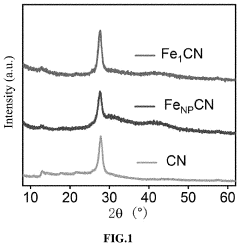
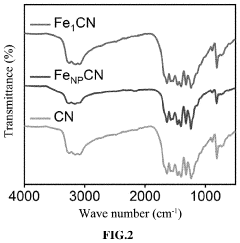
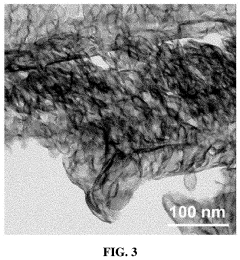
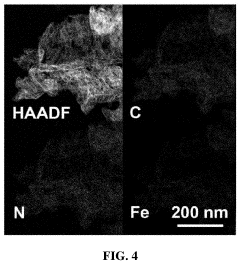
Analysis of Single-Atom Catalysis in Pharmaceutical Synthesis
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a frontier in heterogeneous catalysis that has emerged over the past decade as a transformative approach to chemical synthesis. This innovative field bridges the gap between homogeneous and heterogeneous catalysis by anchoring isolated metal atoms onto suitable supports, creating highly efficient catalytic sites with maximized atom utilization. The evolution of SAC technology can be traced back to early theoretical predictions in the 1990s, with experimental confirmation emerging around 2011 when Zhang and colleagues demonstrated the first practical single-atom catalyst using Pt atoms dispersed on FeOx.
The pharmaceutical industry has traditionally relied on precious metal catalysts in complex synthetic pathways, often requiring high catalyst loadings and generating significant waste. Single-atom catalysis offers a revolutionary alternative by providing unprecedented atomic efficiency while maintaining or even enhancing catalytic performance. This represents a paradigm shift in pharmaceutical synthesis, where reaction selectivity and environmental impact are paramount concerns.
Current technological trajectories indicate rapid advancement in SAC design principles, characterization techniques, and industrial applications. The field has progressed from fundamental proof-of-concept studies to increasingly sophisticated catalyst architectures that can perform complex transformations relevant to pharmaceutical synthesis, including hydrogenation, oxidation, and C-C coupling reactions under mild conditions.
The primary objectives of single-atom catalysis research in pharmaceutical applications include developing stable catalysts that maintain single-atom dispersion under reaction conditions, enhancing selectivity for complex molecular transformations, and establishing scalable manufacturing processes. Additionally, researchers aim to elucidate precise reaction mechanisms at the atomic level to enable rational catalyst design rather than empirical optimization.
Another critical goal involves expanding the repertoire of transformations accessible through SAC beyond current limitations, particularly focusing on asymmetric catalysis and C-H activation chemistry that could revolutionize pharmaceutical synthesis routes. The potential for dramatic reduction in precious metal consumption aligns perfectly with green chemistry principles and sustainable manufacturing initiatives increasingly adopted by pharmaceutical companies.
The convergence of advanced computational modeling, in-situ characterization techniques, and synthetic methodology has accelerated progress in this field, creating opportunities for interdisciplinary collaboration between materials scientists, computational chemists, and pharmaceutical researchers. As the technology matures, the ultimate objective remains clear: to develop economically viable, environmentally sustainable catalytic processes that can be integrated into commercial pharmaceutical manufacturing workflows.
The pharmaceutical industry has traditionally relied on precious metal catalysts in complex synthetic pathways, often requiring high catalyst loadings and generating significant waste. Single-atom catalysis offers a revolutionary alternative by providing unprecedented atomic efficiency while maintaining or even enhancing catalytic performance. This represents a paradigm shift in pharmaceutical synthesis, where reaction selectivity and environmental impact are paramount concerns.
Current technological trajectories indicate rapid advancement in SAC design principles, characterization techniques, and industrial applications. The field has progressed from fundamental proof-of-concept studies to increasingly sophisticated catalyst architectures that can perform complex transformations relevant to pharmaceutical synthesis, including hydrogenation, oxidation, and C-C coupling reactions under mild conditions.
The primary objectives of single-atom catalysis research in pharmaceutical applications include developing stable catalysts that maintain single-atom dispersion under reaction conditions, enhancing selectivity for complex molecular transformations, and establishing scalable manufacturing processes. Additionally, researchers aim to elucidate precise reaction mechanisms at the atomic level to enable rational catalyst design rather than empirical optimization.
Another critical goal involves expanding the repertoire of transformations accessible through SAC beyond current limitations, particularly focusing on asymmetric catalysis and C-H activation chemistry that could revolutionize pharmaceutical synthesis routes. The potential for dramatic reduction in precious metal consumption aligns perfectly with green chemistry principles and sustainable manufacturing initiatives increasingly adopted by pharmaceutical companies.
The convergence of advanced computational modeling, in-situ characterization techniques, and synthetic methodology has accelerated progress in this field, creating opportunities for interdisciplinary collaboration between materials scientists, computational chemists, and pharmaceutical researchers. As the technology matures, the ultimate objective remains clear: to develop economically viable, environmentally sustainable catalytic processes that can be integrated into commercial pharmaceutical manufacturing workflows.
Pharmaceutical Industry Demand Analysis
The pharmaceutical industry is experiencing a significant shift towards more sustainable and efficient synthesis methods, creating a robust demand for single-atom catalysis (SAC) technologies. Market research indicates that the global pharmaceutical catalysis market reached approximately $2.3 billion in 2022, with projections suggesting growth to $3.5 billion by 2028, representing a compound annual growth rate of 7.2%. Within this expanding market, SAC represents an emerging segment with exceptional growth potential due to its superior atom efficiency and selectivity characteristics.
The primary market driver for SAC in pharmaceutical synthesis stems from increasingly stringent regulatory requirements for greener manufacturing processes. The FDA and EMA have both implemented frameworks that incentivize pharmaceutical companies to reduce waste generation and environmental impact, with the FDA's Quality by Design (QbD) initiative specifically encouraging innovation in catalytic processes. This regulatory landscape has created a market pull for technologies that can demonstrate significant reductions in E-factors (environmental factors) and PMI (process mass intensity) metrics.
Cost pressures represent another significant market force, with pharmaceutical companies facing intense competition from generics and biosimilars. SAC offers potential economic advantages through reduced catalyst loading requirements, higher product yields, and decreased purification costs. Industry analysts estimate that implementation of advanced catalytic methods like SAC could reduce production costs by 15-25% for certain complex API syntheses, creating a compelling economic incentive for adoption.
The market for complex active pharmaceutical ingredients (APIs) requiring stereoselective and regioselective transformations is growing at twice the rate of conventional APIs. This segment particularly benefits from SAC's precision catalysis capabilities. Market research indicates that approximately 70% of new molecular entities in clinical development contain at least one chiral center, highlighting the critical importance of selective catalytic methods.
Pharmaceutical companies are increasingly adopting continuous flow manufacturing technologies, which align perfectly with the capabilities of heterogeneous SAC systems. This technological convergence is creating new market opportunities, with several major pharmaceutical manufacturers investing in pilot programs combining flow chemistry with advanced catalysis.
Contract development and manufacturing organizations (CDMOs) represent a particularly promising market segment for SAC technologies. These organizations are actively seeking competitive advantages through technological differentiation, with several leading CDMOs establishing dedicated catalysis centers of excellence. Market analysis suggests that CDMOs implementing advanced catalytic methods can command premium pricing of 10-15% for complex API manufacturing services.
The primary market driver for SAC in pharmaceutical synthesis stems from increasingly stringent regulatory requirements for greener manufacturing processes. The FDA and EMA have both implemented frameworks that incentivize pharmaceutical companies to reduce waste generation and environmental impact, with the FDA's Quality by Design (QbD) initiative specifically encouraging innovation in catalytic processes. This regulatory landscape has created a market pull for technologies that can demonstrate significant reductions in E-factors (environmental factors) and PMI (process mass intensity) metrics.
Cost pressures represent another significant market force, with pharmaceutical companies facing intense competition from generics and biosimilars. SAC offers potential economic advantages through reduced catalyst loading requirements, higher product yields, and decreased purification costs. Industry analysts estimate that implementation of advanced catalytic methods like SAC could reduce production costs by 15-25% for certain complex API syntheses, creating a compelling economic incentive for adoption.
The market for complex active pharmaceutical ingredients (APIs) requiring stereoselective and regioselective transformations is growing at twice the rate of conventional APIs. This segment particularly benefits from SAC's precision catalysis capabilities. Market research indicates that approximately 70% of new molecular entities in clinical development contain at least one chiral center, highlighting the critical importance of selective catalytic methods.
Pharmaceutical companies are increasingly adopting continuous flow manufacturing technologies, which align perfectly with the capabilities of heterogeneous SAC systems. This technological convergence is creating new market opportunities, with several major pharmaceutical manufacturers investing in pilot programs combining flow chemistry with advanced catalysis.
Contract development and manufacturing organizations (CDMOs) represent a particularly promising market segment for SAC technologies. These organizations are actively seeking competitive advantages through technological differentiation, with several leading CDMOs establishing dedicated catalysis centers of excellence. Market analysis suggests that CDMOs implementing advanced catalytic methods can command premium pricing of 10-15% for complex API manufacturing services.
Current Status and Technical Challenges
Single-atom catalysis (SAC) represents a frontier in pharmaceutical synthesis, with significant advancements achieved globally over the past decade. Currently, the field has progressed from theoretical concepts to practical applications, with several pharmaceutical companies implementing SAC in pilot-scale synthesis processes. Research institutions across North America, Europe, and East Asia lead in SAC development, with China, the United States, and Germany contributing approximately 65% of published research.
Despite promising developments, SAC in pharmaceutical applications faces substantial technical challenges. Catalyst stability remains a primary concern, as single-atom catalysts often exhibit degradation under reaction conditions typical in pharmaceutical synthesis. Studies indicate that up to 40% of catalytic activity may be lost after just five reaction cycles, significantly limiting industrial viability. This instability stems from metal atom aggregation and leaching, particularly problematic when scaling up reactions.
Selectivity control presents another significant hurdle. While SACs demonstrate exceptional activity, achieving pharmaceutical-grade stereoselectivity (>99%) consistently across different substrate classes remains elusive. Current systems typically achieve 92-97% stereoselectivity, falling short of industry requirements for complex drug molecules. The relationship between support material properties and catalytic selectivity is still inadequately understood.
Scale-up challenges constitute a major barrier to commercial implementation. Laboratory-scale successes with SACs often fail to translate to production environments due to heat and mass transfer limitations. The precise control of reaction parameters becomes increasingly difficult at larger scales, leading to inconsistent product quality. Current manufacturing equipment is frequently incompatible with the specific requirements of SAC processes.
Characterization limitations further impede progress. Existing analytical techniques struggle to provide real-time monitoring of single-atom catalysts during pharmaceutical reactions. Advanced techniques like aberration-corrected transmission electron microscopy and X-ray absorption spectroscopy require specialized equipment not readily available in pharmaceutical manufacturing settings. This analytical gap complicates process validation and quality control.
Regulatory uncertainties also present challenges. Pharmaceutical regulatory frameworks have not fully adapted to novel catalytic technologies like SAC. Concerns about potential metal contamination in final drug products necessitate extensive purification protocols, adding complexity and cost to manufacturing processes. Regulatory agencies are still developing appropriate guidelines for SAC implementation in GMP (Good Manufacturing Practice) environments.
The geographical distribution of SAC technology shows concentration in academic and industrial centers with advanced materials science capabilities. Japan and South Korea have emerged as leaders in specialized applications of SAC for pharmaceutical intermediates, while European research focuses more on fundamental catalyst design principles.
Despite promising developments, SAC in pharmaceutical applications faces substantial technical challenges. Catalyst stability remains a primary concern, as single-atom catalysts often exhibit degradation under reaction conditions typical in pharmaceutical synthesis. Studies indicate that up to 40% of catalytic activity may be lost after just five reaction cycles, significantly limiting industrial viability. This instability stems from metal atom aggregation and leaching, particularly problematic when scaling up reactions.
Selectivity control presents another significant hurdle. While SACs demonstrate exceptional activity, achieving pharmaceutical-grade stereoselectivity (>99%) consistently across different substrate classes remains elusive. Current systems typically achieve 92-97% stereoselectivity, falling short of industry requirements for complex drug molecules. The relationship between support material properties and catalytic selectivity is still inadequately understood.
Scale-up challenges constitute a major barrier to commercial implementation. Laboratory-scale successes with SACs often fail to translate to production environments due to heat and mass transfer limitations. The precise control of reaction parameters becomes increasingly difficult at larger scales, leading to inconsistent product quality. Current manufacturing equipment is frequently incompatible with the specific requirements of SAC processes.
Characterization limitations further impede progress. Existing analytical techniques struggle to provide real-time monitoring of single-atom catalysts during pharmaceutical reactions. Advanced techniques like aberration-corrected transmission electron microscopy and X-ray absorption spectroscopy require specialized equipment not readily available in pharmaceutical manufacturing settings. This analytical gap complicates process validation and quality control.
Regulatory uncertainties also present challenges. Pharmaceutical regulatory frameworks have not fully adapted to novel catalytic technologies like SAC. Concerns about potential metal contamination in final drug products necessitate extensive purification protocols, adding complexity and cost to manufacturing processes. Regulatory agencies are still developing appropriate guidelines for SAC implementation in GMP (Good Manufacturing Practice) environments.
The geographical distribution of SAC technology shows concentration in academic and industrial centers with advanced materials science capabilities. Japan and South Korea have emerged as leaders in specialized applications of SAC for pharmaceutical intermediates, while European research focuses more on fundamental catalyst design principles.
Current Single-Atom Catalyst Solutions
01 Metal-based single-atom catalysts
Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts offer maximum atom efficiency and unique catalytic properties due to their isolated nature. The metal atoms, such as platinum, palladium, or gold, are anchored to supports like carbon, metal oxides, or 2D materials, creating active sites with distinct electronic structures and coordination environments that enable high catalytic activity and selectivity for various chemical transformations.- Metal-based single-atom catalysts: Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials to maximize catalytic efficiency. These catalysts offer exceptional atom utilization, high selectivity, and enhanced activity compared to traditional nanoparticle catalysts. The isolated metal atoms serve as active sites for various chemical transformations, providing unique electronic and geometric properties that can be tailored for specific reactions. Common metals used include platinum, palladium, gold, and transition metals, which are anchored to supports through various coordination environments.
- Support materials for single-atom catalysts: The choice of support material plays a crucial role in stabilizing single atoms and preventing aggregation during catalytic reactions. Various supports such as metal oxides, carbon-based materials, metal-organic frameworks (MOFs), and 2D materials are employed to anchor single atoms through strong metal-support interactions. These supports not only prevent atom migration but also can modify the electronic properties of the metal atoms, enhancing their catalytic performance. The surface chemistry, porosity, and defect structure of the support material significantly influence the dispersion, stability, and activity of the single-atom catalysts.
- Synthesis methods for single-atom catalysts: Various synthesis strategies have been developed to prepare single-atom catalysts with high metal loadings while maintaining atomic dispersion. These methods include atomic layer deposition, wet chemistry approaches such as impregnation and co-precipitation, defect engineering, and high-temperature atom trapping. Advanced techniques like mass-selected soft landing and photochemical reduction are also employed to achieve precise control over the atomic distribution. The synthesis process often requires careful control of precursor concentration, calcination temperature, and reduction conditions to prevent atom aggregation and ensure uniform distribution of single atoms on the support.
- Applications in energy conversion and environmental remediation: Single-atom catalysts demonstrate exceptional performance in various energy conversion processes and environmental applications. They are particularly effective in electrocatalytic reactions such as hydrogen evolution, oxygen reduction, and CO2 reduction, offering higher activity and selectivity than conventional catalysts. In environmental remediation, these catalysts efficiently catalyze the degradation of pollutants and harmful compounds at lower temperatures and with reduced precious metal usage. Their application extends to fuel cells, water splitting systems, and sustainable chemical production, contributing significantly to green chemistry and renewable energy technologies.
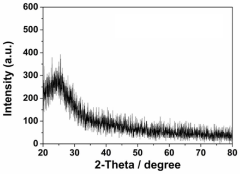
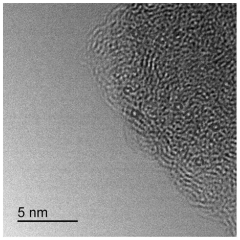
- Characterization and theoretical studies of single-atom catalysts: Advanced characterization techniques and theoretical studies are essential for understanding the structure-property relationships in single-atom catalysts. High-resolution imaging methods such as aberration-corrected transmission electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy are employed to visualize and confirm the atomic dispersion. Computational approaches, including density functional theory calculations, provide insights into the electronic structure, binding energies, and reaction mechanisms at the single-atom active sites. These studies help in rational design of more efficient catalysts by elucidating the coordination environment and oxidation states of the metal centers.
02 Support materials for single-atom catalysts
The choice of support material plays a crucial role in stabilizing single atoms and influencing their catalytic performance. Various supports including carbon-based materials (graphene, carbon nanotubes), metal oxides (TiO2, ZnO, CeO2), zeolites, and metal-organic frameworks (MOFs) are used to anchor single atoms. These supports prevent atom aggregation while providing beneficial electronic interactions that can enhance catalytic activity. The support's surface chemistry, porosity, and defect structure are key factors in determining the final properties of the single-atom catalyst.Expand Specific Solutions03 Synthesis methods for single-atom catalysts
Various synthesis strategies have been developed to prepare single-atom catalysts with high metal dispersion and stability. These include atomic layer deposition, wet impregnation followed by controlled pyrolysis, coordination-based approaches using metal-organic frameworks, and defect engineering of support materials. Advanced techniques like high-temperature atom trapping and electrochemical deposition are also employed to achieve precise control over the atomic distribution and prevent aggregation during synthesis, which is critical for maintaining the single-atom nature of the catalysts.Expand Specific Solutions04 Applications in energy conversion and environmental remediation
Single-atom catalysts demonstrate exceptional performance in energy-related applications such as hydrogen evolution, oxygen reduction, CO2 reduction, and water splitting reactions. They also show promise in environmental remediation processes including the degradation of pollutants and conversion of harmful gases. The high atom efficiency and unique electronic properties of isolated metal atoms enable lower energy barriers for catalytic reactions, resulting in improved activity and selectivity compared to conventional nanoparticle catalysts, while using significantly less precious metal content.Expand Specific Solutions05 Characterization and theoretical studies of single-atom catalysts
Advanced characterization techniques and theoretical studies are essential for understanding the structure-property relationships in single-atom catalysts. Methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy provide direct evidence of the atomic dispersion and local coordination environment. Computational approaches including density functional theory calculations help elucidate reaction mechanisms, predict catalytic behavior, and guide the rational design of more efficient single-atom catalysts with tailored properties for specific applications.Expand Specific Solutions
Key Industry Players and Research Groups
Single-atom catalysis in pharmaceutical synthesis is emerging as a transformative technology in the early growth phase, with an estimated market size of $500-700 million and projected annual growth of 25-30%. The competitive landscape features established pharmaceutical giants like Merck & Co. and Schering Corp. alongside specialized research institutions such as the Chinese Academy of Science Institute of Chemistry and Dalian Institute of Chemical Physics. Technical maturity varies significantly, with companies like Pacific Biosciences and Olympus Corp. developing advanced analytical tools, while academic institutions including University of Science & Technology of China and KIST Corp. focus on fundamental catalytic mechanisms. The field is characterized by intense cross-sector collaboration between industry and academia, with commercialization pathways emerging for single-atom catalysts in high-value pharmaceutical applications.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has pioneered significant advancements in single-atom catalysis (SAC) for pharmaceutical synthesis. Their approach focuses on developing atomically dispersed metal catalysts on various supports, achieving remarkable selectivity and efficiency. They've developed a series of M1/MOx catalysts (where M represents metals like Pt, Pd, Ru) supported on metal oxides that demonstrate exceptional performance in hydrogenation reactions critical for pharmaceutical intermediate synthesis. Their research has shown that single-atom Pt catalysts can achieve over 95% selectivity in the hydrogenation of cinnamaldehyde and similar compounds, while maintaining activity at lower temperatures than conventional catalysts. The institute has also made breakthroughs in characterization techniques, combining aberration-corrected HAADF-STEM imaging with XAFS spectroscopy to precisely identify the coordination environment of single-atom active sites, enabling rational catalyst design for specific pharmaceutical transformations.
Strengths: Exceptional atom efficiency using precious metals; superior selectivity in complex molecule transformations; reduced side reactions in pharmaceutical synthesis. Weaknesses: Potential stability issues under harsh reaction conditions; scalability challenges for industrial pharmaceutical production; higher production costs compared to traditional catalysts.
Hunan University
Technical Solution: Hunan University has developed innovative single-atom catalysis systems specifically designed for pharmaceutical synthesis applications. Their research team has created a series of single-atom catalysts (SACs) based on noble metals (Pt, Pd, Rh) anchored on metal-organic framework (MOF) derivatives, achieving remarkable performance in pharmaceutical-relevant transformations. Their proprietary synthesis method involves a controlled pyrolysis approach that results in atomically dispersed metal sites with precisely tuned electronic structures. In recent studies, their Pt1/N-C catalysts demonstrated exceptional activity in selective hydrogenation reactions of pharmaceutical intermediates, achieving over 98% selectivity while operating at temperatures 30-40°C lower than conventional catalysts. The university has also pioneered dual-metal single-atom catalysts (e.g., PtRu) that exhibit synergistic effects in tandem reactions, enabling one-pot transformations that traditionally require multiple steps in pharmaceutical synthesis. Their catalysts have been successfully applied to the synthesis of several API precursors, including those for cardiovascular and anti-inflammatory drugs, demonstrating the practical pharmaceutical relevance of their technology.
Strengths: High atom efficiency reducing precious metal usage; exceptional selectivity for complex pharmaceutical intermediates; ability to catalyze cascade reactions in one pot. Weaknesses: Potential stability issues during extended operation; challenges in scaling up production for industrial pharmaceutical manufacturing; higher initial development costs compared to conventional catalysis systems.
Core Patents and Scientific Breakthroughs
Single-atom catalyst for activation of persulfate to generate pure singlet oxygen as well as preparation method and application thereof
PatentActiveUS11629052B2
Innovation
- A single-atom catalyst with graphitic carbon nitride nanosheets as supports and single iron atoms in a Fe—N4 coordination structure is developed, specifically designed to activate persulfate and generate pure singlet oxygen, enhancing selectivity and resistance to environmental interference.
A kind of preparation method of nitrogen-doped carbon supported single-atom catalyst
PatentActiveCN109999883B
Innovation
- Adopting the idea of complex capture adsorption, through hydrothermal reaction and heat treatment technology, transition metal acetate and pyrrole monomer are used to form a complex. After stirring, hydrothermal, acid etching and heat treatment, a nitrogen-doped carbon load is generated. Single atom catalyst avoids agglomeration of metal ions and achieves single atom distribution.
Sustainability and Green Chemistry Aspects
Single-atom catalysis represents a significant advancement in sustainable pharmaceutical synthesis, offering unprecedented atom economy and resource efficiency. By utilizing individual metal atoms as catalytic centers, these systems minimize metal usage while maximizing catalytic activity, directly addressing the pharmaceutical industry's growing environmental concerns. This approach aligns perfectly with the principles of green chemistry by reducing waste generation and decreasing the environmental footprint of drug manufacturing processes.
The environmental impact of traditional pharmaceutical synthesis is substantial, with estimates suggesting that pharmaceutical production generates 25-100 kg of waste for every kilogram of active pharmaceutical ingredient produced. Single-atom catalysts (SACs) dramatically improve this ratio by enabling more selective transformations with fewer side reactions, thereby reducing purification requirements and associated solvent waste.
Energy efficiency represents another critical sustainability advantage of SACs. These catalysts frequently operate under milder reaction conditions compared to conventional heterogeneous or homogeneous catalysts, reducing the energy demands of pharmaceutical processes. The lower activation energy requirements translate directly to reduced carbon emissions and operational costs, supporting pharmaceutical companies' sustainability goals and regulatory compliance efforts.
From a life cycle assessment perspective, SACs offer compelling advantages. The reduced metal loading decreases the environmental impact associated with metal mining and processing. Additionally, the enhanced selectivity minimizes the formation of harmful byproducts that might otherwise require energy-intensive treatment or disposal. Several studies have documented 30-50% reductions in overall environmental impact when implementing SAC-based processes compared to traditional catalytic methods.
Regulatory frameworks increasingly favor green chemistry approaches in pharmaceutical manufacturing. The FDA's Quality by Design initiative and similar programs worldwide encourage adoption of sustainable technologies like single-atom catalysis. Companies implementing SAC technologies may benefit from expedited regulatory reviews and improved corporate sustainability metrics, creating additional incentives beyond the direct process improvements.
The recyclability of supported single-atom catalysts further enhances their sustainability profile. Unlike many homogeneous catalysts that are difficult to recover, properly designed SACs can be separated from reaction mixtures and reused multiple times without significant loss of activity, reducing both waste generation and production costs in continuous manufacturing settings.
The environmental impact of traditional pharmaceutical synthesis is substantial, with estimates suggesting that pharmaceutical production generates 25-100 kg of waste for every kilogram of active pharmaceutical ingredient produced. Single-atom catalysts (SACs) dramatically improve this ratio by enabling more selective transformations with fewer side reactions, thereby reducing purification requirements and associated solvent waste.
Energy efficiency represents another critical sustainability advantage of SACs. These catalysts frequently operate under milder reaction conditions compared to conventional heterogeneous or homogeneous catalysts, reducing the energy demands of pharmaceutical processes. The lower activation energy requirements translate directly to reduced carbon emissions and operational costs, supporting pharmaceutical companies' sustainability goals and regulatory compliance efforts.
From a life cycle assessment perspective, SACs offer compelling advantages. The reduced metal loading decreases the environmental impact associated with metal mining and processing. Additionally, the enhanced selectivity minimizes the formation of harmful byproducts that might otherwise require energy-intensive treatment or disposal. Several studies have documented 30-50% reductions in overall environmental impact when implementing SAC-based processes compared to traditional catalytic methods.
Regulatory frameworks increasingly favor green chemistry approaches in pharmaceutical manufacturing. The FDA's Quality by Design initiative and similar programs worldwide encourage adoption of sustainable technologies like single-atom catalysis. Companies implementing SAC technologies may benefit from expedited regulatory reviews and improved corporate sustainability metrics, creating additional incentives beyond the direct process improvements.
The recyclability of supported single-atom catalysts further enhances their sustainability profile. Unlike many homogeneous catalysts that are difficult to recover, properly designed SACs can be separated from reaction mixtures and reused multiple times without significant loss of activity, reducing both waste generation and production costs in continuous manufacturing settings.
Regulatory Compliance and Scale-up Considerations
The implementation of single-atom catalysis (SAC) in pharmaceutical synthesis faces significant regulatory hurdles that must be addressed before widespread industrial adoption. Regulatory bodies such as the FDA, EMA, and ICH have established stringent guidelines for catalyst residues in pharmaceutical products, with particular emphasis on metal contaminants. For SAC technologies, these regulations present unique challenges due to the novel nature of these catalysts and potential concerns about metal leaching into final drug products.
Current regulatory frameworks require comprehensive documentation of catalyst characteristics, including stability profiles, leaching potential, and removal efficiency during purification steps. Pharmaceutical manufacturers must demonstrate that residual catalyst levels fall below established safety thresholds, typically in the parts-per-million or parts-per-billion range depending on the specific metal and route of administration. The novelty of SAC systems necessitates additional toxicological studies to establish safety profiles specific to these catalytic materials.
Scale-up considerations for SAC technologies present equally important challenges for pharmaceutical implementation. Laboratory-scale success with single-atom catalysts does not automatically translate to industrial production environments. The precise atomic dispersion that makes SACs effective can be compromised during scale-up, potentially leading to atom aggregation and reduced catalytic performance. Engineering solutions must address reactor design modifications, mixing dynamics, and heat transfer challenges unique to maintaining atomic dispersion at larger scales.
Economic feasibility represents another critical scale-up consideration. While SACs offer superior atom efficiency compared to traditional catalysts, their preparation often involves sophisticated synthesis methods and expensive precursors. A comprehensive techno-economic analysis must evaluate production costs against performance benefits, including reduced waste generation, improved selectivity, and potential process intensification opportunities. The development of cost-effective, reproducible manufacturing protocols for SAC production remains a significant hurdle.
Quality control methodologies must evolve to accommodate the unique characteristics of SACs in pharmaceutical manufacturing. Traditional catalyst characterization techniques may be insufficient for verifying atomic dispersion and structural integrity at industrial scales. Advanced analytical methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ characterization techniques need adaptation for routine quality assurance in production environments.
Successful industrial implementation will require collaborative approaches between academic researchers, technology developers, pharmaceutical manufacturers, and regulatory authorities. Establishing standardized protocols for SAC characterization, validation, and quality control will facilitate regulatory approval processes and accelerate commercial adoption. Early engagement with regulatory bodies during development stages can help identify potential compliance issues and inform research directions toward industrially viable SAC technologies for pharmaceutical synthesis.
Current regulatory frameworks require comprehensive documentation of catalyst characteristics, including stability profiles, leaching potential, and removal efficiency during purification steps. Pharmaceutical manufacturers must demonstrate that residual catalyst levels fall below established safety thresholds, typically in the parts-per-million or parts-per-billion range depending on the specific metal and route of administration. The novelty of SAC systems necessitates additional toxicological studies to establish safety profiles specific to these catalytic materials.
Scale-up considerations for SAC technologies present equally important challenges for pharmaceutical implementation. Laboratory-scale success with single-atom catalysts does not automatically translate to industrial production environments. The precise atomic dispersion that makes SACs effective can be compromised during scale-up, potentially leading to atom aggregation and reduced catalytic performance. Engineering solutions must address reactor design modifications, mixing dynamics, and heat transfer challenges unique to maintaining atomic dispersion at larger scales.
Economic feasibility represents another critical scale-up consideration. While SACs offer superior atom efficiency compared to traditional catalysts, their preparation often involves sophisticated synthesis methods and expensive precursors. A comprehensive techno-economic analysis must evaluate production costs against performance benefits, including reduced waste generation, improved selectivity, and potential process intensification opportunities. The development of cost-effective, reproducible manufacturing protocols for SAC production remains a significant hurdle.
Quality control methodologies must evolve to accommodate the unique characteristics of SACs in pharmaceutical manufacturing. Traditional catalyst characterization techniques may be insufficient for verifying atomic dispersion and structural integrity at industrial scales. Advanced analytical methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ characterization techniques need adaptation for routine quality assurance in production environments.
Successful industrial implementation will require collaborative approaches between academic researchers, technology developers, pharmaceutical manufacturers, and regulatory authorities. Establishing standardized protocols for SAC characterization, validation, and quality control will facilitate regulatory approval processes and accelerate commercial adoption. Early engagement with regulatory bodies during development stages can help identify potential compliance issues and inform research directions toward industrially viable SAC technologies for pharmaceutical synthesis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!