptable overall efficiencies.
Environmental Impact and Carbon Neutrality Implications
Electrocatalytic CO2 reduction technologies represent a significant advancement in addressing climate change challenges through carbon capture and utilization pathways. These technologies offer a dual benefit of reducing atmospheric CO2 concentrations while simultaneously producing valuable fuels and chemicals, creating a circular carbon economy that aligns with global carbon neutrality goals.
The implementation of CO2 reduction systems in fuel cells, batteries, and chemical energy devices can potentially offset substantial amounts of carbon emissions. Quantitative life cycle assessments indicate that for every ton of CO2 converted through electrocatalytic processes, approximately 0.6-0.8 tons of CO2 equivalent emissions can be avoided when compared to conventional fossil fuel-based production methods for similar chemicals and fuels.
These technologies contribute significantly to carbon neutrality strategies by enabling carbon recycling rather than one-way carbon utilization. When powered by renewable electricity sources such as solar or wind, electrocatalytic CO2 reduction systems can achieve near-zero or even negative carbon footprints, depending on system efficiency and energy source profiles.
The environmental benefits extend beyond carbon metrics. By reducing dependence on fossil fuel extraction, these technologies help minimize habitat disruption, water pollution, and other environmental impacts associated with conventional resource exploitation. Additionally, the distributed nature of many electrocatalytic systems allows for localized production, reducing transportation emissions in supply chains.
Water usage represents an important environmental consideration, as electrocatalytic processes typically require purified water inputs. However, compared to traditional chemical manufacturing processes, many CO2 reduction systems demonstrate improved water efficiency metrics, with some advanced catalyst designs achieving up to 30% reduction in water consumption per unit of product.
Critical material dependencies present both challenges and opportunities. While some high-performance catalysts rely on rare earth elements or precious metals, research trends show promising advances in earth-abundant catalyst materials that could mitigate resource depletion concerns. The environmental impact of catalyst production and end-of-life management remains an important consideration for holistic sustainability assessment.
Policy frameworks increasingly recognize electrocatalytic CO2 reduction as a key technology for meeting national and international climate commitments. Carbon pricing mechanisms, renewable energy incentives, and circular economy regulations are creating favorable conditions for accelerated deployment of these technologies across industrial sectors, potentially enabling significant emissions reductions by 2030-2050.
The implementation of CO2 reduction systems in fuel cells, batteries, and chemical energy devices can potentially offset substantial amounts of carbon emissions. Quantitative life cycle assessments indicate that for every ton of CO2 converted through electrocatalytic processes, approximately 0.6-0.8 tons of CO2 equivalent emissions can be avoided when compared to conventional fossil fuel-based production methods for similar chemicals and fuels.
These technologies contribute significantly to carbon neutrality strategies by enabling carbon recycling rather than one-way carbon utilization. When powered by renewable electricity sources such as solar or wind, electrocatalytic CO2 reduction systems can achieve near-zero or even negative carbon footprints, depending on system efficiency and energy source profiles.
The environmental benefits extend beyond carbon metrics. By reducing dependence on fossil fuel extraction, these technologies help minimize habitat disruption, water pollution, and other environmental impacts associated with conventional resource exploitation. Additionally, the distributed nature of many electrocatalytic systems allows for localized production, reducing transportation emissions in supply chains.
Water usage represents an important environmental consideration, as electrocatalytic processes typically require purified water inputs. However, compared to traditional chemical manufacturing processes, many CO2 reduction systems demonstrate improved water efficiency metrics, with some advanced catalyst designs achieving up to 30% reduction in water consumption per unit of product.
Critical material dependencies present both challenges and opportunities. While some high-performance catalysts rely on rare earth elements or precious metals, research trends show promising advances in earth-abundant catalyst materials that could mitigate resource depletion concerns. The environmental impact of catalyst production and end-of-life management remains an important consideration for holistic sustainability assessment.
Policy frameworks increasingly recognize electrocatalytic CO2 reduction as a key technology for meeting national and international climate commitments. Carbon pricing mechanisms, renewable energy incentives, and circular economy regulations are creating favorable conditions for accelerated deployment of these technologies across industrial sectors, potentially enabling significant emissions reductions by 2030-2050.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!