Single-Atom Catalysis and Its Innovations in Industrial Catalysis
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis that has emerged over the past decade. This innovative field focuses on the dispersion of isolated metal atoms on various supports, creating catalysts with maximum atom efficiency. The concept was first formally introduced in 2011, though earlier studies had observed similar phenomena without explicitly defining the term. Since then, SAC has experienced exponential growth in research interest due to its potential to bridge homogeneous and heterogeneous catalysis while offering unprecedented atom utilization efficiency.
The evolution of catalysis technology has progressed from traditional bulk catalysts to nanoparticles, clusters, and now to the atomic level with single-atom catalysts. This progression reflects the continuous pursuit of higher catalytic efficiency, selectivity, and reduced noble metal usage in industrial processes. Single-atom catalysts represent the ultimate downsizing of metal catalysts, where every metal atom can potentially serve as an active site.
The technological trajectory of SAC has been accelerated by advances in characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, which have enabled direct visualization and electronic structure analysis of isolated atoms. Computational methods have similarly evolved to provide deeper insights into reaction mechanisms at the atomic level, further propelling the field forward.
The primary objective of single-atom catalysis research is to develop highly efficient catalytic systems that minimize precious metal usage while maximizing catalytic performance. This aligns with green chemistry principles and sustainable development goals in the chemical industry. Specific technical goals include enhancing catalyst stability under industrial conditions, developing scalable synthesis methods, and expanding the application scope beyond current limitations.
Another critical objective is understanding the fundamental science behind single-atom catalysis, including metal-support interactions, reaction mechanisms, and the nature of active sites. This knowledge is essential for rational catalyst design rather than empirical development approaches. Researchers aim to establish structure-performance relationships that can guide the development of next-generation catalysts with tailored properties.
From an industrial perspective, the goal is to transition SAC from laboratory curiosities to practical catalysts for large-scale applications. This requires addressing challenges in synthesis scalability, long-term stability, and cost-effectiveness. Industries ranging from petrochemicals to fine chemicals, environmental remediation, and energy conversion are potential beneficiaries of SAC innovations, driving significant research investment in this area.
The evolution of catalysis technology has progressed from traditional bulk catalysts to nanoparticles, clusters, and now to the atomic level with single-atom catalysts. This progression reflects the continuous pursuit of higher catalytic efficiency, selectivity, and reduced noble metal usage in industrial processes. Single-atom catalysts represent the ultimate downsizing of metal catalysts, where every metal atom can potentially serve as an active site.
The technological trajectory of SAC has been accelerated by advances in characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, which have enabled direct visualization and electronic structure analysis of isolated atoms. Computational methods have similarly evolved to provide deeper insights into reaction mechanisms at the atomic level, further propelling the field forward.
The primary objective of single-atom catalysis research is to develop highly efficient catalytic systems that minimize precious metal usage while maximizing catalytic performance. This aligns with green chemistry principles and sustainable development goals in the chemical industry. Specific technical goals include enhancing catalyst stability under industrial conditions, developing scalable synthesis methods, and expanding the application scope beyond current limitations.
Another critical objective is understanding the fundamental science behind single-atom catalysis, including metal-support interactions, reaction mechanisms, and the nature of active sites. This knowledge is essential for rational catalyst design rather than empirical development approaches. Researchers aim to establish structure-performance relationships that can guide the development of next-generation catalysts with tailored properties.
From an industrial perspective, the goal is to transition SAC from laboratory curiosities to practical catalysts for large-scale applications. This requires addressing challenges in synthesis scalability, long-term stability, and cost-effectiveness. Industries ranging from petrochemicals to fine chemicals, environmental remediation, and energy conversion are potential beneficiaries of SAC innovations, driving significant research investment in this area.
Market Demand Analysis for SAC Technologies
The global market for Single-Atom Catalysis (SAC) technologies is experiencing robust growth, driven by increasing demands for sustainable and efficient catalytic processes across multiple industries. Current market analysis indicates that the industrial catalysis sector, valued at over $25 billion globally, is actively seeking innovative solutions to address efficiency limitations and environmental concerns associated with traditional catalysts.
The petroleum refining industry represents the largest market segment for SAC technologies, as refineries worldwide face stringent environmental regulations and pressure to optimize production processes. The ability of single-atom catalysts to achieve near 100% atom utilization offers compelling economic advantages over conventional catalysts, which typically utilize only surface atoms effectively.
Chemical manufacturing constitutes another significant market segment, particularly in fine chemical synthesis and pharmaceutical production. These industries require highly selective catalysts capable of facilitating complex reactions with minimal byproduct formation. SAC technologies demonstrate superior selectivity in numerous reaction pathways, potentially reducing purification costs and waste generation by 30-40% compared to conventional catalytic systems.
Environmental applications represent the fastest-growing market segment for SAC technologies. The automotive industry's transition toward cleaner emission standards has intensified demand for more efficient catalytic converters. Similarly, industrial emission control systems require advanced catalytic solutions to meet increasingly stringent regulations. SAC-based systems have demonstrated capability to reduce precious metal usage by up to 90% while maintaining or improving catalytic performance.
Energy sector applications, particularly in hydrogen production and fuel cell technologies, present substantial growth opportunities. The hydrogen economy's expansion depends critically on cost-effective catalysts for water splitting and fuel cell reactions. SAC technologies offer potential breakthroughs in reducing dependency on platinum group metals, addressing a key economic barrier to widespread hydrogen adoption.
Market forecasts suggest the SAC technology market will grow at a compound annual growth rate exceeding 15% over the next decade. This growth trajectory is supported by increasing research investments from both public and private sectors, with over 200 research institutions and companies actively developing SAC applications worldwide.
Customer demand analysis reveals that industrial end-users prioritize three key performance indicators: catalyst longevity, selectivity, and cost-effectiveness. SAC technologies demonstrate competitive advantages in selectivity and material efficiency, though challenges in stability and scalable manufacturing remain significant market entry barriers that must be addressed to fully capitalize on market opportunities.
The petroleum refining industry represents the largest market segment for SAC technologies, as refineries worldwide face stringent environmental regulations and pressure to optimize production processes. The ability of single-atom catalysts to achieve near 100% atom utilization offers compelling economic advantages over conventional catalysts, which typically utilize only surface atoms effectively.
Chemical manufacturing constitutes another significant market segment, particularly in fine chemical synthesis and pharmaceutical production. These industries require highly selective catalysts capable of facilitating complex reactions with minimal byproduct formation. SAC technologies demonstrate superior selectivity in numerous reaction pathways, potentially reducing purification costs and waste generation by 30-40% compared to conventional catalytic systems.
Environmental applications represent the fastest-growing market segment for SAC technologies. The automotive industry's transition toward cleaner emission standards has intensified demand for more efficient catalytic converters. Similarly, industrial emission control systems require advanced catalytic solutions to meet increasingly stringent regulations. SAC-based systems have demonstrated capability to reduce precious metal usage by up to 90% while maintaining or improving catalytic performance.
Energy sector applications, particularly in hydrogen production and fuel cell technologies, present substantial growth opportunities. The hydrogen economy's expansion depends critically on cost-effective catalysts for water splitting and fuel cell reactions. SAC technologies offer potential breakthroughs in reducing dependency on platinum group metals, addressing a key economic barrier to widespread hydrogen adoption.
Market forecasts suggest the SAC technology market will grow at a compound annual growth rate exceeding 15% over the next decade. This growth trajectory is supported by increasing research investments from both public and private sectors, with over 200 research institutions and companies actively developing SAC applications worldwide.
Customer demand analysis reveals that industrial end-users prioritize three key performance indicators: catalyst longevity, selectivity, and cost-effectiveness. SAC technologies demonstrate competitive advantages in selectivity and material efficiency, though challenges in stability and scalable manufacturing remain significant market entry barriers that must be addressed to fully capitalize on market opportunities.
Current Status and Challenges in Single-Atom Catalysis
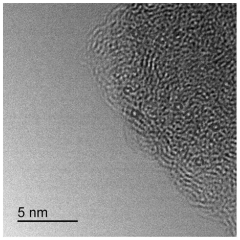
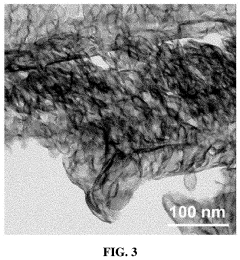
Single-atom catalysis (SAC) represents a frontier in heterogeneous catalysis research, with significant advancements achieved globally over the past decade. Currently, researchers have successfully synthesized single-atom catalysts (SACs) with various metal atoms (Pt, Pd, Ru, Fe, Co, Ni) dispersed on different supports including metal oxides, carbon materials, and metal-organic frameworks. The atomically dispersed active sites in SACs offer maximum atom utilization efficiency and unique catalytic properties that bridge homogeneous and heterogeneous catalysis.
Despite remarkable progress, several critical challenges impede the widespread industrial application of SACs. Stability remains a primary concern, as isolated metal atoms tend to aggregate under reaction conditions, particularly at elevated temperatures or in reducing environments. This sintering effect significantly compromises catalyst longevity and performance in industrial settings where extended operational lifetimes are essential.
Scalable synthesis presents another substantial hurdle. Laboratory methods that produce small quantities of high-quality SACs often involve complex procedures and expensive precursors, making industrial-scale production economically prohibitive. Current synthetic approaches frequently lack reproducibility when scaled up, resulting in inconsistent metal loading and dispersion across batches.
Characterization limitations further complicate SAC development. Even advanced techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy face challenges in precisely identifying the coordination environment and oxidation states of single atoms during catalytic reactions. This knowledge gap hinders rational catalyst design and optimization for specific industrial processes.
The geographic distribution of SAC research shows concentration in China, the United States, and Europe, with China leading in publication output and patent applications. Research institutions like the Chinese Academy of Sciences, University of California system, and Max Planck Society dominate the field, while industrial involvement from companies like BASF, Johnson Matthey, and Sinopec is gradually increasing but remains limited compared to academic efforts.
Performance gaps between laboratory demonstrations and industrial requirements constitute a significant barrier. While SACs show exceptional activity for certain reactions under controlled conditions, they often underperform in complex industrial environments with multiple reactants, byproducts, and potential catalyst poisons. The selectivity advantages demonstrated in laboratory settings frequently diminish when applied to real-world feedstocks.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, chemical engineers, and industrial partners to develop robust synthesis protocols, advanced in-situ characterization methods, and innovative stabilization strategies that can translate the promising fundamental science of single-atom catalysis into transformative industrial applications.
Despite remarkable progress, several critical challenges impede the widespread industrial application of SACs. Stability remains a primary concern, as isolated metal atoms tend to aggregate under reaction conditions, particularly at elevated temperatures or in reducing environments. This sintering effect significantly compromises catalyst longevity and performance in industrial settings where extended operational lifetimes are essential.
Scalable synthesis presents another substantial hurdle. Laboratory methods that produce small quantities of high-quality SACs often involve complex procedures and expensive precursors, making industrial-scale production economically prohibitive. Current synthetic approaches frequently lack reproducibility when scaled up, resulting in inconsistent metal loading and dispersion across batches.
Characterization limitations further complicate SAC development. Even advanced techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy face challenges in precisely identifying the coordination environment and oxidation states of single atoms during catalytic reactions. This knowledge gap hinders rational catalyst design and optimization for specific industrial processes.
The geographic distribution of SAC research shows concentration in China, the United States, and Europe, with China leading in publication output and patent applications. Research institutions like the Chinese Academy of Sciences, University of California system, and Max Planck Society dominate the field, while industrial involvement from companies like BASF, Johnson Matthey, and Sinopec is gradually increasing but remains limited compared to academic efforts.
Performance gaps between laboratory demonstrations and industrial requirements constitute a significant barrier. While SACs show exceptional activity for certain reactions under controlled conditions, they often underperform in complex industrial environments with multiple reactants, byproducts, and potential catalyst poisons. The selectivity advantages demonstrated in laboratory settings frequently diminish when applied to real-world feedstocks.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, chemical engineers, and industrial partners to develop robust synthesis protocols, advanced in-situ characterization methods, and innovative stabilization strategies that can translate the promising fundamental science of single-atom catalysis into transformative industrial applications.
Current Technical Solutions in Single-Atom Catalysis
01 Metal-based single-atom catalysts
Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials to maximize catalytic efficiency. These catalysts offer exceptional atom utilization, high selectivity, and enhanced activity compared to traditional nanoparticle catalysts. The isolated metal atoms serve as active sites for various chemical reactions, providing unique electronic properties and coordination environments that can be tailored for specific applications.- Metal-based single-atom catalysts: Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts offer maximum atom efficiency and unique catalytic properties due to their isolated nature. The metal atoms, typically transition metals, are anchored to supports like carbon, metal oxides, or 2D materials, creating active sites with distinct electronic structures and coordination environments that enable high catalytic activity and selectivity for various chemical reactions.
- Support materials for single-atom catalysts: The choice of support material plays a crucial role in stabilizing single atoms and influencing their catalytic performance. Various supports including carbon-based materials (graphene, carbon nanotubes), metal oxides (TiO2, ZnO, CeO2), zeolites, and metal-organic frameworks (MOFs) are used to anchor single metal atoms. These supports prevent atom aggregation while providing beneficial electronic interactions that can enhance catalytic activity, selectivity, and stability under reaction conditions.
- Synthesis methods for single-atom catalysts: Various synthesis strategies have been developed to prepare single-atom catalysts with high metal dispersion and stability. These include atomic layer deposition, wet chemistry methods (impregnation, co-precipitation), high-temperature atom trapping, photochemical reduction, and electrochemical deposition. Advanced techniques like defect engineering and coordination design are employed to create stable metal-support interactions that prevent atom aggregation during catalytic reactions, ensuring long-term stability and performance.
- Applications in energy conversion and environmental remediation: Single-atom catalysts demonstrate exceptional performance in energy-related applications and environmental remediation processes. They are employed in electrocatalytic reactions like hydrogen evolution, oxygen reduction/evolution, and CO2 reduction, offering higher activity and selectivity than conventional catalysts. In environmental applications, they efficiently catalyze pollutant degradation and transform harmful substances into benign products. Their high atom efficiency makes them particularly valuable for sustainable chemical processes and clean energy technologies.
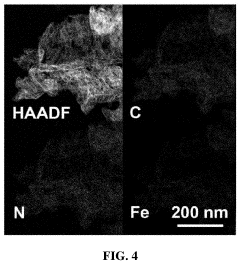
- Characterization and theoretical studies of single-atom catalysts: Advanced characterization techniques and theoretical studies are essential for understanding single-atom catalysts. Methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy enable direct visualization and electronic structure analysis of isolated atoms. Computational approaches including density functional theory calculations provide insights into reaction mechanisms, binding energies, and electronic properties. These combined experimental and theoretical approaches guide the rational design of more efficient single-atom catalysts with tailored properties.
02 Support materials for single-atom catalysts
The choice of support material plays a crucial role in stabilizing single atoms and preventing aggregation while influencing catalytic performance. Common support materials include carbon-based substrates (graphene, carbon nanotubes), metal oxides, and metal-organic frameworks (MOFs). These supports provide anchoring sites for single atoms through various interactions such as coordination bonds or electronic coupling, maintaining the dispersion of isolated atoms even under harsh reaction conditions.Expand Specific Solutions03 Synthesis methods for single-atom catalysts
Various synthesis strategies have been developed to prepare single-atom catalysts with high metal loading while preventing aggregation. These methods include atomic layer deposition, wet chemistry approaches (impregnation, co-precipitation), high-temperature atom trapping, and defect engineering. Advanced techniques like spatial confinement and coordination design enable precise control over the atomic dispersion and coordination environment of the metal centers, which directly influences their catalytic properties.Expand Specific Solutions04 Applications in energy conversion and environmental remediation
Single-atom catalysts demonstrate exceptional performance in energy-related applications such as hydrogen evolution, oxygen reduction/evolution reactions, CO2 reduction, and fuel cells. Their high atom efficiency makes them economically viable for utilizing precious metals in industrial processes. In environmental applications, these catalysts show remarkable activity for pollutant degradation, NOx reduction, and CO oxidation at lower temperatures than conventional catalysts, contributing to more sustainable chemical processes.Expand Specific Solutions05 Characterization and theoretical studies of single-atom catalysts
Advanced characterization techniques are essential for confirming the atomic dispersion and understanding the structure-property relationships in single-atom catalysts. Methods include aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy. Computational studies using density functional theory provide insights into reaction mechanisms, binding energies, and electronic structures of single-atom active sites, guiding rational design of more efficient catalysts with optimized coordination environments.Expand Specific Solutions
Key Industry Players in Single-Atom Catalysis Research
Single-atom catalysis represents a frontier in industrial catalysis, currently transitioning from early development to commercial application phases. The market is experiencing rapid growth, projected to reach significant scale as industries seek more efficient catalytic processes. Technologically, the field shows varying maturity levels across players. Research institutions like KIST, KAIST, and Chinese Academy of Sciences lead fundamental research, while companies including SK Innovation and Mitsubishi Heavy Industries are advancing industrial applications. Universities such as Johns Hopkins, Sun Yat-Sen, and Central South University contribute significantly to the knowledge base. The competitive landscape features strong collaboration between academic and industrial sectors, with Asian institutions particularly prominent in patent activity and technological innovation.
Central South University
Technical Solution: Central South University has developed significant innovations in single-atom catalysis with industrial applications, particularly focusing on non-precious metal single-atom catalysts for energy conversion and environmental remediation. Their research team has pioneered the development of atomically dispersed transition metal catalysts (Fe, Co, Ni) on nitrogen-doped carbon supports through controlled pyrolysis and chemical vapor deposition techniques[5]. A standout innovation is their development of single-atom Fe-N-C catalysts for the oxygen reduction reaction with performance metrics approaching platinum catalysts but at significantly reduced costs, making them viable alternatives for fuel cell applications. The university has also made breakthroughs in single-atom catalysts for CO2 electroreduction, achieving high Faradaic efficiencies for conversion to value-added chemicals like carbon monoxide and formic acid[6]. Their catalysts demonstrate exceptional stability under industrial-relevant conditions, maintaining activity for thousands of hours in accelerated durability tests. Central South University researchers have developed scalable synthesis methods that can produce gram-scale quantities of single-atom catalysts while maintaining atomic dispersion, addressing one of the key challenges in translating SAC technology to industrial applications.
Strengths: Exceptional cost-effectiveness through the use of earth-abundant metals instead of precious metals, making their catalysts economically viable for large-scale applications. Their catalysts show remarkable selectivity and activity for specific reactions, particularly in electrochemical energy conversion. Weaknesses: Some of their catalyst systems still show performance gaps compared to precious metal benchmarks in certain applications, and the long-term stability under variable industrial conditions requires further optimization.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE) at the Chinese Academy of Sciences has pioneered significant advancements in single-atom catalysis (SAC) for industrial applications. Their approach focuses on developing atomically dispersed metal catalysts on various supports, particularly for energy conversion and environmental remediation. IPE has developed a series of innovative preparation methods including atomic layer deposition, wet chemistry approaches, and high-temperature atom trapping techniques to achieve precise single-atom dispersion[1]. Their catalysts demonstrate remarkable activity in CO oxidation, water-gas shift reactions, and electrochemical reactions. A notable innovation is their development of thermally stable Pt1/CeO2 catalysts that maintain single-atom dispersion even at temperatures above 800°C, addressing one of the key challenges in industrial implementation of SACs[2]. IPE has also pioneered the use of in-situ characterization techniques to understand the dynamic structural changes of single-atom catalysts under reaction conditions, providing crucial insights for rational catalyst design.
Strengths: Superior atom efficiency utilizing nearly 100% of metal atoms as active sites, significantly reducing precious metal usage while maintaining or improving catalytic performance. Their catalysts show exceptional selectivity for targeted reactions due to uniform active sites. Weaknesses: Some of their SAC systems still face challenges in large-scale production and long-term stability under harsh industrial conditions, requiring further engineering solutions for commercial deployment.
Core Patents and Literature in SAC Innovation
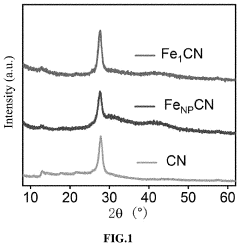
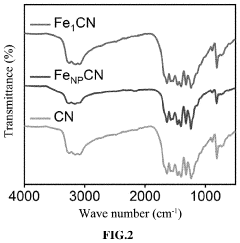
A kind of preparation method of nitrogen-doped carbon supported single-atom catalyst
PatentActiveCN109999883B
Innovation
- Adopting the idea of complex capture adsorption, through hydrothermal reaction and heat treatment technology, transition metal acetate and pyrrole monomer are used to form a complex. After stirring, hydrothermal, acid etching and heat treatment, a nitrogen-doped carbon load is generated. Single atom catalyst avoids agglomeration of metal ions and achieves single atom distribution.
Single-atom catalyst for activation of persulfate to generate pure singlet oxygen as well as preparation method and application thereof
PatentActiveUS11629052B2
Innovation
- A single-atom catalyst with graphitic carbon nitride nanosheets as supports and single iron atoms in a Fe—N4 coordination structure is developed, specifically designed to activate persulfate and generate pure singlet oxygen, enhancing selectivity and resistance to environmental interference.
Sustainability Impact of Single-Atom Catalysis
Single-atom catalysis (SAC) represents a paradigm shift in sustainable industrial practices, offering unprecedented atom efficiency by utilizing individual metal atoms as catalytic sites. This approach dramatically reduces precious metal usage by up to 95% compared to conventional nanoparticle catalysts, addressing critical resource scarcity concerns for platinum group metals and rare earth elements essential to modern industrial processes.
The environmental footprint of SAC extends beyond material conservation. These catalysts demonstrate superior energy efficiency, often operating at lower temperatures than traditional alternatives, thereby reducing industrial energy consumption and associated carbon emissions. In petrochemical processing, SAC systems have demonstrated energy savings of 15-30% while maintaining or improving conversion rates.
Waste reduction constitutes another significant sustainability advantage of SAC technology. The enhanced selectivity of single-atom catalysts minimizes unwanted by-products in chemical synthesis, pharmaceutical manufacturing, and fine chemical production. This selectivity translates to streamlined downstream processing, reduced solvent usage, and decreased waste treatment requirements—all contributing to circular economy principles.
In environmental remediation applications, single-atom catalysts have shown remarkable effectiveness in water purification and air pollution control. Their high activity enables efficient degradation of persistent organic pollutants and conversion of harmful emissions at concentrations where conventional catalysts prove ineffective. Recent field trials have demonstrated SAC-based systems capable of removing trace pharmaceutical contaminants from wastewater at concentrations below parts per billion.
The lifecycle sustainability benefits of SAC technology are particularly noteworthy. The extended catalyst lifespan—often 2-3 times longer than conventional catalysts due to reduced sintering and poisoning—decreases replacement frequency and associated environmental impacts. Additionally, the simplified recovery of precious metals from spent single-atom catalysts facilitates closed-loop material cycles.
From a regulatory perspective, SAC aligns with increasingly stringent environmental legislation worldwide. Its ability to enable cleaner production processes positions industries to meet and exceed compliance requirements while potentially qualifying for green certification programs and sustainability incentives.
The quantifiable sustainability impacts of SAC implementation are compelling: studies across multiple industrial sectors indicate potential reductions of 40-60% in catalyst-related carbon footprints, 50-80% decreases in precious metal consumption, and significant improvements in process mass intensity metrics—a key indicator of green chemistry principles in action.
The environmental footprint of SAC extends beyond material conservation. These catalysts demonstrate superior energy efficiency, often operating at lower temperatures than traditional alternatives, thereby reducing industrial energy consumption and associated carbon emissions. In petrochemical processing, SAC systems have demonstrated energy savings of 15-30% while maintaining or improving conversion rates.
Waste reduction constitutes another significant sustainability advantage of SAC technology. The enhanced selectivity of single-atom catalysts minimizes unwanted by-products in chemical synthesis, pharmaceutical manufacturing, and fine chemical production. This selectivity translates to streamlined downstream processing, reduced solvent usage, and decreased waste treatment requirements—all contributing to circular economy principles.
In environmental remediation applications, single-atom catalysts have shown remarkable effectiveness in water purification and air pollution control. Their high activity enables efficient degradation of persistent organic pollutants and conversion of harmful emissions at concentrations where conventional catalysts prove ineffective. Recent field trials have demonstrated SAC-based systems capable of removing trace pharmaceutical contaminants from wastewater at concentrations below parts per billion.
The lifecycle sustainability benefits of SAC technology are particularly noteworthy. The extended catalyst lifespan—often 2-3 times longer than conventional catalysts due to reduced sintering and poisoning—decreases replacement frequency and associated environmental impacts. Additionally, the simplified recovery of precious metals from spent single-atom catalysts facilitates closed-loop material cycles.
From a regulatory perspective, SAC aligns with increasingly stringent environmental legislation worldwide. Its ability to enable cleaner production processes positions industries to meet and exceed compliance requirements while potentially qualifying for green certification programs and sustainability incentives.
The quantifiable sustainability impacts of SAC implementation are compelling: studies across multiple industrial sectors indicate potential reductions of 40-60% in catalyst-related carbon footprints, 50-80% decreases in precious metal consumption, and significant improvements in process mass intensity metrics—a key indicator of green chemistry principles in action.
Scale-up Challenges and Industrial Implementation
The transition from laboratory-scale single-atom catalysis (SAC) to industrial implementation presents significant challenges that must be addressed for commercial viability. The primary obstacle lies in the scalable synthesis of single-atom catalysts while maintaining their unique atomic dispersion and preventing aggregation. Traditional batch processes often fail to deliver consistent atom distribution when scaled up, resulting in performance degradation and reduced catalytic efficiency.
Manufacturing single-atom catalysts at industrial scale requires precise control over numerous parameters including temperature, pressure, and reaction kinetics. The development of continuous flow processes represents a promising approach, allowing for better control of reaction conditions and potentially more uniform catalyst production. However, these systems demand sophisticated engineering solutions and significant capital investment, creating economic barriers to widespread adoption.
Stability issues present another critical challenge, as single-atom catalysts must maintain their dispersed state under harsh industrial conditions. High temperatures, pressure fluctuations, and exposure to various chemical environments can trigger atom migration and clustering, diminishing the catalytic advantages of isolated active sites. Innovative stabilization strategies, such as stronger metal-support interactions or encapsulation techniques, are being explored to enhance durability in industrial settings.
Cost considerations significantly impact industrial implementation. The synthesis of SACs often requires precious metals and specialized preparation methods, resulting in higher production costs compared to conventional catalysts. Economic viability necessitates either demonstrating substantially improved performance that justifies premium pricing or developing more cost-effective synthesis routes using earth-abundant metals and simplified procedures.
Characterization and quality control present unique challenges at industrial scale. The atomic-level dispersion that defines SACs requires sophisticated analytical techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy, which are difficult to implement as routine quality control measures in production environments. Developing practical, rapid assessment methods remains crucial for industrial adoption.
Regulatory considerations also influence implementation timelines. Novel catalytic materials must undergo safety assessments and environmental impact evaluations before industrial deployment. The unique properties of SACs may require specialized testing protocols to ensure compliance with existing regulations, potentially extending commercialization timelines.
Despite these challenges, several promising approaches are emerging to facilitate industrial implementation. These include the development of hierarchical support structures that enhance stability, the use of atomic layer deposition for precise atom placement, and the integration of computational modeling to optimize synthesis parameters and predict industrial performance.
Manufacturing single-atom catalysts at industrial scale requires precise control over numerous parameters including temperature, pressure, and reaction kinetics. The development of continuous flow processes represents a promising approach, allowing for better control of reaction conditions and potentially more uniform catalyst production. However, these systems demand sophisticated engineering solutions and significant capital investment, creating economic barriers to widespread adoption.
Stability issues present another critical challenge, as single-atom catalysts must maintain their dispersed state under harsh industrial conditions. High temperatures, pressure fluctuations, and exposure to various chemical environments can trigger atom migration and clustering, diminishing the catalytic advantages of isolated active sites. Innovative stabilization strategies, such as stronger metal-support interactions or encapsulation techniques, are being explored to enhance durability in industrial settings.
Cost considerations significantly impact industrial implementation. The synthesis of SACs often requires precious metals and specialized preparation methods, resulting in higher production costs compared to conventional catalysts. Economic viability necessitates either demonstrating substantially improved performance that justifies premium pricing or developing more cost-effective synthesis routes using earth-abundant metals and simplified procedures.
Characterization and quality control present unique challenges at industrial scale. The atomic-level dispersion that defines SACs requires sophisticated analytical techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy, which are difficult to implement as routine quality control measures in production environments. Developing practical, rapid assessment methods remains crucial for industrial adoption.
Regulatory considerations also influence implementation timelines. Novel catalytic materials must undergo safety assessments and environmental impact evaluations before industrial deployment. The unique properties of SACs may require specialized testing protocols to ensure compliance with existing regulations, potentially extending commercialization timelines.
Despite these challenges, several promising approaches are emerging to facilitate industrial implementation. These include the development of hierarchical support structures that enhance stability, the use of atomic layer deposition for precise atom placement, and the integration of computational modeling to optimize synthesis parameters and predict industrial performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!