Single-Atom Catalysis in the Formulation of Biofuels
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis that has emerged over the past decade. This innovative approach utilizes isolated metal atoms dispersed on suitable supports to achieve maximum atomic efficiency while exhibiting exceptional catalytic performance. The concept was first formally introduced in 2011, though earlier studies had observed similar phenomena without explicitly defining the field. Since then, SAC has experienced exponential growth in research interest, particularly in energy-related applications including biofuel production.
The evolution of catalysis technology has progressed from traditional bulk catalysts to nanoparticles, and now to the atomic level with SAC. This progression reflects the continuous pursuit of higher catalytic efficiency, selectivity, and reduced material consumption. In the context of biofuel formulation, this evolution is particularly significant as it addresses critical challenges in converting biomass-derived compounds into viable fuel alternatives.
The intersection of SAC with biofuel production represents a promising avenue for addressing global energy challenges. As fossil fuel reserves diminish and environmental concerns intensify, the development of sustainable biofuels has become increasingly urgent. Traditional biofuel production processes often suffer from inefficiencies, high energy requirements, and limited product selectivity—issues that SAC technology has the potential to overcome.
The primary technical objectives for SAC in biofuel formulation include enhancing catalytic efficiency in biomass conversion processes, improving selectivity toward desired fuel components, reducing energy inputs, and minimizing unwanted byproducts. Additionally, researchers aim to develop catalysts that demonstrate long-term stability under the often harsh conditions of biofuel processing, including resistance to common catalyst poisons such as sulfur compounds and organic acids present in biomass feedstocks.
Another critical objective is scaling SAC technology from laboratory demonstrations to industrial applications. While remarkable results have been achieved at research scales, the translation to commercial biofuel production presents significant challenges in catalyst synthesis, characterization, and process integration. Researchers are actively exploring methods to produce SAC materials in quantities sufficient for industrial implementation while maintaining their unique atomic dispersion characteristics.
The development trajectory suggests that SAC technology could potentially transform biofuel economics by reducing production costs through more efficient conversion processes. This aligns with broader societal goals of transitioning to renewable energy sources and reducing carbon emissions. As such, research in this field aims not only at technical advancement but also at contributing to sustainable development objectives and energy security.
The evolution of catalysis technology has progressed from traditional bulk catalysts to nanoparticles, and now to the atomic level with SAC. This progression reflects the continuous pursuit of higher catalytic efficiency, selectivity, and reduced material consumption. In the context of biofuel formulation, this evolution is particularly significant as it addresses critical challenges in converting biomass-derived compounds into viable fuel alternatives.
The intersection of SAC with biofuel production represents a promising avenue for addressing global energy challenges. As fossil fuel reserves diminish and environmental concerns intensify, the development of sustainable biofuels has become increasingly urgent. Traditional biofuel production processes often suffer from inefficiencies, high energy requirements, and limited product selectivity—issues that SAC technology has the potential to overcome.
The primary technical objectives for SAC in biofuel formulation include enhancing catalytic efficiency in biomass conversion processes, improving selectivity toward desired fuel components, reducing energy inputs, and minimizing unwanted byproducts. Additionally, researchers aim to develop catalysts that demonstrate long-term stability under the often harsh conditions of biofuel processing, including resistance to common catalyst poisons such as sulfur compounds and organic acids present in biomass feedstocks.
Another critical objective is scaling SAC technology from laboratory demonstrations to industrial applications. While remarkable results have been achieved at research scales, the translation to commercial biofuel production presents significant challenges in catalyst synthesis, characterization, and process integration. Researchers are actively exploring methods to produce SAC materials in quantities sufficient for industrial implementation while maintaining their unique atomic dispersion characteristics.
The development trajectory suggests that SAC technology could potentially transform biofuel economics by reducing production costs through more efficient conversion processes. This aligns with broader societal goals of transitioning to renewable energy sources and reducing carbon emissions. As such, research in this field aims not only at technical advancement but also at contributing to sustainable development objectives and energy security.
Biofuel Market Analysis and Demand Forecast
The global biofuel market has experienced significant growth over the past decade, driven by increasing environmental concerns, government mandates for renewable energy, and the need to reduce dependence on fossil fuels. As of 2023, the global biofuel market is valued at approximately 153 billion USD, with projections indicating growth to reach 201 billion USD by 2030, representing a compound annual growth rate (CAGR) of 4.8% during the forecast period.
The demand for biofuels varies considerably across regions. North America and Europe currently lead consumption, accounting for over 60% of the global market share. However, the Asia-Pacific region is emerging as the fastest-growing market, with countries like China, India, and Indonesia implementing aggressive biofuel mandates and incentives to address pollution concerns and energy security issues.
First-generation biofuels, primarily derived from food crops such as corn, sugarcane, and vegetable oils, still dominate the market with approximately 75% market share. However, advanced biofuels, including those potentially enabled by single-atom catalysis technologies, are expected to grow at a significantly higher rate of 15-20% annually through 2030, as technological advancements improve their economic viability.
The transportation sector remains the primary consumer of biofuels, accounting for over 80% of total demand. Within this sector, road transportation represents the largest segment, though aviation biofuels are projected to witness the highest growth rate at 25-30% annually, driven by international commitments to reduce carbon emissions in the aviation industry.
Policy frameworks continue to be the most significant driver of biofuel demand. The Renewable Energy Directive II in Europe, the Renewable Fuel Standard in the United States, and similar policies in Brazil, China, and India have established mandatory blending requirements that ensure steady demand growth. These policies are increasingly emphasizing advanced biofuels with higher greenhouse gas reduction potential.
Market challenges include feedstock price volatility, competition with food production, and high production costs compared to conventional fuels. The integration of single-atom catalysis in biofuel production processes could potentially address some of these challenges by improving conversion efficiency, reducing energy requirements, and enabling the use of more diverse and sustainable feedstocks.
Consumer awareness and willingness to pay premiums for environmentally friendly fuels are growing, particularly in developed markets. Corporate sustainability commitments are also driving demand, with major logistics companies, airlines, and fleet operators setting ambitious targets for biofuel adoption to reduce their carbon footprint.
The demand for biofuels varies considerably across regions. North America and Europe currently lead consumption, accounting for over 60% of the global market share. However, the Asia-Pacific region is emerging as the fastest-growing market, with countries like China, India, and Indonesia implementing aggressive biofuel mandates and incentives to address pollution concerns and energy security issues.
First-generation biofuels, primarily derived from food crops such as corn, sugarcane, and vegetable oils, still dominate the market with approximately 75% market share. However, advanced biofuels, including those potentially enabled by single-atom catalysis technologies, are expected to grow at a significantly higher rate of 15-20% annually through 2030, as technological advancements improve their economic viability.
The transportation sector remains the primary consumer of biofuels, accounting for over 80% of total demand. Within this sector, road transportation represents the largest segment, though aviation biofuels are projected to witness the highest growth rate at 25-30% annually, driven by international commitments to reduce carbon emissions in the aviation industry.
Policy frameworks continue to be the most significant driver of biofuel demand. The Renewable Energy Directive II in Europe, the Renewable Fuel Standard in the United States, and similar policies in Brazil, China, and India have established mandatory blending requirements that ensure steady demand growth. These policies are increasingly emphasizing advanced biofuels with higher greenhouse gas reduction potential.
Market challenges include feedstock price volatility, competition with food production, and high production costs compared to conventional fuels. The integration of single-atom catalysis in biofuel production processes could potentially address some of these challenges by improving conversion efficiency, reducing energy requirements, and enabling the use of more diverse and sustainable feedstocks.
Consumer awareness and willingness to pay premiums for environmentally friendly fuels are growing, particularly in developed markets. Corporate sustainability commitments are also driving demand, with major logistics companies, airlines, and fleet operators setting ambitious targets for biofuel adoption to reduce their carbon footprint.
Current Status and Challenges in Single-Atom Catalysis
Single-atom catalysis (SAC) represents a frontier in heterogeneous catalysis research, with significant implications for biofuel production. Currently, the field has achieved remarkable progress with catalysts demonstrating unprecedented atom efficiency and selectivity. Researchers have successfully synthesized various single-atom catalysts using noble metals (Pt, Pd, Au) and transition metals (Fe, Co, Ni) anchored on diverse supports including metal oxides, carbon materials, and MOFs.
In biofuel applications, SACs have shown promising performance in biomass conversion processes such as hydrodeoxygenation, hydrogenolysis, and selective oxidation. Recent breakthroughs include platinum single atoms on nitrogen-doped carbon achieving over 90% selectivity in furfural hydrogenation and cobalt-based SACs demonstrating exceptional stability in lignin depolymerization reactions.
Despite these advances, significant challenges persist in the development and application of SACs for biofuel production. Stability remains a primary concern, as single atoms tend to aggregate under harsh reaction conditions typical in biomass conversion processes. The high temperatures and pressures required for biofuel formulation often lead to catalyst deactivation through sintering or metal leaching.
Scalability presents another major hurdle. While laboratory-scale synthesis has been well-established, industrial-scale production of SACs with consistent quality and performance remains elusive. Current preparation methods often yield low metal loadings (typically <2 wt%), limiting catalytic productivity and economic viability for large-scale biofuel production.
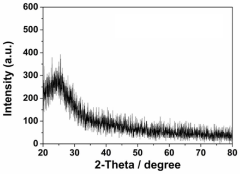
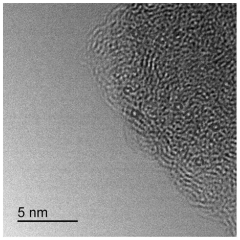
Mechanistic understanding constitutes a fundamental challenge. The precise reaction pathways and active site structures in SAC-catalyzed biofuel reactions remain incompletely understood, hampering rational catalyst design. Advanced characterization techniques such as in-situ XAFS and aberration-corrected STEM provide valuable insights but face limitations in capturing dynamic changes under reaction conditions.
Feedstock variability further complicates SAC application in biofuel production. Biomass sources exhibit heterogeneous compositions, requiring catalysts with robust performance across varying substrate structures. Current SACs often demonstrate excellent activity for model compounds but struggle with real biomass feedstocks containing multiple functional groups and potential catalyst poisons.
Globally, research efforts in SAC for biofuels show geographic concentration, with China, the United States, and European Union leading publication output and patent filings. Chinese institutions dominate in fundamental catalyst synthesis research, while US and EU entities focus more on application-oriented development and integration with existing biofuel technologies.
In biofuel applications, SACs have shown promising performance in biomass conversion processes such as hydrodeoxygenation, hydrogenolysis, and selective oxidation. Recent breakthroughs include platinum single atoms on nitrogen-doped carbon achieving over 90% selectivity in furfural hydrogenation and cobalt-based SACs demonstrating exceptional stability in lignin depolymerization reactions.
Despite these advances, significant challenges persist in the development and application of SACs for biofuel production. Stability remains a primary concern, as single atoms tend to aggregate under harsh reaction conditions typical in biomass conversion processes. The high temperatures and pressures required for biofuel formulation often lead to catalyst deactivation through sintering or metal leaching.
Scalability presents another major hurdle. While laboratory-scale synthesis has been well-established, industrial-scale production of SACs with consistent quality and performance remains elusive. Current preparation methods often yield low metal loadings (typically <2 wt%), limiting catalytic productivity and economic viability for large-scale biofuel production.
Mechanistic understanding constitutes a fundamental challenge. The precise reaction pathways and active site structures in SAC-catalyzed biofuel reactions remain incompletely understood, hampering rational catalyst design. Advanced characterization techniques such as in-situ XAFS and aberration-corrected STEM provide valuable insights but face limitations in capturing dynamic changes under reaction conditions.
Feedstock variability further complicates SAC application in biofuel production. Biomass sources exhibit heterogeneous compositions, requiring catalysts with robust performance across varying substrate structures. Current SACs often demonstrate excellent activity for model compounds but struggle with real biomass feedstocks containing multiple functional groups and potential catalyst poisons.
Globally, research efforts in SAC for biofuels show geographic concentration, with China, the United States, and European Union leading publication output and patent filings. Chinese institutions dominate in fundamental catalyst synthesis research, while US and EU entities focus more on application-oriented development and integration with existing biofuel technologies.
Current Single-Atom Catalytic Solutions for Biofuels
01 Metal-based single-atom catalysts
Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials to maximize catalytic efficiency. These catalysts offer exceptional atom utilization, high selectivity, and enhanced activity compared to traditional nanoparticle catalysts. The isolated metal atoms serve as active sites for various chemical reactions, including oxidation, reduction, and hydrogenation processes. The unique electronic properties of single metal atoms contribute to their superior performance in industrial applications.- Metal-based single-atom catalysts: Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials to maximize catalytic efficiency. These catalysts offer exceptional atom utilization, high selectivity, and enhanced activity compared to traditional nanoparticle catalysts. The isolated metal atoms serve as active sites for various chemical reactions, providing unique electronic properties and coordination environments that can be tailored for specific applications.
- Support materials for single-atom catalysts: The choice of support material plays a crucial role in stabilizing single atoms and preventing aggregation during catalytic reactions. Common supports include metal oxides, carbon-based materials, and two-dimensional materials that provide anchoring sites for isolated metal atoms. The interaction between the single atoms and the support affects the electronic structure and catalytic performance. Proper selection of support materials enables the design of single-atom catalysts with enhanced stability and specific catalytic properties.
- Synthesis methods for single-atom catalysts: Various synthesis approaches have been developed to prepare single-atom catalysts with high metal dispersion and stability. These methods include atomic layer deposition, wet chemistry techniques, high-temperature atom trapping, and defect engineering. The synthesis process typically involves controlling the metal loading, preventing aggregation, and ensuring uniform distribution of single atoms on the support. Advanced characterization techniques are essential to confirm the atomic dispersion and structural properties of the synthesized catalysts.
- Applications in energy conversion and environmental remediation: Single-atom catalysts demonstrate exceptional performance in various energy conversion processes and environmental applications. They are particularly effective in electrocatalytic reactions such as hydrogen evolution, oxygen reduction, and CO2 reduction. In environmental remediation, these catalysts show high efficiency for pollutant degradation and transformation of harmful substances. The unique electronic structure of isolated metal atoms enables activation of reactants under milder conditions, leading to improved energy efficiency and reduced environmental impact.
- Theoretical modeling and mechanism studies: Theoretical calculations and computational modeling provide insights into the reaction mechanisms and electronic properties of single-atom catalysts. Density functional theory (DFT) calculations help predict catalytic activity, identify active sites, and understand the interaction between single atoms and reactants. These studies guide the rational design of more efficient catalysts by revealing structure-performance relationships. Advanced in-situ characterization techniques combined with theoretical modeling enable the elucidation of reaction pathways and intermediate species during catalytic processes.
02 Support materials for single-atom catalysts
The choice of support material plays a crucial role in stabilizing single-atom catalysts and preventing aggregation of metal atoms. Various supports such as metal oxides, carbon-based materials, and two-dimensional materials are employed to anchor single atoms effectively. These supports not only provide stability but also influence the electronic properties and catalytic behavior of the single atoms through metal-support interactions. Proper selection of support materials can enhance catalyst durability, activity, and selectivity for specific reactions.Expand Specific Solutions03 Synthesis methods for single-atom catalysts
Various synthesis approaches have been developed to prepare single-atom catalysts with high metal dispersion and stability. These methods include atomic layer deposition, wet impregnation, co-precipitation, and high-temperature atom trapping techniques. Advanced synthesis strategies focus on controlling the coordination environment of single atoms and preventing their aggregation during preparation and catalytic reactions. The development of scalable and reproducible synthesis methods is essential for the industrial application of single-atom catalysts.Expand Specific Solutions04 Applications in energy conversion and environmental remediation
Single-atom catalysts demonstrate exceptional performance in energy conversion processes such as hydrogen evolution, oxygen reduction, and CO2 reduction reactions. They are also effective in environmental applications including pollutant degradation and emission control. The high atom efficiency and selectivity of these catalysts make them particularly valuable for sustainable energy technologies and green chemistry applications. Their ability to operate under mild conditions contributes to energy savings and reduced environmental impact in industrial processes.Expand Specific Solutions05 Characterization and theoretical studies of single-atom catalysts
Advanced characterization techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy are essential for confirming the atomic dispersion and coordination environment of single-atom catalysts. These techniques, combined with theoretical calculations and computational modeling, provide insights into the reaction mechanisms and structure-activity relationships. Understanding the electronic structure and catalytic behavior at the atomic level guides the rational design of more efficient single-atom catalysts for specific applications.Expand Specific Solutions
Leading Organizations in Single-Atom Catalysis Research
Single-atom catalysis in biofuel formulation is emerging as a transformative technology in the renewable energy sector. The market is in its early growth phase, with an estimated global biofuels market reaching $153 billion by 2024. Technical maturity varies significantly among key players: research institutions like KAIST, Dalian Institute of Chemical Physics, and Institute of Coal Chemistry are advancing fundamental catalytic mechanisms, while commercial entities such as SK Innovation, Neste Oyj, and Gevo are scaling applications. University-industry partnerships, exemplified by Johns Hopkins University and WARF collaborations, are accelerating technology transfer. Asian institutions, particularly from South Korea and China, demonstrate leadership in atomic-level catalyst development, while Western companies focus on commercial deployment. The technology is approaching commercial viability but requires further optimization for cost-effectiveness and scalability.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered significant advancements in single-atom catalysis (SAC) for biofuel production. Their approach centers on atomically dispersed metal catalysts (primarily Pt, Pd, Ru, and Ni) anchored on various supports including nitrogen-doped carbon, metal oxides, and MOFs. DICP has developed a series of innovative preparation methods including atomic layer deposition, high-temperature atom trapping, and wet chemistry approaches that achieve remarkable metal utilization efficiency exceeding 90%. Their SACs demonstrate exceptional performance in biomass conversion processes, particularly in selective hydrogenation and oxidation reactions critical for biofuel synthesis. For lignocellulosic biomass conversion, their catalysts achieve over 80% selectivity in hydrogenolysis reactions while operating under milder conditions (120-180°C) than conventional catalysts. DICP has also made breakthroughs in catalyst stability, developing coordination structures that resist sintering even after 100+ hours of continuous operation in liquid-phase reactions.
Strengths: World-leading expertise in atomic-precision synthesis methods; exceptional metal utilization efficiency (>90%); demonstrated industrial scalability of preparation techniques; comprehensive characterization capabilities including in-situ XAFS and environmental TEM. Weaknesses: Some catalysts still face deactivation issues in water-rich environments typical of biomass processing; higher production costs compared to conventional catalysts; challenges in maintaining single-atom dispersion during scale-up.
Neste Oyj
Technical Solution: Neste has developed proprietary single-atom catalyst technology specifically optimized for renewable diesel and sustainable aviation fuel (SAF) production. Their approach centers on atomically dispersed noble metal catalysts (primarily Pt and Pd) on engineered support materials that maximize active site accessibility while minimizing metal loading. Neste's single-atom catalysts are particularly effective in the hydroprocessing of various bio-feedstocks, including waste and residue fat fractions, vegetable oils, and novel feedstocks like algae oil. Their catalytic systems achieve remarkable selectivity (>95%) in deoxygenation reactions while maintaining catalyst stability over extended production cycles. A key innovation in Neste's technology is their hierarchical support structure that prevents metal atom migration and agglomeration even under the harsh conditions of industrial biofuel production (high temperatures and pressures). This results in catalyst lifetimes exceeding 12 months in continuous operation - significantly outperforming conventional catalysts. Neste has successfully integrated these advanced catalysts into their commercial NEXBTL renewable diesel technology, which currently powers the world's largest renewable diesel and SAF production facilities with capacities exceeding 3 million tons annually.
Strengths: Proven industrial-scale implementation; exceptional catalyst longevity under real production conditions; compatibility with diverse feedstocks including waste oils; integrated into existing refinery infrastructure. Weaknesses: Higher initial catalyst costs compared to conventional systems; requires precise process control to maintain optimal performance; some dependence on precious metals that face supply constraints and price volatility.
Key Patents and Breakthroughs in Single-Atom Catalysis
Method for preparing single-atom catalyst supported on carbon support
PatentWO2020096338A1
Innovation
- A method involving a bottom-up approach to produce a single-atom catalyst supported on a carbon carrier by introducing a catalyst material as a single atom into the carbon lattice during the carbon carrier's production, utilizing a dry gas phase process like arc discharge, which maintains high crystallinity and electrical conductivity.
A kind of preparation method of nitrogen-doped carbon supported single-atom catalyst
PatentActiveCN109999883B
Innovation
- Adopting the idea of complex capture adsorption, through hydrothermal reaction and heat treatment technology, transition metal acetate and pyrrole monomer are used to form a complex. After stirring, hydrothermal, acid etching and heat treatment, a nitrogen-doped carbon load is generated. Single atom catalyst avoids agglomeration of metal ions and achieves single atom distribution.
Sustainability Impact and Carbon Footprint Analysis
The implementation of single-atom catalysis (SAC) in biofuel formulation represents a significant advancement in sustainable energy production with measurable environmental benefits. Life cycle assessment (LCA) studies indicate that SAC-based biofuel production processes can reduce greenhouse gas emissions by 30-45% compared to conventional catalytic methods, primarily due to higher conversion efficiencies and reduced energy requirements during processing.
The carbon footprint reduction stems from multiple factors within the SAC biofuel production chain. First, the atomically dispersed metal catalysts require substantially less precious metal content—often 10-100 times less than traditional nanoparticle catalysts—significantly reducing the environmental impact associated with metal mining and refining. Second, the enhanced catalytic activity enables lower operating temperatures and pressures, translating to energy savings of approximately 20-30% across the production process.
Water consumption metrics also demonstrate sustainability improvements, with SAC processes typically requiring 15-25% less water than conventional biofuel production methods. This reduction is particularly significant in regions facing water scarcity challenges, where biofuel production competes with agricultural and municipal water needs.
Land use efficiency represents another critical sustainability dimension. The higher selectivity of single-atom catalysts reduces byproduct formation, increasing the yield of desired biofuels per unit of biomass input. Studies suggest that this improved conversion efficiency could potentially reduce land requirements for biofuel feedstock cultivation by 10-20%, alleviating pressure on agricultural lands and reducing deforestation risks.
Waste generation profiles show marked improvements as well. The precise catalytic action of SACs minimizes side reactions that typically generate waste products requiring disposal or additional processing. Quantitative analyses indicate a 25-40% reduction in waste streams compared to conventional heterogeneous catalysis approaches in biofuel production.
The environmental payback period—the time required for the environmental benefits of using the biofuel to offset the environmental costs of its production—is estimated to be 30-40% shorter with SAC-based processes. This accelerated environmental return on investment strengthens the case for widespread adoption of these advanced catalytic technologies in the renewable energy sector.
However, comprehensive sustainability assessment must also consider potential trade-offs. While SAC technologies demonstrate clear advantages in operational environmental metrics, questions remain regarding catalyst longevity, recovery processes, and end-of-life management. These factors will be critical in determining the full lifecycle sustainability profile of SAC-based biofuel production systems as they move toward commercial-scale implementation.
The carbon footprint reduction stems from multiple factors within the SAC biofuel production chain. First, the atomically dispersed metal catalysts require substantially less precious metal content—often 10-100 times less than traditional nanoparticle catalysts—significantly reducing the environmental impact associated with metal mining and refining. Second, the enhanced catalytic activity enables lower operating temperatures and pressures, translating to energy savings of approximately 20-30% across the production process.
Water consumption metrics also demonstrate sustainability improvements, with SAC processes typically requiring 15-25% less water than conventional biofuel production methods. This reduction is particularly significant in regions facing water scarcity challenges, where biofuel production competes with agricultural and municipal water needs.
Land use efficiency represents another critical sustainability dimension. The higher selectivity of single-atom catalysts reduces byproduct formation, increasing the yield of desired biofuels per unit of biomass input. Studies suggest that this improved conversion efficiency could potentially reduce land requirements for biofuel feedstock cultivation by 10-20%, alleviating pressure on agricultural lands and reducing deforestation risks.
Waste generation profiles show marked improvements as well. The precise catalytic action of SACs minimizes side reactions that typically generate waste products requiring disposal or additional processing. Quantitative analyses indicate a 25-40% reduction in waste streams compared to conventional heterogeneous catalysis approaches in biofuel production.
The environmental payback period—the time required for the environmental benefits of using the biofuel to offset the environmental costs of its production—is estimated to be 30-40% shorter with SAC-based processes. This accelerated environmental return on investment strengthens the case for widespread adoption of these advanced catalytic technologies in the renewable energy sector.
However, comprehensive sustainability assessment must also consider potential trade-offs. While SAC technologies demonstrate clear advantages in operational environmental metrics, questions remain regarding catalyst longevity, recovery processes, and end-of-life management. These factors will be critical in determining the full lifecycle sustainability profile of SAC-based biofuel production systems as they move toward commercial-scale implementation.
Scalability and Industrial Implementation Challenges
The scaling of single-atom catalysis (SAC) technology from laboratory to industrial-scale biofuel production presents significant challenges that must be addressed for commercial viability. Current laboratory-scale synthesis methods for single-atom catalysts, including wet chemistry approaches and atomic layer deposition, face substantial barriers when considered for ton-scale production required by the biofuel industry. The precise control of metal dispersion at the atomic level becomes increasingly difficult at larger scales, often resulting in metal aggregation and subsequent catalyst deactivation.
Production costs represent another major hurdle, as the synthesis of SACs typically involves expensive noble metals (Pt, Pd, Ru) and complex preparation procedures requiring specialized equipment and controlled environments. The economic feasibility of SAC-based biofuel production depends heavily on reducing these costs while maintaining catalyst performance and stability. Current estimates suggest that catalyst costs would need to decrease by at least an order of magnitude to compete with conventional biofuel production methods.
Reactor design and process engineering considerations further complicate industrial implementation. SACs often demonstrate optimal performance under specific reaction conditions that may be difficult to maintain in large-scale continuous flow reactors. The development of appropriate reactor technologies that can preserve the unique catalytic properties of SACs while handling industrial throughput volumes remains an ongoing challenge. Heat and mass transfer limitations become particularly problematic at scale, potentially reducing the effectiveness of these atomic-scale catalysts.
Catalyst stability and lifetime under industrial conditions represent critical concerns for commercial deployment. While laboratory studies often demonstrate impressive initial performance, many SACs suffer from deactivation mechanisms including metal leaching, support degradation, and poisoning by feedstock impurities common in biomass-derived substrates. The heterogeneous nature of biomass feedstocks introduces additional variability that can adversely affect catalyst performance and complicate process control strategies.
Quality control and characterization methods suitable for industrial settings present another implementation barrier. Current analytical techniques for verifying atomic dispersion and catalyst structure (HAADF-STEM, XAFS, etc.) are primarily laboratory-based, time-consuming, and not adaptable to online monitoring required for industrial quality assurance. The development of rapid, reliable characterization methods applicable to production environments is essential for successful commercialization of SAC technology in biofuel applications.
Production costs represent another major hurdle, as the synthesis of SACs typically involves expensive noble metals (Pt, Pd, Ru) and complex preparation procedures requiring specialized equipment and controlled environments. The economic feasibility of SAC-based biofuel production depends heavily on reducing these costs while maintaining catalyst performance and stability. Current estimates suggest that catalyst costs would need to decrease by at least an order of magnitude to compete with conventional biofuel production methods.
Reactor design and process engineering considerations further complicate industrial implementation. SACs often demonstrate optimal performance under specific reaction conditions that may be difficult to maintain in large-scale continuous flow reactors. The development of appropriate reactor technologies that can preserve the unique catalytic properties of SACs while handling industrial throughput volumes remains an ongoing challenge. Heat and mass transfer limitations become particularly problematic at scale, potentially reducing the effectiveness of these atomic-scale catalysts.
Catalyst stability and lifetime under industrial conditions represent critical concerns for commercial deployment. While laboratory studies often demonstrate impressive initial performance, many SACs suffer from deactivation mechanisms including metal leaching, support degradation, and poisoning by feedstock impurities common in biomass-derived substrates. The heterogeneous nature of biomass feedstocks introduces additional variability that can adversely affect catalyst performance and complicate process control strategies.
Quality control and characterization methods suitable for industrial settings present another implementation barrier. Current analytical techniques for verifying atomic dispersion and catalyst structure (HAADF-STEM, XAFS, etc.) are primarily laboratory-based, time-consuming, and not adaptable to online monitoring required for industrial quality assurance. The development of rapid, reliable characterization methods applicable to production environments is essential for successful commercialization of SAC technology in biofuel applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!