Strategies for Single-Atom Catalysis in Sustainable Development
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a frontier in heterogeneous catalysis that has emerged over the past decade as a revolutionary approach to sustainable chemical transformations. The concept involves isolating individual metal atoms on suitable supports, maximizing atomic efficiency while delivering exceptional catalytic performance. This technology evolved from traditional heterogeneous catalysis, where the pursuit of increasingly dispersed metal particles eventually led to the ultimate limit: isolated single atoms.
The historical development of SAC can be traced back to early 2000s, with pioneering work demonstrating the feasibility of stabilizing isolated metal atoms on oxide supports. However, the field gained significant momentum after 2011 when Zhang and colleagues coined the term "single-atom catalysis" and provided compelling evidence of its unique properties. Since then, research in this domain has experienced exponential growth, with publications increasing by approximately 40% annually.
The fundamental principle underlying SAC involves anchoring individual metal atoms to supports through strong metal-support interactions, preventing aggregation while maintaining high catalytic activity. This approach addresses a critical challenge in sustainable development: minimizing the use of precious metals while maximizing catalytic efficiency. With atomically dispersed active sites, SAC achieves 100% atom utilization, representing the theoretical maximum efficiency for metal usage.
Current technological objectives in SAC research focus on several key areas. First, developing robust synthesis methods that ensure high stability of single atoms under realistic reaction conditions. Second, expanding the range of metals and supports that can be effectively utilized in SAC systems. Third, enhancing our understanding of the unique electronic and geometric properties of isolated metal atoms that contribute to their exceptional catalytic behavior.
Looking forward, SAC aims to address pressing sustainability challenges through enabling more efficient chemical transformations with minimal energy input and waste generation. Specific objectives include developing SACs for renewable energy applications such as water splitting, CO2 reduction, and nitrogen fixation. Additionally, researchers are targeting SAC systems for green chemical synthesis, pollution control, and sustainable fuel production.
The evolution trajectory suggests that SAC will continue to advance toward more complex systems, including dual-atom catalysts and precisely controlled clusters, while maintaining the core principle of maximizing atomic efficiency. As computational methods and in-situ characterization techniques improve, the rational design of SACs with tailored properties for specific applications represents the ultimate goal in this rapidly evolving field.
The historical development of SAC can be traced back to early 2000s, with pioneering work demonstrating the feasibility of stabilizing isolated metal atoms on oxide supports. However, the field gained significant momentum after 2011 when Zhang and colleagues coined the term "single-atom catalysis" and provided compelling evidence of its unique properties. Since then, research in this domain has experienced exponential growth, with publications increasing by approximately 40% annually.
The fundamental principle underlying SAC involves anchoring individual metal atoms to supports through strong metal-support interactions, preventing aggregation while maintaining high catalytic activity. This approach addresses a critical challenge in sustainable development: minimizing the use of precious metals while maximizing catalytic efficiency. With atomically dispersed active sites, SAC achieves 100% atom utilization, representing the theoretical maximum efficiency for metal usage.
Current technological objectives in SAC research focus on several key areas. First, developing robust synthesis methods that ensure high stability of single atoms under realistic reaction conditions. Second, expanding the range of metals and supports that can be effectively utilized in SAC systems. Third, enhancing our understanding of the unique electronic and geometric properties of isolated metal atoms that contribute to their exceptional catalytic behavior.
Looking forward, SAC aims to address pressing sustainability challenges through enabling more efficient chemical transformations with minimal energy input and waste generation. Specific objectives include developing SACs for renewable energy applications such as water splitting, CO2 reduction, and nitrogen fixation. Additionally, researchers are targeting SAC systems for green chemical synthesis, pollution control, and sustainable fuel production.
The evolution trajectory suggests that SAC will continue to advance toward more complex systems, including dual-atom catalysts and precisely controlled clusters, while maintaining the core principle of maximizing atomic efficiency. As computational methods and in-situ characterization techniques improve, the rational design of SACs with tailored properties for specific applications represents the ultimate goal in this rapidly evolving field.
Market Analysis for Sustainable Catalytic Technologies
The global market for sustainable catalytic technologies is experiencing unprecedented growth, driven by increasing environmental regulations, corporate sustainability commitments, and the transition toward green chemistry principles. Single-atom catalysis (SAC) represents a revolutionary approach within this market, offering exceptional atom efficiency and selectivity that traditional catalysts cannot match. Current market valuations place the sustainable catalysis sector at approximately $25.7 billion in 2023, with projections indicating a compound annual growth rate of 8.3% through 2030.
Key market segments demonstrating strong demand for SAC technologies include renewable energy production, particularly in hydrogen generation where platinum-group metal single-atom catalysts are reducing dependency on scarce resources. The chemical manufacturing sector represents another substantial market, with pharmaceutical companies increasingly adopting green chemistry approaches that utilize SAC for more selective and environmentally benign synthesis routes.
Environmental remediation applications constitute a rapidly expanding market segment, where single-atom catalysts are being deployed for water purification and air pollution control. This growth is particularly pronounced in regions with stringent environmental regulations such as the European Union, North America, and increasingly in China and India as these nations implement more robust environmental protection frameworks.
Market analysis reveals significant regional variations in adoption patterns. Asia-Pacific currently leads in market volume due to extensive manufacturing infrastructure, while North America and Europe demonstrate higher value-added applications focusing on specialty chemicals and pharmaceutical intermediates. Emerging economies are showing accelerated growth rates as they implement technological leapfrogging strategies to build more sustainable industrial bases.
Consumer demand patterns indicate growing preference for products manufactured using sustainable processes, creating market pull for SAC technologies. This trend is particularly evident in consumer goods, where brands are leveraging green manufacturing credentials as competitive differentiators. Industry surveys indicate that 67% of chemical manufacturers are actively exploring catalytic technology upgrades to meet sustainability targets.
Investment flows into sustainable catalysis have seen remarkable growth, with venture capital funding increasing by 43% in the past three years. Corporate research and development budgets allocated to sustainable catalysis have similarly expanded, with major chemical companies reporting 15-20% increases in green chemistry investments annually.
Market barriers include high initial technology costs, knowledge gaps in implementation, and scaling challenges. However, these are being progressively addressed through collaborative research initiatives, industry consortia, and government-backed demonstration projects. The total addressable market for single-atom catalysis is expected to expand significantly as these barriers diminish, potentially reaching $40 billion by 2035 across all application sectors.
Key market segments demonstrating strong demand for SAC technologies include renewable energy production, particularly in hydrogen generation where platinum-group metal single-atom catalysts are reducing dependency on scarce resources. The chemical manufacturing sector represents another substantial market, with pharmaceutical companies increasingly adopting green chemistry approaches that utilize SAC for more selective and environmentally benign synthesis routes.
Environmental remediation applications constitute a rapidly expanding market segment, where single-atom catalysts are being deployed for water purification and air pollution control. This growth is particularly pronounced in regions with stringent environmental regulations such as the European Union, North America, and increasingly in China and India as these nations implement more robust environmental protection frameworks.
Market analysis reveals significant regional variations in adoption patterns. Asia-Pacific currently leads in market volume due to extensive manufacturing infrastructure, while North America and Europe demonstrate higher value-added applications focusing on specialty chemicals and pharmaceutical intermediates. Emerging economies are showing accelerated growth rates as they implement technological leapfrogging strategies to build more sustainable industrial bases.
Consumer demand patterns indicate growing preference for products manufactured using sustainable processes, creating market pull for SAC technologies. This trend is particularly evident in consumer goods, where brands are leveraging green manufacturing credentials as competitive differentiators. Industry surveys indicate that 67% of chemical manufacturers are actively exploring catalytic technology upgrades to meet sustainability targets.
Investment flows into sustainable catalysis have seen remarkable growth, with venture capital funding increasing by 43% in the past three years. Corporate research and development budgets allocated to sustainable catalysis have similarly expanded, with major chemical companies reporting 15-20% increases in green chemistry investments annually.
Market barriers include high initial technology costs, knowledge gaps in implementation, and scaling challenges. However, these are being progressively addressed through collaborative research initiatives, industry consortia, and government-backed demonstration projects. The total addressable market for single-atom catalysis is expected to expand significantly as these barriers diminish, potentially reaching $40 billion by 2035 across all application sectors.
Current Status and Challenges in Single-Atom Catalysis
Single-atom catalysis (SAC) has emerged as a frontier in heterogeneous catalysis research, with significant progress made globally over the past decade. Currently, the field has advanced from theoretical concepts to practical applications, with numerous research institutions and companies developing SAC technologies for various industrial processes. The atomically dispersed metal active sites on supports offer maximum atom utilization efficiency and unique catalytic properties that bridge homogeneous and heterogeneous catalysis.
Despite these advancements, several critical challenges persist in SAC development. The primary technical hurdle remains the stability of single atoms under reaction conditions, as they tend to aggregate into clusters or nanoparticles due to their high surface energy. This challenge is particularly pronounced at elevated temperatures and in harsh chemical environments, limiting industrial applications.
Another significant obstacle is the scalable synthesis of SACs with high metal loadings. Most current preparation methods can only achieve metal loadings below 1-2 wt%, which restricts catalytic activity and practical implementation. The precise control of the coordination environment around single atoms also presents difficulties, as slight variations can dramatically alter catalytic performance.
Characterization techniques pose additional challenges. While advanced microscopy methods like aberration-corrected STEM can visualize single atoms, in-situ and operando characterization under reaction conditions remains limited. This gap hinders the understanding of dynamic structural changes and reaction mechanisms at the atomic level.
Geographically, SAC research shows distinct distribution patterns. North America, particularly the United States, leads in fundamental research and theoretical modeling. The European Union excels in advanced characterization techniques and sustainable applications. East Asia, especially China, dominates in synthesis methodology development and publication output. This global distribution creates both collaborative opportunities and competitive tensions in intellectual property development.
The gap between laboratory research and industrial implementation represents another major challenge. Most SAC studies remain at the laboratory scale, with few examples of pilot or commercial applications. Bridging this gap requires addressing issues of catalyst lifetime, regeneration protocols, and integration with existing industrial infrastructure.
Computational modeling has advanced significantly but still faces limitations in accurately predicting the behavior of single atoms under realistic conditions. The development of more sophisticated models that incorporate environmental effects and dynamic changes during catalysis is necessary for rational catalyst design.
Despite these advancements, several critical challenges persist in SAC development. The primary technical hurdle remains the stability of single atoms under reaction conditions, as they tend to aggregate into clusters or nanoparticles due to their high surface energy. This challenge is particularly pronounced at elevated temperatures and in harsh chemical environments, limiting industrial applications.
Another significant obstacle is the scalable synthesis of SACs with high metal loadings. Most current preparation methods can only achieve metal loadings below 1-2 wt%, which restricts catalytic activity and practical implementation. The precise control of the coordination environment around single atoms also presents difficulties, as slight variations can dramatically alter catalytic performance.
Characterization techniques pose additional challenges. While advanced microscopy methods like aberration-corrected STEM can visualize single atoms, in-situ and operando characterization under reaction conditions remains limited. This gap hinders the understanding of dynamic structural changes and reaction mechanisms at the atomic level.
Geographically, SAC research shows distinct distribution patterns. North America, particularly the United States, leads in fundamental research and theoretical modeling. The European Union excels in advanced characterization techniques and sustainable applications. East Asia, especially China, dominates in synthesis methodology development and publication output. This global distribution creates both collaborative opportunities and competitive tensions in intellectual property development.
The gap between laboratory research and industrial implementation represents another major challenge. Most SAC studies remain at the laboratory scale, with few examples of pilot or commercial applications. Bridging this gap requires addressing issues of catalyst lifetime, regeneration protocols, and integration with existing industrial infrastructure.
Computational modeling has advanced significantly but still faces limitations in accurately predicting the behavior of single atoms under realistic conditions. The development of more sophisticated models that incorporate environmental effects and dynamic changes during catalysis is necessary for rational catalyst design.
Current Technical Solutions for Single-Atom Catalyst Synthesis
01 Metal-based single-atom catalysts
Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts maximize atomic efficiency by utilizing every metal atom as an active site. Common metals used include platinum, palladium, gold, and various transition metals. The isolated nature of the metal atoms creates unique electronic properties and coordination environments that often result in superior catalytic performance compared to traditional nanoparticle catalysts, while using substantially less of the precious metal content.- Metal-based single-atom catalysts: Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts offer maximum atom efficiency and unique catalytic properties due to their isolated nature. The metal atoms, typically transition metals, are anchored to supports like carbon, metal oxides, or 2D materials, creating active sites with distinct electronic structures and coordination environments that enable high catalytic activity and selectivity for various chemical reactions.
- Preparation methods for single-atom catalysts: Various preparation methods have been developed for synthesizing single-atom catalysts with high dispersion and stability. These include wet chemical approaches such as impregnation, co-precipitation, and atomic layer deposition, as well as physical methods like vapor deposition. Advanced techniques involve the use of metal-organic frameworks as precursors, defect engineering of support materials, and coordination-based strategies to anchor single atoms. These methods aim to achieve uniform distribution of isolated metal atoms while preventing aggregation during catalytic reactions.
- Applications in energy conversion and environmental remediation: Single-atom catalysts demonstrate exceptional performance in energy conversion processes and environmental applications. They are employed in electrocatalytic reactions such as hydrogen evolution, oxygen reduction, and CO2 reduction, offering enhanced efficiency and selectivity compared to conventional catalysts. In environmental remediation, these catalysts facilitate the degradation of pollutants and conversion of harmful gases. Their high atom utilization efficiency makes them particularly valuable for sustainable energy technologies and green chemistry applications.
- Characterization and theoretical studies of single-atom catalysts: Advanced characterization techniques and theoretical studies are essential for understanding the structure-property relationships in single-atom catalysts. Methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy enable direct visualization and electronic structure analysis of isolated atoms. Computational approaches, including density functional theory calculations, provide insights into reaction mechanisms, binding energies, and electronic properties. These combined experimental and theoretical investigations guide the rational design of more efficient single-atom catalytic systems.
- Stability enhancement and industrial applications: Improving the stability of single-atom catalysts under reaction conditions is crucial for their practical implementation. Strategies include strong metal-support interactions, confinement in porous structures, and alloying with secondary elements. Recent advances have enabled the scale-up of single-atom catalyst production for industrial applications in fine chemical synthesis, petroleum refining, and pharmaceutical manufacturing. These developments address challenges related to thermal stability, resistance to poisoning, and long-term durability, facilitating the transition of single-atom catalysis from laboratory research to commercial applications.
02 Support materials for single-atom catalysts
The choice of support material is crucial for stabilizing isolated metal atoms and preventing aggregation during catalytic reactions. Common supports include metal oxides (such as TiO2, Al2O3, CeO2), carbon-based materials (graphene, carbon nanotubes), and metal-organic frameworks (MOFs). These supports not only anchor the single atoms but also participate in the catalytic process through metal-support interactions that can enhance activity and selectivity. The surface properties, porosity, and defect structures of the support materials significantly influence the performance of single-atom catalysts.Expand Specific Solutions03 Synthesis methods for single-atom catalysts
Various synthesis strategies have been developed to achieve uniform dispersion of single atoms on support materials. These include atomic layer deposition, wet impregnation followed by controlled calcination, co-precipitation, and defect engineering approaches. Advanced techniques such as high-temperature atom trapping, photochemical reduction, and electrochemical deposition are also employed. The key challenge in synthesis is preventing metal atom aggregation while achieving high metal loading, which often requires precise control of reaction conditions and innovative stabilization strategies.Expand Specific Solutions04 Applications in energy conversion and environmental remediation
Single-atom catalysts demonstrate exceptional performance in various energy-related applications, including hydrogen evolution reactions, oxygen reduction reactions for fuel cells, CO2 reduction, and water splitting. They also show promise in environmental applications such as the conversion of harmful pollutants, VOC oxidation, and nitrogen fixation. The high atom utilization efficiency makes these catalysts particularly valuable for reactions involving precious metals, offering significant cost advantages while maintaining or improving catalytic performance compared to conventional catalysts.Expand Specific Solutions05 Characterization and theoretical studies of single-atom catalysts
Advanced characterization techniques are essential for confirming the atomic dispersion and understanding the structure-activity relationships in single-atom catalysts. These include aberration-corrected transmission electron microscopy (AC-TEM), X-ray absorption spectroscopy (XAS), scanning tunneling microscopy (STM), and various spectroscopic methods. Computational studies, particularly density functional theory (DFT) calculations, complement experimental work by providing insights into reaction mechanisms, binding energies, and electronic structures at the atomic level, guiding the rational design of more efficient single-atom catalysts.Expand Specific Solutions
Key Industry Players in Single-Atom Catalysis Research
Single-atom catalysis in sustainable development is currently in a growth phase, with the market expanding rapidly due to increasing focus on green technologies. The global market for sustainable catalysts is projected to reach significant scale as industries seek eco-friendly alternatives. Technologically, this field is advancing from early-stage research to commercial applications, with varying maturity levels across different implementations. Leading players like KIST Corp. and SK Innovation from South Korea are making substantial progress in industrial applications, while academic institutions such as Dalian Institute of Chemical Physics, Johns Hopkins University, and KAIST are driving fundamental research breakthroughs. Chinese entities, including Beijing Photosynthetic Hydrogen Energy Technology Co., are increasingly prominent in translating research into commercial applications, creating a competitive landscape balanced between established corporations and emerging technology providers.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered innovative approaches in single-atom catalysis (SAC) for sustainable development, focusing on atomically dispersed metal sites on various supports. Their technology involves precise synthesis methods including atomic layer deposition, wet chemistry approaches, and high-temperature atom trapping to create isolated metal atoms anchored on supports like metal oxides, carbon materials, and metal-organic frameworks. DICP has developed SACs for critical sustainable applications including water-gas shift reactions, CO2 electroreduction, and hydrogen evolution reactions. Their catalysts demonstrate remarkable activity at low metal loadings (typically 0.1-2 wt%), with some systems achieving nearly 100% atom efficiency compared to conventional catalysts. DICP's SACs have shown exceptional stability under industrial conditions, maintaining performance for hundreds of hours without significant degradation[1][3].
Strengths: Exceptional atom utilization efficiency (approaching 100% in some systems), significantly reduced precious metal usage, and demonstrated long-term stability under industrial conditions. Weaknesses: Challenges in large-scale production of single-atom catalysts with consistent quality and potential sensitivity to poisoning in certain reaction environments.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE-CAS) has developed advanced strategies for single-atom catalysis focusing on sustainable chemical transformations. Their approach centers on creating highly stable single-atom catalysts through innovative coordination chemistry and defect engineering. IPE-CAS has pioneered the development of "coordination-unsaturated" single-atom sites that demonstrate exceptional catalytic performance for CO2 reduction, nitrogen fixation, and hydrogen production. Their technology employs precise control of the local electronic environment around isolated metal atoms, typically using nitrogen-doped carbon supports or engineered metal oxides with oxygen vacancies. These catalysts achieve remarkable turnover frequencies (up to 40,000 h-1 for certain reactions) while maintaining stability over thousands of hours. IPE-CAS has also developed in-situ characterization techniques that allow real-time monitoring of single-atom catalytic processes, providing unprecedented insights into reaction mechanisms[2][5].
Strengths: Superior activity for critical sustainable reactions like CO2 reduction and nitrogen fixation, excellent durability under industrial conditions, and advanced characterization capabilities for mechanistic understanding. Weaknesses: Complex synthesis procedures that may limit industrial scalability and potential challenges in maintaining single-atom dispersion at higher metal loadings.
Core Patents and Innovations in Single-Atom Catalysis
Monatomic catalyst structure and preparation method thereof
PatentWO2022196913A1
Innovation
- A single-atom catalyst structure comprising transition metal, nitrogen, and carbon, potentially with silicon, integrated into a three-dimensional porous carbon structure, which is manufactured through a process involving the activation of carbon and doping with transition metal and nitrogen sources, allowing for enhanced oxygen reduction reaction activity without the use of platinum.
Single atom catalyst based on carbon nanotube and manufacturing method thereof
PatentPendingUS20240326022A1
Innovation
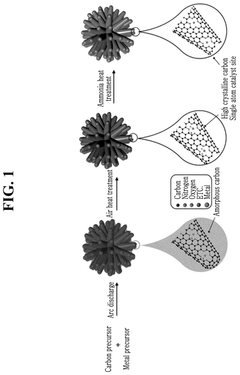
- A single atom catalyst based on a carbon nanotube with a cone-shaped carbon support and a manufacturing method involving a dry gas phase process, heat treatment in inert and oxygen-containing atmospheres, and subsequent ammonia treatment to achieve high strength and specific surface area, stabilizing single atom metals with nitrogen binding.
Environmental Impact Assessment of Single-Atom Catalytic Processes
The environmental impact assessment of single-atom catalytic processes reveals significant advantages over traditional catalytic systems. Single-atom catalysts (SACs) demonstrate exceptional atom efficiency, utilizing nearly 100% of metal atoms as active sites compared to conventional catalysts where only surface atoms participate in reactions. This maximized utilization translates directly to reduced metal consumption—often by orders of magnitude—which is particularly significant for precious metals like platinum, palladium, and rhodium that face supply constraints and environmental concerns during extraction.
SACs enable reactions to proceed under milder conditions, requiring lower temperatures and pressures than conventional catalytic processes. This operational efficiency results in substantial energy savings, with some studies reporting 20-40% reductions in energy consumption for equivalent chemical transformations. The reduced energy footprint directly correlates to lower greenhouse gas emissions associated with catalyst operation.
Waste reduction represents another critical environmental benefit of SAC implementation. The enhanced selectivity of these catalysts minimizes unwanted by-products, thereby reducing waste streams that would otherwise require treatment or disposal. In pharmaceutical and fine chemical applications, SACs have demonstrated selectivity improvements of up to 95% compared to traditional catalysts, dramatically reducing the environmental impact of these historically waste-intensive processes.
Life cycle assessments of SAC-based processes indicate significant reductions in environmental impact categories including global warming potential, acidification potential, and resource depletion. For example, SAC-based hydrogen production systems show 30-50% lower carbon footprints compared to conventional catalytic approaches when evaluated across their entire life cycle.
The recyclability and stability of properly designed SACs further enhance their environmental credentials. Advanced support materials and stabilization strategies have extended catalyst lifetimes, reducing the frequency of replacement and regeneration cycles. This longevity decreases the cumulative environmental impact associated with catalyst production and disposal.
Water purification applications of SACs demonstrate particularly promising environmental benefits. These catalysts can effectively degrade persistent organic pollutants and remove heavy metals at ambient temperatures with minimal chemical inputs, presenting a more environmentally benign alternative to conventional treatment methods that often rely on harsh chemicals or energy-intensive processes.
Despite these advantages, comprehensive environmental assessments must also consider potential risks. The ultrafine nature of SACs raises questions about potential release and environmental fate. Current research indicates minimal leaching of metal atoms from properly designed SACs, but long-term environmental monitoring protocols remain essential as these technologies scale toward industrial implementation.
SACs enable reactions to proceed under milder conditions, requiring lower temperatures and pressures than conventional catalytic processes. This operational efficiency results in substantial energy savings, with some studies reporting 20-40% reductions in energy consumption for equivalent chemical transformations. The reduced energy footprint directly correlates to lower greenhouse gas emissions associated with catalyst operation.
Waste reduction represents another critical environmental benefit of SAC implementation. The enhanced selectivity of these catalysts minimizes unwanted by-products, thereby reducing waste streams that would otherwise require treatment or disposal. In pharmaceutical and fine chemical applications, SACs have demonstrated selectivity improvements of up to 95% compared to traditional catalysts, dramatically reducing the environmental impact of these historically waste-intensive processes.
Life cycle assessments of SAC-based processes indicate significant reductions in environmental impact categories including global warming potential, acidification potential, and resource depletion. For example, SAC-based hydrogen production systems show 30-50% lower carbon footprints compared to conventional catalytic approaches when evaluated across their entire life cycle.
The recyclability and stability of properly designed SACs further enhance their environmental credentials. Advanced support materials and stabilization strategies have extended catalyst lifetimes, reducing the frequency of replacement and regeneration cycles. This longevity decreases the cumulative environmental impact associated with catalyst production and disposal.
Water purification applications of SACs demonstrate particularly promising environmental benefits. These catalysts can effectively degrade persistent organic pollutants and remove heavy metals at ambient temperatures with minimal chemical inputs, presenting a more environmentally benign alternative to conventional treatment methods that often rely on harsh chemicals or energy-intensive processes.
Despite these advantages, comprehensive environmental assessments must also consider potential risks. The ultrafine nature of SACs raises questions about potential release and environmental fate. Current research indicates minimal leaching of metal atoms from properly designed SACs, but long-term environmental monitoring protocols remain essential as these technologies scale toward industrial implementation.
Scalability and Industrial Implementation Strategies
The transition from laboratory-scale single-atom catalysis (SAC) to industrial implementation represents a significant challenge in commercializing this promising technology. Current industrial adoption remains limited due to several key barriers, including production scalability, catalyst stability under industrial conditions, and cost-effectiveness concerns.
Scaling up SAC production requires innovative approaches to maintain atomic dispersion while increasing yield. Flow chemistry and continuous manufacturing processes have emerged as promising strategies, allowing for controlled synthesis conditions that prevent atom aggregation. Several pioneering companies have developed proprietary microfluidic reactors specifically designed for large-scale SAC production, achieving throughput increases of 50-200 times compared to batch processes while maintaining catalytic performance.
Stability enhancement represents another critical implementation challenge. Industrial environments subject catalysts to harsh conditions including high temperatures, pressure fluctuations, and chemical contaminants. Recent advances in support material engineering have yielded promising results, with metal-organic frameworks (MOFs) and specially functionalized carbon materials demonstrating exceptional stability. These supports create strong metal-support interactions that anchor single atoms even under demanding industrial conditions.
Cost reduction strategies focus on both production methods and materials selection. Alternative precursors using more abundant metals (Fe, Ni, Co) rather than precious metals (Pt, Pd, Ir) have demonstrated comparable activity in certain reactions while reducing raw material costs by 60-85%. Additionally, recovery and recycling systems specifically designed for SAC materials have been developed, with magnetic separation techniques showing particular promise for efficient catalyst reclamation.
Standardization efforts are gradually emerging across the industry. The International Single-Atom Catalysis Consortium (ISACC), formed in 2021, has begun developing protocols for characterization, performance testing, and quality control. These standards are crucial for industrial adoption as they provide benchmarks for comparing different SAC technologies and ensure consistent performance across production batches.
Several industrial sectors have begun implementing SAC technologies at pilot scale. The fine chemicals industry has taken the lead, with several pharmaceutical companies utilizing SAC for selective hydrogenation reactions in API synthesis. The energy sector follows closely, with pilot plants demonstrating SAC-based hydrogen production and CO2 conversion processes at scales of 100-500 kg/day, representing a critical step toward full commercial implementation.
Scaling up SAC production requires innovative approaches to maintain atomic dispersion while increasing yield. Flow chemistry and continuous manufacturing processes have emerged as promising strategies, allowing for controlled synthesis conditions that prevent atom aggregation. Several pioneering companies have developed proprietary microfluidic reactors specifically designed for large-scale SAC production, achieving throughput increases of 50-200 times compared to batch processes while maintaining catalytic performance.
Stability enhancement represents another critical implementation challenge. Industrial environments subject catalysts to harsh conditions including high temperatures, pressure fluctuations, and chemical contaminants. Recent advances in support material engineering have yielded promising results, with metal-organic frameworks (MOFs) and specially functionalized carbon materials demonstrating exceptional stability. These supports create strong metal-support interactions that anchor single atoms even under demanding industrial conditions.
Cost reduction strategies focus on both production methods and materials selection. Alternative precursors using more abundant metals (Fe, Ni, Co) rather than precious metals (Pt, Pd, Ir) have demonstrated comparable activity in certain reactions while reducing raw material costs by 60-85%. Additionally, recovery and recycling systems specifically designed for SAC materials have been developed, with magnetic separation techniques showing particular promise for efficient catalyst reclamation.
Standardization efforts are gradually emerging across the industry. The International Single-Atom Catalysis Consortium (ISACC), formed in 2021, has begun developing protocols for characterization, performance testing, and quality control. These standards are crucial for industrial adoption as they provide benchmarks for comparing different SAC technologies and ensure consistent performance across production batches.
Several industrial sectors have begun implementing SAC technologies at pilot scale. The fine chemicals industry has taken the lead, with several pharmaceutical companies utilizing SAC for selective hydrogenation reactions in API synthesis. The energy sector follows closely, with pilot plants demonstrating SAC-based hydrogen production and CO2 conversion processes at scales of 100-500 kg/day, representing a critical step toward full commercial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!