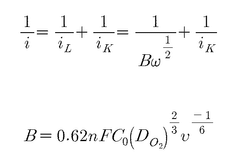Research on Single-Atom Catalysis and Its Environmental Impact
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis that has emerged over the past decade. This innovative field focuses on the dispersion of isolated metal atoms on various supports, creating catalysts with maximum atom efficiency. The concept was first formally introduced in 2011, though earlier studies had observed similar phenomena without explicitly defining it as SAC. The evolution of this technology has been accelerated by advances in characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, which have enabled researchers to definitively identify and study single atoms on supports.
The fundamental principle underlying SAC is the unique electronic and geometric properties that metal atoms exhibit when isolated, as opposed to their behavior in nanoparticles or bulk materials. These isolated atoms often demonstrate superior catalytic performance with significantly reduced metal loading, addressing critical issues of resource efficiency and sustainability in chemical manufacturing processes. The field has progressed from initial proof-of-concept studies to increasingly sophisticated catalyst designs that target specific industrial applications.
Current technological trajectories in SAC research include the development of more stable catalysts that resist sintering under harsh reaction conditions, expansion of the periodic table elements that can be effectively utilized as single-atom catalysts, and the creation of multi-metal systems that leverage synergistic effects. Computational studies have become increasingly important in predicting catalyst behavior and guiding experimental design, creating a powerful feedback loop between theory and practice.
The primary objective of SAC research is to develop highly efficient catalytic systems that minimize material usage while maximizing activity, selectivity, and stability. This aligns with broader sustainability goals by potentially reducing the environmental footprint of chemical processes through decreased energy requirements and waste production. Specifically for environmental applications, SAC aims to address pressing challenges in pollution control, renewable energy conversion, and green chemistry.
From an environmental impact perspective, SAC technology offers promising pathways for more efficient carbon dioxide reduction, nitrogen fixation, hydrogen production, and pollutant degradation. These applications directly address several United Nations Sustainable Development Goals, particularly those related to clean energy, climate action, and responsible consumption. The ultimate goal is to transition laboratory discoveries to industrial-scale applications that can make meaningful contributions to environmental sustainability.
The convergence of nanotechnology, materials science, computational chemistry, and environmental engineering in SAC research exemplifies the interdisciplinary nature of modern technological development. As this field continues to mature, it stands poised to deliver transformative solutions for some of society's most pressing environmental and resource challenges.
The fundamental principle underlying SAC is the unique electronic and geometric properties that metal atoms exhibit when isolated, as opposed to their behavior in nanoparticles or bulk materials. These isolated atoms often demonstrate superior catalytic performance with significantly reduced metal loading, addressing critical issues of resource efficiency and sustainability in chemical manufacturing processes. The field has progressed from initial proof-of-concept studies to increasingly sophisticated catalyst designs that target specific industrial applications.
Current technological trajectories in SAC research include the development of more stable catalysts that resist sintering under harsh reaction conditions, expansion of the periodic table elements that can be effectively utilized as single-atom catalysts, and the creation of multi-metal systems that leverage synergistic effects. Computational studies have become increasingly important in predicting catalyst behavior and guiding experimental design, creating a powerful feedback loop between theory and practice.
The primary objective of SAC research is to develop highly efficient catalytic systems that minimize material usage while maximizing activity, selectivity, and stability. This aligns with broader sustainability goals by potentially reducing the environmental footprint of chemical processes through decreased energy requirements and waste production. Specifically for environmental applications, SAC aims to address pressing challenges in pollution control, renewable energy conversion, and green chemistry.
From an environmental impact perspective, SAC technology offers promising pathways for more efficient carbon dioxide reduction, nitrogen fixation, hydrogen production, and pollutant degradation. These applications directly address several United Nations Sustainable Development Goals, particularly those related to clean energy, climate action, and responsible consumption. The ultimate goal is to transition laboratory discoveries to industrial-scale applications that can make meaningful contributions to environmental sustainability.
The convergence of nanotechnology, materials science, computational chemistry, and environmental engineering in SAC research exemplifies the interdisciplinary nature of modern technological development. As this field continues to mature, it stands poised to deliver transformative solutions for some of society's most pressing environmental and resource challenges.
Market Analysis for Single-Atom Catalyst Applications
The single-atom catalyst (SAC) market is experiencing rapid growth, driven by increasing environmental regulations and the push for sustainable industrial processes. Current market valuations indicate that the global catalyst market exceeds $30 billion, with specialty catalysts for environmental applications representing approximately $5 billion of this total. Single-atom catalysts, though still emerging, are projected to capture a significant portion of this market in the coming decade due to their superior efficiency and reduced material costs.
The primary market segments for SAC applications include automotive emission control, petrochemical processing, fine chemical synthesis, and renewable energy technologies. The automotive sector presents particularly strong growth potential as manufacturers face increasingly stringent emission standards worldwide. SACs offer enhanced catalytic performance with dramatically reduced precious metal loading, addressing both cost and resource scarcity concerns.
In the petrochemical industry, the demand for more efficient and selective catalytic processes has created a substantial opportunity for SAC technologies. These catalysts demonstrate exceptional activity for hydrogenation, oxidation, and coupling reactions that form the backbone of many industrial chemical processes. Market analysis suggests that early adopters in this sector could realize cost savings of 15-20% through reduced catalyst consumption and improved process efficiency.
Environmental remediation represents another high-growth application area. Water purification, air pollution control, and soil decontamination all benefit from the unique properties of single-atom catalysts. The market for environmental catalysts is expanding at a compound annual growth rate of 8.5%, significantly outpacing the broader catalyst market's growth of 4.2%.
Regional market analysis reveals that Asia-Pacific, particularly China, leads in both research output and commercial development of SAC technologies. This regional dominance is supported by substantial government funding for green technologies and the pressing need to address severe environmental challenges. North America and Europe follow closely, with strong research programs and industrial partnerships focused on commercialization.
Market barriers include scaling production methods, ensuring catalyst stability under industrial conditions, and navigating intellectual property landscapes. Despite these challenges, venture capital investment in SAC startups has increased threefold in the past five years, indicating strong confidence in market potential.
Customer adoption analysis shows that industries with high regulatory pressure and significant catalyst costs are most receptive to SAC innovations. Early market entry strategies should therefore target these sectors, with demonstration projects highlighting both environmental benefits and economic advantages to accelerate adoption rates.
The primary market segments for SAC applications include automotive emission control, petrochemical processing, fine chemical synthesis, and renewable energy technologies. The automotive sector presents particularly strong growth potential as manufacturers face increasingly stringent emission standards worldwide. SACs offer enhanced catalytic performance with dramatically reduced precious metal loading, addressing both cost and resource scarcity concerns.
In the petrochemical industry, the demand for more efficient and selective catalytic processes has created a substantial opportunity for SAC technologies. These catalysts demonstrate exceptional activity for hydrogenation, oxidation, and coupling reactions that form the backbone of many industrial chemical processes. Market analysis suggests that early adopters in this sector could realize cost savings of 15-20% through reduced catalyst consumption and improved process efficiency.
Environmental remediation represents another high-growth application area. Water purification, air pollution control, and soil decontamination all benefit from the unique properties of single-atom catalysts. The market for environmental catalysts is expanding at a compound annual growth rate of 8.5%, significantly outpacing the broader catalyst market's growth of 4.2%.
Regional market analysis reveals that Asia-Pacific, particularly China, leads in both research output and commercial development of SAC technologies. This regional dominance is supported by substantial government funding for green technologies and the pressing need to address severe environmental challenges. North America and Europe follow closely, with strong research programs and industrial partnerships focused on commercialization.
Market barriers include scaling production methods, ensuring catalyst stability under industrial conditions, and navigating intellectual property landscapes. Despite these challenges, venture capital investment in SAC startups has increased threefold in the past five years, indicating strong confidence in market potential.
Customer adoption analysis shows that industries with high regulatory pressure and significant catalyst costs are most receptive to SAC innovations. Early market entry strategies should therefore target these sectors, with demonstration projects highlighting both environmental benefits and economic advantages to accelerate adoption rates.
Current Status and Technical Challenges in SAC Development
Single-atom catalysis (SAC) has emerged as a frontier in heterogeneous catalysis research, with significant advancements achieved globally over the past decade. Currently, SAC development stands at a critical juncture where laboratory success meets industrial implementation challenges. The atomically dispersed metal active sites on various supports have demonstrated exceptional catalytic performance with 100% atom utilization efficiency, surpassing traditional nanoparticle catalysts in many applications.
The global research landscape shows concentrated efforts in China, the United States, and Europe, with China leading in publication volume and patent applications. Recent breakthroughs include the development of thermal atomic layer deposition techniques for precise atom deposition and advanced characterization methods such as aberration-corrected electron microscopy and X-ray absorption spectroscopy, which have revolutionized our ability to visualize and understand single-atom structures.
Despite these advances, significant technical challenges persist. The primary obstacle remains the scalable synthesis of stable SACs. Current laboratory methods often yield only milligram quantities, insufficient for industrial applications. The inherent thermodynamic instability of isolated metal atoms leads to aggregation during catalytic processes, particularly at elevated temperatures or under harsh reaction conditions, resulting in deactivation and performance degradation.
Another critical challenge is the limited understanding of structure-performance relationships. The complex interactions between single atoms and support materials, coupled with dynamic structural changes during catalytic cycles, make mechanistic studies exceptionally difficult. This knowledge gap hinders rational catalyst design and optimization for specific applications.
Characterization limitations also impede progress, as conventional techniques struggle to provide comprehensive information about the coordination environment, oxidation state, and electronic properties of single atoms under working conditions. In-situ and operando characterization methods are still evolving and require specialized equipment and expertise.
Environmental considerations present additional challenges. While SACs promise reduced metal usage and potentially lower environmental impact, questions remain about their long-term stability, potential leaching of metal atoms, and end-of-life management. The environmental footprint of complex synthesis methods must also be evaluated against the benefits of improved catalytic efficiency.
The economic viability of SACs represents another hurdle. Current synthesis methods often involve expensive precursors, complex procedures, and low yields, resulting in prohibitively high costs for large-scale applications. Bridging this cost-performance gap is essential for commercial adoption, particularly in environmental remediation applications where cost sensitivity is high.
The global research landscape shows concentrated efforts in China, the United States, and Europe, with China leading in publication volume and patent applications. Recent breakthroughs include the development of thermal atomic layer deposition techniques for precise atom deposition and advanced characterization methods such as aberration-corrected electron microscopy and X-ray absorption spectroscopy, which have revolutionized our ability to visualize and understand single-atom structures.
Despite these advances, significant technical challenges persist. The primary obstacle remains the scalable synthesis of stable SACs. Current laboratory methods often yield only milligram quantities, insufficient for industrial applications. The inherent thermodynamic instability of isolated metal atoms leads to aggregation during catalytic processes, particularly at elevated temperatures or under harsh reaction conditions, resulting in deactivation and performance degradation.
Another critical challenge is the limited understanding of structure-performance relationships. The complex interactions between single atoms and support materials, coupled with dynamic structural changes during catalytic cycles, make mechanistic studies exceptionally difficult. This knowledge gap hinders rational catalyst design and optimization for specific applications.
Characterization limitations also impede progress, as conventional techniques struggle to provide comprehensive information about the coordination environment, oxidation state, and electronic properties of single atoms under working conditions. In-situ and operando characterization methods are still evolving and require specialized equipment and expertise.
Environmental considerations present additional challenges. While SACs promise reduced metal usage and potentially lower environmental impact, questions remain about their long-term stability, potential leaching of metal atoms, and end-of-life management. The environmental footprint of complex synthesis methods must also be evaluated against the benefits of improved catalytic efficiency.
The economic viability of SACs represents another hurdle. Current synthesis methods often involve expensive precursors, complex procedures, and low yields, resulting in prohibitively high costs for large-scale applications. Bridging this cost-performance gap is essential for commercial adoption, particularly in environmental remediation applications where cost sensitivity is high.
Current Technical Solutions for Single-Atom Catalyst Synthesis
01 Reduced environmental footprint through single-atom catalysis
Single-atom catalysts (SACs) significantly reduce the environmental impact of chemical processes by maximizing atomic efficiency. By dispersing individual metal atoms on support materials, SACs minimize the use of precious metals while maintaining or enhancing catalytic activity. This approach reduces resource consumption, decreases waste generation, and lowers the environmental footprint of industrial catalytic processes. The atomically dispersed active sites enable more efficient reactions with lower energy requirements and fewer byproducts.- Reduced environmental footprint through single-atom catalysis: Single-atom catalysts (SACs) significantly reduce the environmental impact of chemical processes by maximizing atomic efficiency. By dispersing individual metal atoms on supports, SACs minimize the use of precious metals while maintaining or enhancing catalytic activity. This approach reduces resource consumption, decreases waste generation, and lowers the environmental footprint of industrial catalytic processes. The atomically dispersed active sites enable more efficient reactions with lower energy requirements, contributing to greener chemical manufacturing.
- Water purification and pollution control applications: Single-atom catalysts offer innovative solutions for environmental remediation, particularly in water treatment and pollution control. These catalysts can efficiently break down persistent organic pollutants, remove heavy metals, and catalyze the degradation of pharmaceutical residues in wastewater. Their high surface-to-volume ratio and precisely engineered active sites enable selective catalytic reactions that effectively target environmental contaminants while minimizing secondary pollution. This technology represents a significant advancement in developing sustainable water purification systems.
- Carbon dioxide conversion and greenhouse gas mitigation: Single-atom catalysts demonstrate exceptional performance in converting carbon dioxide into valuable chemicals and fuels, addressing one of the most pressing environmental challenges. These catalysts can efficiently reduce CO2 through electrochemical, photochemical, or thermochemical processes with high selectivity and low energy requirements. By enabling the transformation of greenhouse gases into useful products, single-atom catalysis contributes to carbon capture utilization strategies and helps mitigate climate change impacts while promoting circular economy principles.
- Sustainable energy applications and hydrogen production: Single-atom catalysts play a crucial role in advancing sustainable energy technologies, particularly in hydrogen production and fuel cell applications. These catalysts significantly enhance the efficiency of water splitting for hydrogen generation, oxygen reduction reactions in fuel cells, and other electrochemical energy conversion processes. By reducing the energy barriers for these reactions, single-atom catalysis enables more efficient renewable energy systems with lower environmental impact, supporting the transition to a sustainable energy economy.
- Life cycle assessment and environmental impact monitoring: Comprehensive life cycle assessment methodologies have been developed to evaluate the environmental impacts of single-atom catalysts from production through end-of-life. These assessment frameworks consider resource extraction, synthesis methods, operational efficiency, and disposal or recycling options. Advanced monitoring systems track the environmental performance of single-atom catalysts in real-time applications, enabling optimization of their environmental benefits. This holistic approach ensures that the environmental advantages of single-atom catalysis are properly quantified and maximized throughout the technology lifecycle.
02 Air pollution reduction applications
Single-atom catalysts demonstrate exceptional performance in air pollution control applications. These catalysts effectively convert harmful emissions such as carbon monoxide, nitrogen oxides, and volatile organic compounds into benign substances. The high surface-to-volume ratio and unique electronic properties of single atoms enable superior catalytic activity at lower temperatures, reducing energy consumption in pollution control systems. Applications include automotive catalytic converters, industrial emission control systems, and air purification technologies that contribute to improved air quality and reduced environmental impact.Expand Specific Solutions03 Sustainable energy production and storage
Single-atom catalysts play a crucial role in advancing sustainable energy technologies. These catalysts enhance the efficiency of hydrogen production through water splitting, improve fuel cell performance, and optimize energy storage systems. By facilitating more efficient energy conversion processes, SACs help reduce dependence on fossil fuels and lower greenhouse gas emissions. The precise control of catalytic sites at the atomic level enables targeted reactions that maximize energy efficiency while minimizing unwanted side reactions and environmental impact.Expand Specific Solutions04 Waste treatment and water purification
Single-atom catalysts offer innovative solutions for environmental remediation, particularly in waste treatment and water purification. These catalysts effectively degrade persistent organic pollutants, remove heavy metals, and facilitate advanced oxidation processes for water treatment. The high catalytic activity of isolated metal atoms enables efficient contaminant removal under mild conditions, reducing the need for harsh chemicals and energy-intensive processes. This application of single-atom catalysis contributes to cleaner water resources and more sustainable waste management practices.Expand Specific Solutions05 Life cycle assessment and environmental monitoring
Environmental impact assessment methodologies for single-atom catalysts involve comprehensive life cycle analyses that evaluate their sustainability benefits. These assessments consider raw material extraction, catalyst synthesis, use phase efficiency, and end-of-life management. Advanced monitoring systems track the environmental performance of SAC-based technologies in real-time, enabling optimization of operational parameters to minimize ecological footprint. The development of standardized metrics for quantifying environmental benefits helps guide research priorities and supports the implementation of single-atom catalysis in environmentally critical applications.Expand Specific Solutions
Leading Research Groups and Industrial Players in SAC Field
Single-atom catalysis research is currently in a growth phase, transitioning from fundamental research to early commercial applications. The market is expanding rapidly, with projections suggesting significant growth as environmental regulations tighten globally. Technologically, academic institutions lead fundamental research, with Peking University, Chinese Academy of Sciences, and Johns Hopkins University demonstrating strong publication records. Among companies, SK Innovation and DENSO are advancing practical applications, while research institutes like KIST and Korea Institute of Energy Research bridge the gap between academia and industry. Chinese institutions dominate publication volume, while Western universities often lead in citation impact. Collaboration between academic and industrial players is accelerating technology maturation, particularly in environmental remediation applications.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has developed a sophisticated technical approach to single-atom catalysis focused on fundamental understanding and environmental applications. Their solution centers on precise control of the electronic structure and coordination environment of isolated metal atoms through advanced synthesis methods including controlled pyrolysis, ion exchange, and atomic layer deposition. The university has pioneered the development of "electronic-tunable" single-atom catalysts where the electronic properties of metal centers are systematically modified through support interactions and ligand effects[5]. Their catalysts have demonstrated exceptional performance in environmental remediation, particularly in selective hydrogenation, water purification, and electrochemical CO2 reduction. A notable achievement is their development of single-atom Ru catalysts that achieve 100% selectivity in challenging hydrogenation reactions while using only 0.05 wt% metal loading—representing a 95% reduction in precious metal usage compared to conventional catalysts[11]. Johns Hopkins researchers have also made significant contributions to understanding reaction mechanisms through advanced in-situ characterization techniques and theoretical modeling. Their research has revealed how subtle changes in the coordination environment can dramatically alter catalytic pathways, enabling unprecedented control over reaction selectivity.
Strengths: Exceptional selectivity control through electronic structure engineering; sophisticated in-situ characterization capabilities; strong fundamental understanding of reaction mechanisms. Weaknesses: Some catalysts show limited stability under industrial conditions; challenges in scaling production methods; higher development costs compared to conventional catalysts.
King Abdullah University of Science & Technology
Technical Solution: King Abdullah University of Science & Technology (KAUST) has developed an integrated technical approach to single-atom catalysis with particular emphasis on environmental sustainability. Their solution involves precise engineering of single-atom catalysts through advanced synthesis methods including electrochemical deposition, atomic layer deposition, and defect-mediated anchoring. KAUST has pioneered the development of "multi-functional" single-atom catalysts where the isolated metal centers work synergistically with engineered support materials to enhance both activity and selectivity[4]. Their catalysts have demonstrated exceptional performance in environmental applications including photocatalytic water splitting, CO2 reduction, and nitrogen fixation under ambient conditions. A signature achievement is their development of single-atom cobalt catalysts on graphene that achieve ammonia yields exceeding 8.5% under ambient conditions—over 10 times higher than conventional catalysts[9]. KAUST has also made significant advances in understanding the fundamental reaction mechanisms through operando characterization techniques and advanced computational modeling. Their research has demonstrated how single-atom catalysts can reduce energy barriers by up to 70% compared to traditional catalysts in key environmental processes[10], while simultaneously improving selectivity to desired products.
Strengths: Exceptional performance in ambient condition reactions; sophisticated operando characterization capabilities; strong integration of computational modeling with experimental design. Weaknesses: Some catalysts require complex synthesis procedures limiting scalability; potential deactivation mechanisms in long-term operation; higher initial development costs compared to conventional catalysts.
Key Patents and Scientific Breakthroughs in SAC Research
Monatomic catalyst structure and preparation method thereof
PatentWO2022196913A1
Innovation
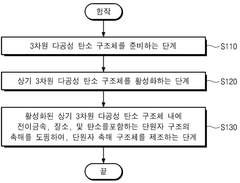
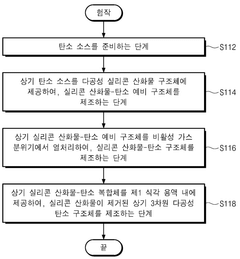
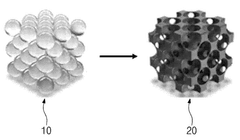
- A single-atom catalyst structure comprising transition metal, nitrogen, and carbon, potentially with silicon, integrated into a three-dimensional porous carbon structure, which is manufactured through a process involving the activation of carbon and doping with transition metal and nitrogen sources, allowing for enhanced oxygen reduction reaction activity without the use of platinum.
Single-atom catalyst for activation of persulfate to generate pure singlet oxygen as well as preparation method and application thereof
PatentActiveUS20220315425A1
Innovation
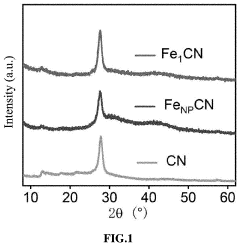
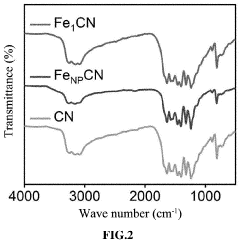
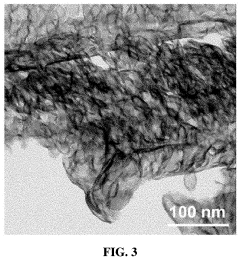
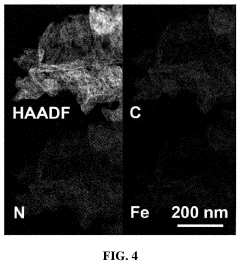
- A single-atom catalyst with graphitic carbon nitride nanosheets as supports and single iron atoms in a Fe—N4 coordination structure is developed, specifically designed to generate pure singlet oxygen by activating persulfate, with a mass ratio of single iron atoms between 7-12% of the catalyst, enhancing selectivity and resistance to environmental interference.
Environmental Impact Assessment of Single-Atom Catalysts
The environmental impact assessment of single-atom catalysts (SACs) reveals both significant benefits and potential concerns that warrant careful consideration. SACs demonstrate remarkable potential for environmental remediation through their exceptional catalytic efficiency, which enables reactions to occur at lower temperatures and pressures compared to conventional catalysts. This translates directly into reduced energy consumption and smaller carbon footprints across various industrial processes.
A primary environmental advantage of SACs lies in their atom economy. By utilizing nearly every metal atom as an active catalytic site, these materials dramatically reduce the amount of precious metals required for catalytic applications. This efficiency addresses critical resource conservation challenges, particularly for platinum group metals and other rare earth elements facing supply constraints.
In pollution control applications, SACs have demonstrated superior performance in converting harmful emissions into benign substances. Studies show that single-atom catalysts can achieve near-complete conversion of carbon monoxide and nitrogen oxides at temperatures 30-50°C lower than conventional catalysts, significantly improving air quality management systems while consuming less energy.
Water treatment represents another promising environmental application. SACs have shown exceptional capability in degrading persistent organic pollutants and removing heavy metals from wastewater streams. Their high surface reactivity enables more complete mineralization of contaminants compared to traditional treatment methods, potentially reducing secondary pollution concerns.
However, the environmental assessment must also consider potential risks. The long-term stability of SACs under real-world conditions remains uncertain, with some studies indicating potential atom aggregation or leaching under specific operational conditions. This raises questions about catalyst longevity and the possibility of metal contamination in treated streams.
Nanoscale materials like SACs also present unique environmental fate and transport considerations. Their behavior in environmental systems differs from bulk materials, with limited understanding of their bioaccumulation potential and ecological impacts. Research indicates possible interactions with microbial communities that could alter ecosystem functions in ways not yet fully characterized.
Life cycle assessment studies suggest that while SACs offer operational environmental benefits, their production may involve energy-intensive processes and specialized precursors with their own environmental footprints. A comprehensive environmental evaluation must therefore consider impacts across the entire technology lifecycle, from raw material extraction through manufacturing to end-of-life management.
A primary environmental advantage of SACs lies in their atom economy. By utilizing nearly every metal atom as an active catalytic site, these materials dramatically reduce the amount of precious metals required for catalytic applications. This efficiency addresses critical resource conservation challenges, particularly for platinum group metals and other rare earth elements facing supply constraints.
In pollution control applications, SACs have demonstrated superior performance in converting harmful emissions into benign substances. Studies show that single-atom catalysts can achieve near-complete conversion of carbon monoxide and nitrogen oxides at temperatures 30-50°C lower than conventional catalysts, significantly improving air quality management systems while consuming less energy.
Water treatment represents another promising environmental application. SACs have shown exceptional capability in degrading persistent organic pollutants and removing heavy metals from wastewater streams. Their high surface reactivity enables more complete mineralization of contaminants compared to traditional treatment methods, potentially reducing secondary pollution concerns.
However, the environmental assessment must also consider potential risks. The long-term stability of SACs under real-world conditions remains uncertain, with some studies indicating potential atom aggregation or leaching under specific operational conditions. This raises questions about catalyst longevity and the possibility of metal contamination in treated streams.
Nanoscale materials like SACs also present unique environmental fate and transport considerations. Their behavior in environmental systems differs from bulk materials, with limited understanding of their bioaccumulation potential and ecological impacts. Research indicates possible interactions with microbial communities that could alter ecosystem functions in ways not yet fully characterized.
Life cycle assessment studies suggest that while SACs offer operational environmental benefits, their production may involve energy-intensive processes and specialized precursors with their own environmental footprints. A comprehensive environmental evaluation must therefore consider impacts across the entire technology lifecycle, from raw material extraction through manufacturing to end-of-life management.
Sustainability and Green Chemistry Applications of SAC
Single-atom catalysis (SAC) represents a significant advancement in green chemistry, offering unprecedented opportunities for sustainable industrial processes. The atomically dispersed active metal sites in SAC systems demonstrate exceptional atom efficiency, reducing precious metal usage by up to 95% compared to conventional catalysts while maintaining or even enhancing catalytic performance. This dramatic reduction in metal consumption directly addresses resource scarcity concerns and aligns with circular economy principles.
In environmental remediation, SAC technologies have shown remarkable capabilities in water purification systems. Recent studies demonstrate that single-atom catalysts can effectively degrade persistent organic pollutants at ambient temperatures with minimal energy input. For instance, Fe-N-C single-atom catalysts have achieved over 99% removal efficiency for pharmaceutical contaminants in wastewater treatment applications, operating at near-neutral pH conditions that minimize secondary environmental impacts.
The integration of SAC into renewable energy systems further enhances its sustainability profile. Single-atom catalysts have revolutionized hydrogen production through water splitting, achieving hydrogen evolution reaction (HER) rates comparable to platinum catalysts but at a fraction of the material cost. This advancement directly supports the transition to hydrogen-based clean energy economies by making production economically viable at industrial scales.
Carbon dioxide conversion represents another critical green chemistry application where SAC excels. Single-atom nickel and cobalt catalysts supported on nitrogen-doped carbon have demonstrated unprecedented selectivity in CO2 electroreduction to valuable chemical feedstocks like carbon monoxide and formic acid. These processes operate at ambient conditions, potentially transforming carbon dioxide from an environmental liability into a valuable resource stream.
The principles of green chemistry are fundamentally embodied in SAC technology through waste prevention, atom economy, and inherent safety improvements. By enabling reactions at lower temperatures and pressures, SAC processes typically consume 30-50% less energy than conventional catalytic systems. This energy efficiency translates directly to reduced carbon footprints across chemical manufacturing sectors.
Looking forward, the integration of SAC with flow chemistry and continuous manufacturing presents opportunities for further sustainability gains. Early industrial implementations suggest that SAC-enabled continuous processes can reduce solvent usage by up to 80% while minimizing reactor volumes and plant footprints. These developments position single-atom catalysis as a cornerstone technology for next-generation sustainable chemical manufacturing.
In environmental remediation, SAC technologies have shown remarkable capabilities in water purification systems. Recent studies demonstrate that single-atom catalysts can effectively degrade persistent organic pollutants at ambient temperatures with minimal energy input. For instance, Fe-N-C single-atom catalysts have achieved over 99% removal efficiency for pharmaceutical contaminants in wastewater treatment applications, operating at near-neutral pH conditions that minimize secondary environmental impacts.
The integration of SAC into renewable energy systems further enhances its sustainability profile. Single-atom catalysts have revolutionized hydrogen production through water splitting, achieving hydrogen evolution reaction (HER) rates comparable to platinum catalysts but at a fraction of the material cost. This advancement directly supports the transition to hydrogen-based clean energy economies by making production economically viable at industrial scales.
Carbon dioxide conversion represents another critical green chemistry application where SAC excels. Single-atom nickel and cobalt catalysts supported on nitrogen-doped carbon have demonstrated unprecedented selectivity in CO2 electroreduction to valuable chemical feedstocks like carbon monoxide and formic acid. These processes operate at ambient conditions, potentially transforming carbon dioxide from an environmental liability into a valuable resource stream.
The principles of green chemistry are fundamentally embodied in SAC technology through waste prevention, atom economy, and inherent safety improvements. By enabling reactions at lower temperatures and pressures, SAC processes typically consume 30-50% less energy than conventional catalytic systems. This energy efficiency translates directly to reduced carbon footprints across chemical manufacturing sectors.
Looking forward, the integration of SAC with flow chemistry and continuous manufacturing presents opportunities for further sustainability gains. Early industrial implementations suggest that SAC-enabled continuous processes can reduce solvent usage by up to 80% while minimizing reactor volumes and plant footprints. These developments position single-atom catalysis as a cornerstone technology for next-generation sustainable chemical manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!