Single-Atom Catalysis in the Chemistry of Carbon Utilization
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis that has emerged over the past decade. This innovative approach involves dispersing individual metal atoms onto suitable supports, maximizing atomic efficiency while exhibiting unique catalytic properties distinct from traditional nanoparticle catalysts. The concept was first formally introduced in 2011, though earlier studies had observed similar phenomena without explicitly defining the field.
The evolution of SAC technology has been driven by advances in synthetic methodologies, characterization techniques, and computational modeling. Early developments focused primarily on noble metals (Pt, Pd, Au), while recent years have witnessed expansion to non-noble metals (Fe, Co, Ni) and even non-metals, significantly broadening the application scope while reducing costs.
Carbon utilization represents one of the most pressing challenges of our time, encompassing CO2 conversion, methane activation, and biomass valorization. Traditional catalytic processes for these transformations often suffer from low efficiency, poor selectivity, and high energy requirements. The intersection of SAC with carbon chemistry offers unprecedented opportunities to address these limitations through precise atomic-level control of catalytic sites.
The primary technical objectives in this field include developing stable single-atom catalysts capable of activating carbon-containing molecules under mild conditions, understanding the fundamental mechanisms governing their exceptional performance, and scaling production methods for industrial implementation. Particular emphasis is placed on designing catalysts that can selectively break C-H bonds, form C-C bonds, or reduce CO2 with minimal energy input.
Recent breakthroughs have demonstrated SACs' remarkable ability to catalyze CO2 hydrogenation to value-added chemicals, methane partial oxidation to methanol, and biomass conversion to platform chemicals with significantly improved efficiency compared to conventional catalysts. These advances highlight the transformative potential of SACs in establishing sustainable carbon cycles.
The technical trajectory indicates a shift from empirical discovery toward rational design, enabled by advanced characterization techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy, coupled with density functional theory calculations. This progression has facilitated deeper understanding of structure-performance relationships and active site identification.
Looking forward, the field aims to develop multi-functional SACs capable of cascade reactions, improve long-term stability under industrial conditions, and establish scalable, economically viable synthesis protocols. The ultimate goal remains harnessing the unique properties of atomically dispersed metal centers to revolutionize carbon utilization pathways, contributing to circular carbon economy and climate change mitigation efforts.
The evolution of SAC technology has been driven by advances in synthetic methodologies, characterization techniques, and computational modeling. Early developments focused primarily on noble metals (Pt, Pd, Au), while recent years have witnessed expansion to non-noble metals (Fe, Co, Ni) and even non-metals, significantly broadening the application scope while reducing costs.
Carbon utilization represents one of the most pressing challenges of our time, encompassing CO2 conversion, methane activation, and biomass valorization. Traditional catalytic processes for these transformations often suffer from low efficiency, poor selectivity, and high energy requirements. The intersection of SAC with carbon chemistry offers unprecedented opportunities to address these limitations through precise atomic-level control of catalytic sites.
The primary technical objectives in this field include developing stable single-atom catalysts capable of activating carbon-containing molecules under mild conditions, understanding the fundamental mechanisms governing their exceptional performance, and scaling production methods for industrial implementation. Particular emphasis is placed on designing catalysts that can selectively break C-H bonds, form C-C bonds, or reduce CO2 with minimal energy input.
Recent breakthroughs have demonstrated SACs' remarkable ability to catalyze CO2 hydrogenation to value-added chemicals, methane partial oxidation to methanol, and biomass conversion to platform chemicals with significantly improved efficiency compared to conventional catalysts. These advances highlight the transformative potential of SACs in establishing sustainable carbon cycles.
The technical trajectory indicates a shift from empirical discovery toward rational design, enabled by advanced characterization techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy, coupled with density functional theory calculations. This progression has facilitated deeper understanding of structure-performance relationships and active site identification.
Looking forward, the field aims to develop multi-functional SACs capable of cascade reactions, improve long-term stability under industrial conditions, and establish scalable, economically viable synthesis protocols. The ultimate goal remains harnessing the unique properties of atomically dispersed metal centers to revolutionize carbon utilization pathways, contributing to circular carbon economy and climate change mitigation efforts.
Carbon Utilization Market Demand Analysis
The global carbon utilization market is experiencing significant growth driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. Current market valuations indicate that carbon capture, utilization, and storage (CCUS) technologies represent a rapidly expanding sector, with projections suggesting the market could reach $70 billion by 2030. This growth trajectory is supported by substantial investments from both public and private sectors, with governments worldwide allocating billions in funding for carbon management initiatives.
Single-atom catalysis (SAC) is emerging as a critical technology within this market landscape, offering unprecedented efficiency in carbon conversion processes. The demand for SAC technologies is particularly strong in regions with ambitious carbon neutrality targets, including the European Union, North America, and increasingly, China and Japan. These regions are implementing stringent carbon pricing mechanisms and emissions trading systems that create economic incentives for carbon utilization technologies.
Industrial sectors represent the primary demand drivers for carbon utilization technologies. The chemical manufacturing industry shows particular interest in SAC for carbon utilization, as it enables the production of value-added chemicals from CO2 rather than fossil resources. Market research indicates that approximately 25% of the chemical industry's carbon footprint could potentially be addressed through advanced catalytic carbon conversion technologies.
Energy production represents another significant market segment, with growing demand for technologies that can convert captured carbon into synthetic fuels. This application aligns with the transportation sector's need for low-carbon fuel alternatives, creating a substantial potential market for SAC-enabled carbon utilization processes.
Construction materials manufacturing has emerged as an unexpected growth area for carbon utilization, with technologies that incorporate CO2 into concrete and other building materials gaining commercial traction. This sector alone could potentially utilize millions of tons of CO2 annually, representing a multi-billion dollar opportunity for carbon conversion technologies.
Market analysis reveals that end-users are increasingly willing to pay premium prices for products manufactured using carbon utilization technologies, particularly when these products offer performance advantages or contribute to sustainability goals. This consumer preference is creating pull-through demand that further accelerates market growth.
The competitive landscape for carbon utilization technologies is becoming increasingly crowded, with both established chemical companies and specialized startups developing proprietary SAC solutions. Venture capital funding in this space has grown substantially, with investment in carbon utilization startups exceeding $1.5 billion in recent years, indicating strong market confidence in the commercial potential of these technologies.
Single-atom catalysis (SAC) is emerging as a critical technology within this market landscape, offering unprecedented efficiency in carbon conversion processes. The demand for SAC technologies is particularly strong in regions with ambitious carbon neutrality targets, including the European Union, North America, and increasingly, China and Japan. These regions are implementing stringent carbon pricing mechanisms and emissions trading systems that create economic incentives for carbon utilization technologies.
Industrial sectors represent the primary demand drivers for carbon utilization technologies. The chemical manufacturing industry shows particular interest in SAC for carbon utilization, as it enables the production of value-added chemicals from CO2 rather than fossil resources. Market research indicates that approximately 25% of the chemical industry's carbon footprint could potentially be addressed through advanced catalytic carbon conversion technologies.
Energy production represents another significant market segment, with growing demand for technologies that can convert captured carbon into synthetic fuels. This application aligns with the transportation sector's need for low-carbon fuel alternatives, creating a substantial potential market for SAC-enabled carbon utilization processes.
Construction materials manufacturing has emerged as an unexpected growth area for carbon utilization, with technologies that incorporate CO2 into concrete and other building materials gaining commercial traction. This sector alone could potentially utilize millions of tons of CO2 annually, representing a multi-billion dollar opportunity for carbon conversion technologies.
Market analysis reveals that end-users are increasingly willing to pay premium prices for products manufactured using carbon utilization technologies, particularly when these products offer performance advantages or contribute to sustainability goals. This consumer preference is creating pull-through demand that further accelerates market growth.
The competitive landscape for carbon utilization technologies is becoming increasingly crowded, with both established chemical companies and specialized startups developing proprietary SAC solutions. Venture capital funding in this space has grown substantially, with investment in carbon utilization startups exceeding $1.5 billion in recent years, indicating strong market confidence in the commercial potential of these technologies.
Current Status and Challenges in Single-Atom Catalysis
Single-atom catalysis (SAC) represents a frontier in heterogeneous catalysis research, with significant implications for carbon utilization chemistry. Currently, the field has achieved remarkable progress in catalyst synthesis methodologies, with controlled atom dispersion techniques enabling precise placement of isolated metal atoms on various supports. Atomic layer deposition, wet chemistry methods, and high-temperature atom trapping have emerged as predominant synthesis routes, each with distinct advantages for specific applications.
The characterization of single-atom catalysts has advanced substantially through sophisticated techniques including aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ/operando methods. These tools have enabled unprecedented visualization and understanding of active sites during catalytic processes involving carbon-containing molecules. For carbon utilization specifically, single-atom catalysts have demonstrated exceptional performance in CO2 reduction, methane activation, and biomass conversion.
Despite these advances, significant challenges persist in the field. Stability remains a primary concern, as isolated metal atoms tend to aggregate under reaction conditions, particularly at elevated temperatures or in reducing environments common in carbon conversion processes. This sintering phenomenon compromises the single-atom nature of catalysts and diminishes their unique catalytic properties. The development of robust anchoring strategies that maintain dispersion under harsh reaction conditions represents a critical research direction.
Another major challenge involves the scalable production of single-atom catalysts. While laboratory-scale synthesis has been well-established, industrial-scale manufacturing faces substantial hurdles in maintaining uniform atom dispersion and controlling metal loading. This scaling challenge directly impacts the practical application of SACs in large-scale carbon utilization processes such as industrial CO2 conversion or biomass valorization.
The mechanistic understanding of single-atom catalysis in carbon chemistry remains incomplete. The complex interplay between the metal center, support material, and carbon-containing substrates creates reaction pathways that differ significantly from traditional heterogeneous catalysts. Elucidating these mechanisms requires advanced computational modeling coupled with cutting-edge spectroscopic techniques, an area where significant knowledge gaps persist.
Geographically, research in single-atom catalysis shows distinct patterns, with China, the United States, and Europe leading in publication output and patent filings. Chinese institutions have particularly focused on energy-related applications of SACs, while US research often emphasizes fundamental mechanistic studies. This distribution influences the direction of technological development and potential commercialization pathways for carbon utilization applications.
The characterization of single-atom catalysts has advanced substantially through sophisticated techniques including aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ/operando methods. These tools have enabled unprecedented visualization and understanding of active sites during catalytic processes involving carbon-containing molecules. For carbon utilization specifically, single-atom catalysts have demonstrated exceptional performance in CO2 reduction, methane activation, and biomass conversion.
Despite these advances, significant challenges persist in the field. Stability remains a primary concern, as isolated metal atoms tend to aggregate under reaction conditions, particularly at elevated temperatures or in reducing environments common in carbon conversion processes. This sintering phenomenon compromises the single-atom nature of catalysts and diminishes their unique catalytic properties. The development of robust anchoring strategies that maintain dispersion under harsh reaction conditions represents a critical research direction.
Another major challenge involves the scalable production of single-atom catalysts. While laboratory-scale synthesis has been well-established, industrial-scale manufacturing faces substantial hurdles in maintaining uniform atom dispersion and controlling metal loading. This scaling challenge directly impacts the practical application of SACs in large-scale carbon utilization processes such as industrial CO2 conversion or biomass valorization.
The mechanistic understanding of single-atom catalysis in carbon chemistry remains incomplete. The complex interplay between the metal center, support material, and carbon-containing substrates creates reaction pathways that differ significantly from traditional heterogeneous catalysts. Elucidating these mechanisms requires advanced computational modeling coupled with cutting-edge spectroscopic techniques, an area where significant knowledge gaps persist.
Geographically, research in single-atom catalysis shows distinct patterns, with China, the United States, and Europe leading in publication output and patent filings. Chinese institutions have particularly focused on energy-related applications of SACs, while US research often emphasizes fundamental mechanistic studies. This distribution influences the direction of technological development and potential commercialization pathways for carbon utilization applications.
Current Single-Atom Catalyst Solutions for Carbon Conversion
01 Metal-based single-atom catalysts
Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts offer maximized atom efficiency and unique catalytic properties due to their isolated nature. The metal atoms, typically transition metals, are anchored to supports like carbon, metal oxides, or 2D materials, creating active sites with distinct electronic structures and coordination environments that enable high catalytic activity and selectivity for various chemical transformations.- Metal-based single-atom catalysts: Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts maximize atom efficiency by utilizing every metal atom as an active site. Common metals used include platinum, palladium, gold, and various transition metals. The isolated nature of the metal atoms creates unique electronic properties and coordination environments that often result in superior catalytic performance compared to traditional nanoparticle catalysts, with enhanced selectivity and activity for various chemical transformations.
- Support materials for single-atom catalysts: The choice of support material is crucial for stabilizing isolated metal atoms and preventing aggregation in single-atom catalysts. Common supports include carbon-based materials (graphene, carbon nanotubes), metal oxides (TiO2, ZnO, Al2O3), and metal-organic frameworks (MOFs). These supports not only anchor the single atoms but also influence their electronic properties and catalytic behavior through metal-support interactions. The surface chemistry and porosity of the support material play vital roles in determining catalyst stability, accessibility of active sites, and overall catalytic performance.
- Synthesis methods for single-atom catalysts: Various synthesis strategies have been developed to achieve uniform dispersion of single atoms on support materials. These include atomic layer deposition, wet impregnation followed by controlled pyrolysis, coordination-based approaches using metal-organic frameworks, and defect engineering of support materials. Advanced techniques like high-temperature atom trapping and electrochemical deposition are also employed. The key challenge in synthesis is preventing metal atom aggregation while achieving high metal loading, which requires precise control of reaction conditions and careful selection of precursors and support materials.
- Applications in energy conversion and environmental remediation: Single-atom catalysts demonstrate exceptional performance in energy-related applications such as hydrogen evolution, oxygen reduction/evolution reactions, CO2 reduction, and fuel cells. Their high atom efficiency makes them economically viable for utilizing precious metals in large-scale applications. In environmental remediation, these catalysts show superior activity for pollutant degradation, including the conversion of harmful gases like CO and NOx, and the breakdown of organic pollutants in wastewater. The tunable electronic structure of single-atom catalysts allows for optimized binding energies with reactants, resulting in enhanced catalytic efficiency.
- Characterization and theoretical studies of single-atom catalysts: Advanced characterization techniques are essential for confirming the atomic dispersion and understanding the structure-property relationships in single-atom catalysts. These include aberration-corrected transmission electron microscopy (AC-TEM), X-ray absorption spectroscopy (XAS), and scanning tunneling microscopy (STM). Computational methods such as density functional theory (DFT) calculations provide insights into reaction mechanisms, binding energies, and electronic structures at the atomic level. These theoretical studies guide the rational design of more efficient single-atom catalysts by predicting optimal metal-support combinations and reaction pathways.
02 Support materials for single-atom catalysts
The choice of support material plays a crucial role in stabilizing single atoms and influencing their catalytic performance. Various supports including carbon-based materials (graphene, carbon nanotubes), metal oxides (TiO2, ZnO, CeO2), zeolites, and metal-organic frameworks (MOFs) are used to anchor single atoms. These supports prevent atom aggregation while providing electronic interactions that can tune the catalytic properties of the single atoms. The support-atom interface often determines stability, activity, and selectivity in catalytic reactions.Expand Specific Solutions03 Synthesis methods for single-atom catalysts
Various synthesis strategies have been developed to prepare single-atom catalysts with high metal dispersion and stability. These include atomic layer deposition, wet chemistry methods (impregnation, co-precipitation), high-temperature atom trapping, photochemical reduction, and electrochemical deposition. Advanced techniques like defect engineering and coordination design are employed to create stable metal-support interactions that prevent atom aggregation during synthesis and catalytic reactions. The synthesis approach significantly influences the final structure and performance of the catalyst.Expand Specific Solutions04 Applications in energy conversion and environmental remediation
Single-atom catalysts demonstrate exceptional performance in energy-related applications and environmental remediation processes. They are employed in electrocatalytic reactions such as hydrogen evolution, oxygen reduction/evolution, and CO2 reduction for clean energy production. In environmental applications, these catalysts efficiently catalyze pollutant degradation, CO oxidation, and NOx reduction. Their high atom efficiency and tunable selectivity make them particularly valuable for sustainable chemical processes where resource utilization and environmental impact are critical considerations.Expand Specific Solutions05 Characterization and theoretical studies of single-atom catalysts
Advanced characterization techniques and theoretical studies are essential for understanding single-atom catalysts. Atomic-resolution imaging methods like aberration-corrected transmission electron microscopy (AC-TEM), X-ray absorption spectroscopy (XAS), and scanning tunneling microscopy (STM) are used to confirm the atomic dispersion and coordination environment. Computational methods including density functional theory (DFT) help elucidate reaction mechanisms, predict catalytic behavior, and guide rational design of more efficient catalysts by revealing electronic structures and energy profiles of catalytic processes.Expand Specific Solutions
Key Industry Players in Single-Atom Catalysis Research
Single-atom catalysis in carbon utilization is emerging as a transformative technology in the early commercialization phase, with a projected market growth to $5-7 billion by 2030. The competitive landscape features research institutions leading fundamental innovation, including China's Dalian Institute of Chemical Physics and South China University of Technology, alongside corporate players like KIST Corp. and SK Innovation developing industrial applications. Technical maturity varies across applications, with CO2 conversion showing promising advances. Chinese institutions dominate research output, while South Korean entities like KIST demonstrate strong patent portfolios. Western organizations, including Johns Hopkins University and Northwestern University, contribute significant fundamental research but lag in commercialization compared to Asian counterparts.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered significant advancements in single-atom catalysis (SAC) for carbon utilization. Their approach focuses on atomically dispersed metal catalysts anchored on various supports for CO2 conversion and carbon-based chemical transformations. DICP has developed innovative synthesis methods including atomic layer deposition and high-temperature atom trapping techniques to create stable single-atom catalysts with precisely controlled active sites. Their catalysts demonstrate exceptional activity for CO2 hydrogenation to methanol, formic acid, and CO with near 100% selectivity in some cases. DICP researchers have also engineered dual-metal single-atom catalysts that leverage synergistic effects between different metal centers to enhance catalytic performance. Their work includes comprehensive mechanistic studies using in-situ spectroscopy and DFT calculations to understand reaction pathways at the atomic level, enabling rational catalyst design with optimized coordination environments for specific carbon utilization reactions.
Strengths: Exceptional atomic-level control over catalyst structure, leading to superior selectivity and activity compared to traditional catalysts. Their advanced characterization capabilities allow for precise understanding of structure-function relationships. Weaknesses: Some synthesis methods may face scalability challenges for industrial applications, and the long-term stability of single-atom catalysts under industrial conditions remains a concern.
South China University of Technology
Technical Solution: South China University of Technology has developed innovative approaches to single-atom catalysis for carbon utilization, focusing particularly on CO2 electroreduction and conversion of carbon-based feedstocks. Their research team has pioneered the development of non-precious metal single-atom catalysts, particularly using abundant metals like Fe, Co, and Ni anchored on nitrogen-doped carbon supports. Their catalytic systems demonstrate remarkable CO2 electroreduction performance with Faradaic efficiencies exceeding 95% for CO production and stability over 100+ hours of continuous operation. A distinctive aspect of their approach is the development of dual-atom catalysts where two metal atoms work cooperatively to activate CO2 molecules more effectively than isolated single atoms. The university has also made significant advances in mechanistic understanding through operando spectroscopy techniques and theoretical calculations, revealing how the local coordination environment and electronic structure of single-atom sites determine catalytic pathways. Their recent work includes developing photocatalytic systems incorporating single-atom catalysts for solar-driven CO2 reduction with enhanced quantum efficiencies.
Strengths: Focus on earth-abundant metals makes their catalysts economically viable for large-scale applications; their dual-atom catalyst designs show superior performance compared to conventional single-atom catalysts for certain reactions. Weaknesses: Some of their catalysts show activity decay under industrial-relevant conditions, and the complex synthesis procedures may present challenges for standardization and mass production.
Core Patents and Innovations in Single-Atom Catalysis
A kind of preparation method of nitrogen-doped carbon supported single-atom catalyst
PatentActiveCN109999883B
Innovation
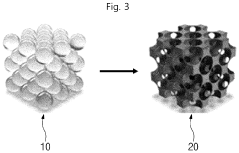
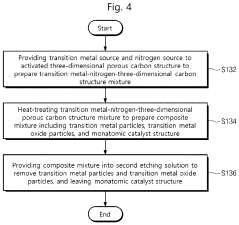
- Adopting the idea of complex capture adsorption, through hydrothermal reaction and heat treatment technology, transition metal acetate and pyrrole monomer are used to form a complex. After stirring, hydrothermal, acid etching and heat treatment, a nitrogen-doped carbon load is generated. Single atom catalyst avoids agglomeration of metal ions and achieves single atom distribution.
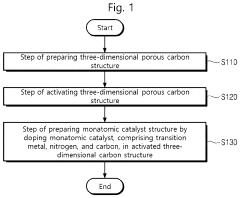
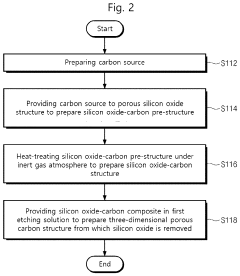
Single-atom catalyst structure and preparation method thereof
PatentPendingUS20230420693A1
Innovation
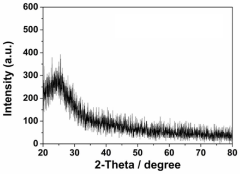
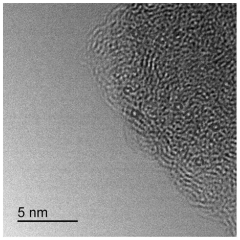
- A single-atom catalyst structure is developed by doping transition metal, nitrogen, and carbon into a three-dimensional ordered mesoporous carbon structure, which includes silicon, enhancing oxygen reduction reaction activity and reducing platinum usage, while maintaining low preparation costs and enabling mass production.
Environmental Impact Assessment of SAC Technologies
The environmental implications of Single-Atom Catalysis (SAC) technologies in carbon utilization represent a critical dimension for evaluating their sustainability and long-term viability. SAC systems demonstrate remarkable potential for reducing environmental footprints across multiple industrial processes by enabling more efficient carbon conversion pathways with minimal waste generation.
Primary environmental benefits of SAC technologies include significant reductions in energy consumption compared to conventional catalytic processes. The atomically dispersed active sites in SACs operate at lower activation energies, potentially decreasing process temperatures by 50-150°C in carbon dioxide hydrogenation and carbon monoxide conversion reactions. This translates to substantial energy savings and associated greenhouse gas emission reductions when implemented at industrial scales.
Waste minimization constitutes another environmental advantage of SAC technologies. Traditional heterogeneous catalysts often generate byproducts requiring additional separation and disposal processes. In contrast, SACs demonstrate superior selectivity in carbon utilization reactions, with some systems achieving over 95% selectivity toward target products. This precision reduces downstream purification requirements and minimizes chemical waste streams.
Life cycle assessments of SAC technologies reveal promising sustainability metrics. Preliminary studies indicate that carbon dioxide utilization processes employing single-atom catalysts can achieve carbon footprint reductions of 30-60% compared to conventional routes. However, these assessments must account for catalyst synthesis methods, which sometimes involve energy-intensive procedures or rare metal precursors that may offset environmental gains.
Resource efficiency represents a particularly significant environmental benefit. SACs maximize atomic efficiency of precious metals, with utilization rates approaching 100% compared to 10-30% in conventional nanoparticle catalysts. This dramatic improvement reduces mining impacts and resource depletion associated with critical metals like platinum, palladium, and rhodium.
Potential environmental concerns include the unknown long-term ecological impacts of atomically dispersed metals should they enter natural systems. While preliminary ecotoxicity studies suggest minimal bioaccumulation risks compared to metal nanoparticles, comprehensive environmental fate analyses remain limited. Additionally, the stability of support materials under reaction conditions requires further investigation to prevent unintended releases.
Regulatory frameworks for SAC technologies are still evolving, with current environmental standards primarily focused on conventional catalytic systems. Future policy development will need to address the unique characteristics of atomically dispersed catalysts, particularly regarding end-of-life management and potential environmental release scenarios.
Primary environmental benefits of SAC technologies include significant reductions in energy consumption compared to conventional catalytic processes. The atomically dispersed active sites in SACs operate at lower activation energies, potentially decreasing process temperatures by 50-150°C in carbon dioxide hydrogenation and carbon monoxide conversion reactions. This translates to substantial energy savings and associated greenhouse gas emission reductions when implemented at industrial scales.
Waste minimization constitutes another environmental advantage of SAC technologies. Traditional heterogeneous catalysts often generate byproducts requiring additional separation and disposal processes. In contrast, SACs demonstrate superior selectivity in carbon utilization reactions, with some systems achieving over 95% selectivity toward target products. This precision reduces downstream purification requirements and minimizes chemical waste streams.
Life cycle assessments of SAC technologies reveal promising sustainability metrics. Preliminary studies indicate that carbon dioxide utilization processes employing single-atom catalysts can achieve carbon footprint reductions of 30-60% compared to conventional routes. However, these assessments must account for catalyst synthesis methods, which sometimes involve energy-intensive procedures or rare metal precursors that may offset environmental gains.
Resource efficiency represents a particularly significant environmental benefit. SACs maximize atomic efficiency of precious metals, with utilization rates approaching 100% compared to 10-30% in conventional nanoparticle catalysts. This dramatic improvement reduces mining impacts and resource depletion associated with critical metals like platinum, palladium, and rhodium.
Potential environmental concerns include the unknown long-term ecological impacts of atomically dispersed metals should they enter natural systems. While preliminary ecotoxicity studies suggest minimal bioaccumulation risks compared to metal nanoparticles, comprehensive environmental fate analyses remain limited. Additionally, the stability of support materials under reaction conditions requires further investigation to prevent unintended releases.
Regulatory frameworks for SAC technologies are still evolving, with current environmental standards primarily focused on conventional catalytic systems. Future policy development will need to address the unique characteristics of atomically dispersed catalysts, particularly regarding end-of-life management and potential environmental release scenarios.
Scalability and Industrial Implementation Roadmap
The transition of single-atom catalysis (SAC) from laboratory research to industrial implementation requires a comprehensive roadmap addressing multiple technical and economic challenges. Current industrial applications of SAC in carbon utilization remain limited to pilot-scale demonstrations, with full commercialization hindered by several key factors that must be systematically addressed.
Scale-up production represents the primary challenge, as laboratory synthesis methods typically yield only gram-scale quantities of catalysts. Industrial implementation necessitates kilogram to ton-scale production while maintaining the critical single-atom dispersion that defines SAC performance. Recent advances in continuous flow synthesis and automated production systems show promise for addressing this challenge, with several research groups demonstrating semi-continuous production methods achieving up to 100g batches with consistent atom dispersion.
Economic viability constitutes another significant barrier, particularly regarding precious metal catalysts like platinum and palladium commonly used in SAC. Cost reduction strategies include developing more efficient synthesis routes with higher metal loading efficiencies, exploring earth-abundant alternatives such as iron and nickel-based SACs, and implementing catalyst recovery systems. Economic modeling suggests that for carbon utilization applications, SAC implementation becomes commercially viable when production costs decrease below $500 per kilogram of catalyst.
Stability and longevity under industrial conditions present ongoing challenges, as many SACs demonstrate excellent performance in controlled laboratory environments but suffer deactivation in real-world applications. Engineering solutions include developing protective support structures, optimizing reaction conditions to minimize sintering, and designing regeneration protocols that restore catalyst activity without requiring complete replacement.
Standardization and quality control frameworks are emerging as critical components of the implementation roadmap. Several international consortia are developing standardized testing protocols and characterization methods specifically for SACs, which will facilitate regulatory approval and quality assurance in industrial settings. These standards focus particularly on verifying single-atom dispersion, quantifying active site density, and establishing performance benchmarks for carbon conversion efficiency.
The projected timeline for widespread industrial implementation follows a three-phase approach: near-term (1-3 years) focusing on specialized high-value applications where current SAC technology already demonstrates economic advantages; mid-term (3-7 years) expanding to broader carbon utilization processes as production scales increase and costs decrease; and long-term (7-10+ years) integration into mainstream carbon capture and utilization infrastructure as the technology matures and policy frameworks evolve to support carbon utilization technologies.
Scale-up production represents the primary challenge, as laboratory synthesis methods typically yield only gram-scale quantities of catalysts. Industrial implementation necessitates kilogram to ton-scale production while maintaining the critical single-atom dispersion that defines SAC performance. Recent advances in continuous flow synthesis and automated production systems show promise for addressing this challenge, with several research groups demonstrating semi-continuous production methods achieving up to 100g batches with consistent atom dispersion.
Economic viability constitutes another significant barrier, particularly regarding precious metal catalysts like platinum and palladium commonly used in SAC. Cost reduction strategies include developing more efficient synthesis routes with higher metal loading efficiencies, exploring earth-abundant alternatives such as iron and nickel-based SACs, and implementing catalyst recovery systems. Economic modeling suggests that for carbon utilization applications, SAC implementation becomes commercially viable when production costs decrease below $500 per kilogram of catalyst.
Stability and longevity under industrial conditions present ongoing challenges, as many SACs demonstrate excellent performance in controlled laboratory environments but suffer deactivation in real-world applications. Engineering solutions include developing protective support structures, optimizing reaction conditions to minimize sintering, and designing regeneration protocols that restore catalyst activity without requiring complete replacement.
Standardization and quality control frameworks are emerging as critical components of the implementation roadmap. Several international consortia are developing standardized testing protocols and characterization methods specifically for SACs, which will facilitate regulatory approval and quality assurance in industrial settings. These standards focus particularly on verifying single-atom dispersion, quantifying active site density, and establishing performance benchmarks for carbon conversion efficiency.
The projected timeline for widespread industrial implementation follows a three-phase approach: near-term (1-3 years) focusing on specialized high-value applications where current SAC technology already demonstrates economic advantages; mid-term (3-7 years) expanding to broader carbon utilization processes as production scales increase and costs decrease; and long-term (7-10+ years) integration into mainstream carbon capture and utilization infrastructure as the technology matures and policy frameworks evolve to support carbon utilization technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!