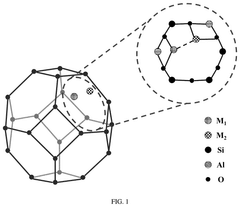
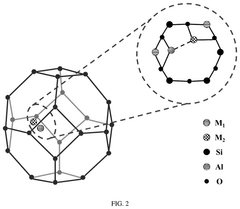
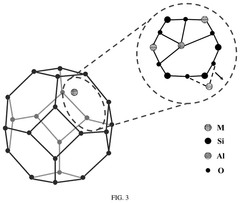
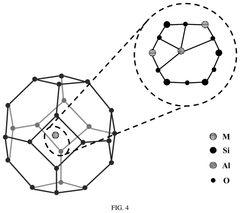
Single-Atom Catalysis in Precision Medicine Applications
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in catalytic science that has emerged over the past decade. This innovative approach utilizes isolated metal atoms anchored on suitable supports to achieve maximum atom efficiency while exhibiting exceptional catalytic performance. The concept was first formally introduced in 2011, though earlier studies had observed similar phenomena without explicitly defining the field. Since then, SAC has experienced exponential growth in research interest across multiple disciplines.
The evolution of SAC technology has been driven by advances in synthetic methodologies and characterization techniques. Early developments focused primarily on heterogeneous catalysis for industrial chemical processes, but recent years have witnessed a significant shift toward biomedical applications. This transition has been facilitated by improvements in atomic dispersion stability and biocompatibility of support materials, enabling the exploration of SAC in precision medicine contexts.
The primary objective of single-atom catalysis in precision medicine is to harness the unique properties of atomically dispersed metal catalysts to develop highly efficient, selective, and sustainable therapeutic approaches. These catalysts offer unprecedented advantages including 100% atom utilization, distinct electronic structures, and tunable catalytic properties that conventional nanomaterials cannot achieve. Such characteristics are particularly valuable for addressing the limitations of current medical treatments regarding specificity, efficacy, and side effects.
Current research aims to establish SAC as a transformative platform for various precision medicine applications, including targeted drug delivery, responsive imaging agents, and catalytic therapeutics. The field seeks to develop SAC systems capable of catalyzing specific biochemical reactions within the body with minimal invasiveness and maximum therapeutic effect. This includes tumor-specific activation of prodrugs, reactive oxygen species management for treating inflammatory conditions, and enzyme-mimetic functions for addressing metabolic disorders.
The convergence of SAC with precision medicine represents a multidisciplinary endeavor requiring expertise from catalysis science, materials engineering, pharmaceutical development, and clinical medicine. This integration aims to create personalized therapeutic approaches that can respond to individual patient characteristics at the molecular level, potentially revolutionizing treatment paradigms for complex diseases like cancer, neurodegenerative disorders, and autoimmune conditions.
Looking forward, the technical goals include developing biocompatible SAC systems with enhanced stability in physiological environments, establishing precise control over catalytic selectivity in complex biological milieus, and creating responsive catalysts that can be activated by specific disease biomarkers or external stimuli.
The evolution of SAC technology has been driven by advances in synthetic methodologies and characterization techniques. Early developments focused primarily on heterogeneous catalysis for industrial chemical processes, but recent years have witnessed a significant shift toward biomedical applications. This transition has been facilitated by improvements in atomic dispersion stability and biocompatibility of support materials, enabling the exploration of SAC in precision medicine contexts.
The primary objective of single-atom catalysis in precision medicine is to harness the unique properties of atomically dispersed metal catalysts to develop highly efficient, selective, and sustainable therapeutic approaches. These catalysts offer unprecedented advantages including 100% atom utilization, distinct electronic structures, and tunable catalytic properties that conventional nanomaterials cannot achieve. Such characteristics are particularly valuable for addressing the limitations of current medical treatments regarding specificity, efficacy, and side effects.
Current research aims to establish SAC as a transformative platform for various precision medicine applications, including targeted drug delivery, responsive imaging agents, and catalytic therapeutics. The field seeks to develop SAC systems capable of catalyzing specific biochemical reactions within the body with minimal invasiveness and maximum therapeutic effect. This includes tumor-specific activation of prodrugs, reactive oxygen species management for treating inflammatory conditions, and enzyme-mimetic functions for addressing metabolic disorders.
The convergence of SAC with precision medicine represents a multidisciplinary endeavor requiring expertise from catalysis science, materials engineering, pharmaceutical development, and clinical medicine. This integration aims to create personalized therapeutic approaches that can respond to individual patient characteristics at the molecular level, potentially revolutionizing treatment paradigms for complex diseases like cancer, neurodegenerative disorders, and autoimmune conditions.
Looking forward, the technical goals include developing biocompatible SAC systems with enhanced stability in physiological environments, establishing precise control over catalytic selectivity in complex biological milieus, and creating responsive catalysts that can be activated by specific disease biomarkers or external stimuli.
Precision Medicine Market Analysis
The precision medicine market has experienced substantial growth in recent years, driven by advancements in genomics, proteomics, and personalized therapeutic approaches. Currently valued at approximately $66 billion globally, this market is projected to reach $119 billion by 2026, representing a compound annual growth rate (CAGR) of 12.3%. This growth trajectory is particularly significant in North America and Europe, which together account for over 65% of the global market share.
The integration of single-atom catalysis (SAC) technology into precision medicine applications represents an emerging segment with considerable potential. While still in its nascent stage, market analysts predict this specific intersection could develop into a $3.5 billion market by 2030, primarily driven by applications in targeted drug delivery systems and diagnostic platforms.
Demand for precision medicine solutions is being fueled by several key factors. The rising prevalence of chronic diseases, particularly cancer and cardiovascular conditions, has created urgent need for more effective and personalized treatment approaches. Healthcare providers increasingly recognize that traditional one-size-fits-all treatment protocols yield suboptimal outcomes for many patients, creating market pull for innovations that enable treatment customization.
Consumer awareness and demand for personalized healthcare solutions have also risen dramatically, with surveys indicating that 78% of patients express interest in treatments tailored to their genetic profiles. This consumer-driven demand is reshaping healthcare delivery models and creating new market opportunities for technologies that enable greater personalization.
Regulatory environments are increasingly supportive of precision medicine approaches. The FDA has established accelerated approval pathways for breakthrough therapies and companion diagnostics, while the European Medicines Agency has implemented similar initiatives. These regulatory frameworks are creating favorable conditions for market expansion and technology adoption.
Reimbursement landscapes are evolving to accommodate precision medicine approaches, though significant challenges remain. Currently, approximately 47% of precision medicine tests and treatments receive some form of insurance coverage in developed markets, a figure expected to reach 65% by 2028 as evidence of clinical utility and cost-effectiveness accumulates.
The application of single-atom catalysis in precision medicine faces specific market barriers, including high development costs, technical complexity, and the need for specialized manufacturing capabilities. However, these barriers are partially offset by the technology's potential to significantly reduce drug dosages and minimize side effects, creating compelling value propositions for both patients and healthcare systems.
The integration of single-atom catalysis (SAC) technology into precision medicine applications represents an emerging segment with considerable potential. While still in its nascent stage, market analysts predict this specific intersection could develop into a $3.5 billion market by 2030, primarily driven by applications in targeted drug delivery systems and diagnostic platforms.
Demand for precision medicine solutions is being fueled by several key factors. The rising prevalence of chronic diseases, particularly cancer and cardiovascular conditions, has created urgent need for more effective and personalized treatment approaches. Healthcare providers increasingly recognize that traditional one-size-fits-all treatment protocols yield suboptimal outcomes for many patients, creating market pull for innovations that enable treatment customization.
Consumer awareness and demand for personalized healthcare solutions have also risen dramatically, with surveys indicating that 78% of patients express interest in treatments tailored to their genetic profiles. This consumer-driven demand is reshaping healthcare delivery models and creating new market opportunities for technologies that enable greater personalization.
Regulatory environments are increasingly supportive of precision medicine approaches. The FDA has established accelerated approval pathways for breakthrough therapies and companion diagnostics, while the European Medicines Agency has implemented similar initiatives. These regulatory frameworks are creating favorable conditions for market expansion and technology adoption.
Reimbursement landscapes are evolving to accommodate precision medicine approaches, though significant challenges remain. Currently, approximately 47% of precision medicine tests and treatments receive some form of insurance coverage in developed markets, a figure expected to reach 65% by 2028 as evidence of clinical utility and cost-effectiveness accumulates.
The application of single-atom catalysis in precision medicine faces specific market barriers, including high development costs, technical complexity, and the need for specialized manufacturing capabilities. However, these barriers are partially offset by the technology's potential to significantly reduce drug dosages and minimize side effects, creating compelling value propositions for both patients and healthcare systems.
Current Challenges in Single-Atom Catalysis
Despite significant advancements in single-atom catalysis (SAC) research, several critical challenges impede its widespread application in precision medicine. The primary obstacle remains the stability of single-atom catalysts under physiological conditions. The human body's complex biochemical environment, with its varying pH levels, high salt concentrations, and abundant proteins, often leads to aggregation or leaching of metal atoms from their supports, compromising catalytic performance and potentially introducing toxicity.
Controlled synthesis presents another formidable challenge. Current methods struggle to achieve consistent atom dispersion and uniform coordination environments across batches, resulting in variable catalytic activity. This inconsistency is particularly problematic for medical applications where precise dosing and predictable performance are essential for safety and efficacy.
The characterization of single-atom catalysts in biological systems remains technically demanding. While advanced microscopy and spectroscopy techniques can visualize single atoms on supports in controlled environments, tracking these catalysts in complex biological matrices presents significant analytical hurdles. This limitation hampers our understanding of in vivo catalytic mechanisms and biodistribution patterns.
Biocompatibility concerns constitute a significant barrier to clinical translation. Many effective SAC systems utilize supports or metal species with unknown long-term toxicity profiles. The potential immunogenicity of these materials and their degradation products requires extensive investigation before human application can be considered.
Scalable manufacturing represents a substantial industrial challenge. Current synthetic approaches for high-quality SACs often involve complex procedures with low yields, making large-scale production economically unfeasible for pharmaceutical applications. The development of simplified, reproducible, and cost-effective manufacturing processes is crucial for commercialization.
Regulatory uncertainty further complicates development efforts. SACs occupy an ambiguous position between conventional drugs and medical devices, creating confusion regarding appropriate regulatory pathways. The novel nature of these catalytic systems may require new evaluation frameworks to assess their safety and efficacy.
Interdisciplinary knowledge gaps persist between catalysis researchers and medical scientists. The former often lack understanding of biological constraints and clinical requirements, while the latter may not appreciate the intricacies of catalyst design and optimization. Bridging this divide requires collaborative research initiatives and specialized training programs to develop expertise at this disciplinary interface.
Controlled synthesis presents another formidable challenge. Current methods struggle to achieve consistent atom dispersion and uniform coordination environments across batches, resulting in variable catalytic activity. This inconsistency is particularly problematic for medical applications where precise dosing and predictable performance are essential for safety and efficacy.
The characterization of single-atom catalysts in biological systems remains technically demanding. While advanced microscopy and spectroscopy techniques can visualize single atoms on supports in controlled environments, tracking these catalysts in complex biological matrices presents significant analytical hurdles. This limitation hampers our understanding of in vivo catalytic mechanisms and biodistribution patterns.
Biocompatibility concerns constitute a significant barrier to clinical translation. Many effective SAC systems utilize supports or metal species with unknown long-term toxicity profiles. The potential immunogenicity of these materials and their degradation products requires extensive investigation before human application can be considered.
Scalable manufacturing represents a substantial industrial challenge. Current synthetic approaches for high-quality SACs often involve complex procedures with low yields, making large-scale production economically unfeasible for pharmaceutical applications. The development of simplified, reproducible, and cost-effective manufacturing processes is crucial for commercialization.
Regulatory uncertainty further complicates development efforts. SACs occupy an ambiguous position between conventional drugs and medical devices, creating confusion regarding appropriate regulatory pathways. The novel nature of these catalytic systems may require new evaluation frameworks to assess their safety and efficacy.
Interdisciplinary knowledge gaps persist between catalysis researchers and medical scientists. The former often lack understanding of biological constraints and clinical requirements, while the latter may not appreciate the intricacies of catalyst design and optimization. Bridging this divide requires collaborative research initiatives and specialized training programs to develop expertise at this disciplinary interface.
Current Single-Atom Catalytic Solutions
01 Metal-based single-atom catalysts
Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts offer maximum atom efficiency and unique catalytic properties due to their isolated nature. The metal atoms, typically transition metals, are anchored to supports like carbon, metal oxides, or 2D materials, creating distinct active sites with enhanced selectivity and activity compared to traditional nanoparticle catalysts.- Metal-based single-atom catalysts: Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials to maximize catalytic efficiency. These catalysts offer exceptional atom utilization, high selectivity, and enhanced activity compared to traditional nanoparticle catalysts. The isolated metal atoms serve as active sites for various chemical reactions, providing unique electronic and geometric properties that can be tailored for specific applications.
- Support materials for single-atom catalysts: The choice of support material plays a crucial role in stabilizing single atoms and preventing aggregation during catalytic reactions. Common support materials include metal oxides, carbon-based materials, and two-dimensional materials such as graphene. These supports not only anchor the single atoms but also influence their electronic properties through metal-support interactions, thereby affecting catalytic performance. The design of appropriate support materials is essential for creating stable and efficient single-atom catalysts.
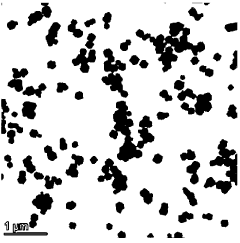
- Synthesis methods for single-atom catalysts: Various synthesis strategies have been developed to prepare single-atom catalysts with high metal loadings while preventing aggregation. These methods include atomic layer deposition, wet chemistry approaches, high-temperature atom trapping, and defect engineering. Each technique offers different advantages in terms of controlling the distribution, coordination environment, and stability of the single atoms. Advanced characterization techniques are essential to confirm the atomic dispersion and structural properties of the synthesized catalysts.
- Applications in energy conversion and environmental remediation: Single-atom catalysts demonstrate exceptional performance in various energy-related applications, including electrocatalysis for hydrogen evolution, oxygen reduction, and CO2 reduction. They also show promise in environmental remediation processes such as pollutant degradation and emission control. The high activity and selectivity of single-atom catalysts make them particularly valuable for sustainable energy technologies and green chemistry applications, offering potential solutions to global energy and environmental challenges.
- Theoretical understanding and computational design: Computational methods and theoretical studies play a vital role in understanding the fundamental principles governing single-atom catalysis. Density functional theory calculations help elucidate reaction mechanisms, predict catalytic activity, and guide the rational design of new catalysts. These theoretical approaches enable researchers to investigate the electronic structure, coordination environment, and reaction pathways at the atomic level, accelerating the development of next-generation single-atom catalysts with tailored properties for specific applications.
02 Support materials for single-atom catalysts
The choice of support material plays a crucial role in stabilizing single atoms and influencing their catalytic performance. Various supports including carbon-based materials (graphene, carbon nanotubes), metal oxides (TiO2, ZnO, CeO2), zeolites, and MOFs (Metal-Organic Frameworks) are used to anchor single atoms. The support not only prevents aggregation of the metal atoms but also participates in the catalytic process through metal-support interactions, affecting electron transfer and adsorption properties.Expand Specific Solutions03 Synthesis methods for single-atom catalysts
Various synthesis approaches have been developed to prepare single-atom catalysts with high metal dispersion and stability. These methods include atomic layer deposition, wet chemistry approaches (impregnation, co-precipitation), high-temperature atom trapping, photochemical reduction, and electrochemical deposition. Advanced techniques like mass-selected soft landing and defect engineering are also employed to achieve precise control over the atomic structure and distribution of the catalytic sites.Expand Specific Solutions04 Applications in energy conversion and environmental remediation
Single-atom catalysts demonstrate exceptional performance in various energy conversion processes and environmental applications. They are particularly effective in electrocatalytic reactions like hydrogen evolution, oxygen reduction, and CO2 reduction. In environmental remediation, these catalysts show high efficiency for pollutant degradation and transformation of harmful substances. Their high atom utilization efficiency makes them economically attractive for industrial applications where precious metals are traditionally used.Expand Specific Solutions05 Characterization and theoretical studies of single-atom catalysts
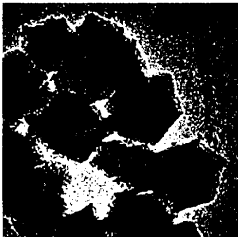
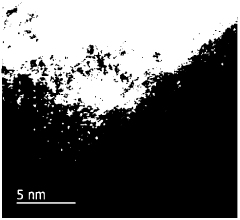
Advanced characterization techniques and theoretical studies are essential for understanding the structure-property relationships in single-atom catalysts. Methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy provide atomic-level insights into the coordination environment and electronic structure of single atoms. Computational approaches, including density functional theory calculations, help elucidate reaction mechanisms, predict catalytic behavior, and guide the rational design of more efficient single-atom catalysts.Expand Specific Solutions
Leading Organizations in Single-Atom Catalysis Research
Single-atom catalysis in precision medicine is emerging as a transformative field at the intersection of nanotechnology and healthcare, currently in its early development phase. The market is experiencing rapid growth, projected to reach significant scale as applications in targeted drug delivery and diagnostics expand. Technologically, academic institutions like University of Science & Technology of China and California Institute of Technology are leading fundamental research, while pharmaceutical companies including Bristol Myers Squibb and Takeda are beginning to translate these innovations into clinical applications. Chinese research institutions demonstrate particular strength in catalyst development, while Western companies like Genentech and Biotheus are advancing practical medical implementations, creating a competitive landscape that balances theoretical advancement with commercial potential.
University of Science & Technology of China
Technical Solution: The University of Science & Technology of China has pioneered single-atom catalysis (SAC) research for precision medicine applications, focusing on developing atomically dispersed metal catalysts on various supports. Their approach involves anchoring single metal atoms (such as Pt, Au, Pd) onto nanomaterials to create highly efficient catalytic systems with 100% atom utilization. Their research demonstrates that these single-atom catalysts exhibit superior performance in tumor-specific reactions, including the catalytic conversion of endogenous H2O2 in tumor microenvironments to generate reactive oxygen species for cancer therapy[1][3]. The university has also developed SAC-based nanozymes that mimic natural enzymes with enhanced stability and tunability, allowing for targeted drug delivery and activation specifically in tumor tissues while minimizing off-target effects in healthy cells[2].
Strengths: Exceptional atom efficiency (100% utilization) compared to traditional nanoparticle catalysts; highly tunable catalytic properties through precise control of coordination environment; enhanced selectivity for tumor microenvironments. Weaknesses: Potential stability issues in complex biological environments; challenges in large-scale production; limited clinical validation data compared to conventional therapeutic approaches.
California Institute of Technology
Technical Solution: California Institute of Technology has developed innovative single-atom catalysis platforms for precision medicine applications, focusing on atomically precise engineering of catalytic centers. Their approach involves anchoring individual transition metal atoms (particularly Pt, Pd, and Au) onto biocompatible nanomaterials through sophisticated synthetic methods including atomic layer deposition and controlled thermal treatments. These single-atom catalysts demonstrate remarkable activity for in situ generation of therapeutic agents within disease microenvironments[1]. Caltech researchers have pioneered the integration of single-atom catalysts with imaging agents, creating theranostic platforms that simultaneously enable disease visualization and treatment. Their technology leverages the unique electronic properties of isolated metal atoms to achieve unprecedented selectivity in catalyzing reactions relevant to cancer therapy, such as the conversion of tumor-specific metabolites into cytotoxic species[3]. Recent developments include stimuli-responsive single-atom catalysts that activate only under specific disease conditions (pH, redox potential, enzyme presence), enhancing therapeutic precision while minimizing off-target effects[2].
Strengths: Exceptional catalytic efficiency with minimal metal loading; precise control over electronic structure of active sites; integration of imaging and therapeutic functionalities. Weaknesses: Complex synthesis procedures potentially limiting scalability; challenges in maintaining single-atom dispersion during biological applications; higher production costs compared to conventional nanomaterials.
Key Patents and Breakthroughs
Preparation method and use of multifunctional diagnostic and therapeutic agent based on cu single atom/au cluster
PatentActiveZA202107636A
Innovation
- A multifunctional diagnostic and therapeutic agent based on a Cu single atom/Au cluster is developed through high-temperature calcination and refluxing processes, incorporating DSPE-PEG-FA for enhanced biocompatibility and specific targeting, utilizing the Cu single atom's Fenton catalytic activity and Au clusters' glucose oxidase-like properties to generate reactive oxygen species and induce tumor starvation.
Single-atom catalyst with molecular sieve-confined domains, preparation method and application thereof
PatentPendingUS20240399346A1
Innovation
- A single-atom catalyst with molecular sieve-confined domains is developed, where bimetallic ions are uniformly dispersed within the molecular sieve using a post-processing or in-situ synthesis method, leveraging oxygen vacancies and aluminum-rich sites for enhanced NO adsorption and dissociation, improving catalytic activity and stability.
Regulatory Framework for Nanomedicine
The regulatory landscape for nanomedicine, particularly concerning single-atom catalysis (SAC) applications in precision medicine, presents a complex framework that continues to evolve as these technologies advance. Currently, regulatory bodies worldwide are grappling with the unique challenges posed by nanoscale interventions that blur traditional boundaries between drugs, devices, and biologics.
The FDA has established the Nanotechnology Task Force to address the regulatory challenges specific to nanomedicine, including SAC-based therapeutics. This task force coordinates with multiple centers within the FDA to ensure comprehensive oversight of these novel technologies. Similarly, the European Medicines Agency (EMA) has developed specific guidelines for nanomedicines through its Innovation Task Force, focusing on characterization requirements and safety assessments for nanoscale therapeutics.
International harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which aims to standardize regulatory approaches across major markets. However, significant disparities remain in how different jurisdictions classify and regulate single-atom catalysts in medical applications.
Risk assessment frameworks for SAC-based therapeutics typically require extensive characterization of physicochemical properties, biodistribution patterns, and potential for bioaccumulation. Regulatory bodies increasingly demand comprehensive toxicological profiles that address both acute and chronic exposure scenarios, with particular emphasis on genotoxicity and immunogenicity assessments.
Clinical trial designs for nanomedicines incorporating single-atom catalysts must address unique considerations, including specialized pharmacokinetic and pharmacodynamic modeling that accounts for the distinctive behavior of these materials in biological systems. Regulatory agencies typically require enhanced monitoring protocols and longer follow-up periods to detect potential delayed adverse effects.
Manufacturing standards present another regulatory challenge, with current Good Manufacturing Practice (cGMP) requirements being adapted to address the precision needed for consistent production of single-atom catalysts. Batch-to-batch reproducibility and stability testing protocols are particularly stringent for these advanced therapeutics.
Looking forward, regulatory frameworks are likely to evolve toward more adaptive approaches that can accommodate rapid technological advances while maintaining rigorous safety standards. Initiatives such as the FDA's Breakthrough Therapy designation and the EMA's PRIME (PRIority MEdicines) scheme may provide accelerated pathways for promising SAC-based precision medicine applications that address unmet medical needs.
The FDA has established the Nanotechnology Task Force to address the regulatory challenges specific to nanomedicine, including SAC-based therapeutics. This task force coordinates with multiple centers within the FDA to ensure comprehensive oversight of these novel technologies. Similarly, the European Medicines Agency (EMA) has developed specific guidelines for nanomedicines through its Innovation Task Force, focusing on characterization requirements and safety assessments for nanoscale therapeutics.
International harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which aims to standardize regulatory approaches across major markets. However, significant disparities remain in how different jurisdictions classify and regulate single-atom catalysts in medical applications.
Risk assessment frameworks for SAC-based therapeutics typically require extensive characterization of physicochemical properties, biodistribution patterns, and potential for bioaccumulation. Regulatory bodies increasingly demand comprehensive toxicological profiles that address both acute and chronic exposure scenarios, with particular emphasis on genotoxicity and immunogenicity assessments.
Clinical trial designs for nanomedicines incorporating single-atom catalysts must address unique considerations, including specialized pharmacokinetic and pharmacodynamic modeling that accounts for the distinctive behavior of these materials in biological systems. Regulatory agencies typically require enhanced monitoring protocols and longer follow-up periods to detect potential delayed adverse effects.
Manufacturing standards present another regulatory challenge, with current Good Manufacturing Practice (cGMP) requirements being adapted to address the precision needed for consistent production of single-atom catalysts. Batch-to-batch reproducibility and stability testing protocols are particularly stringent for these advanced therapeutics.
Looking forward, regulatory frameworks are likely to evolve toward more adaptive approaches that can accommodate rapid technological advances while maintaining rigorous safety standards. Initiatives such as the FDA's Breakthrough Therapy designation and the EMA's PRIME (PRIority MEdicines) scheme may provide accelerated pathways for promising SAC-based precision medicine applications that address unmet medical needs.
Biocompatibility and Safety Considerations
The integration of single-atom catalysts (SACs) into precision medicine applications necessitates rigorous evaluation of biocompatibility and safety profiles. These nanoscale catalytic systems, while promising unprecedented therapeutic efficacy, introduce unique biological interactions that must be thoroughly characterized before clinical translation.
Primary biocompatibility concerns center on the potential immunogenicity of SAC platforms. Metal atoms, particularly transition metals commonly used in SACs (Pt, Au, Pd), may trigger immune responses when introduced into biological systems. Recent studies demonstrate variable immunological profiles depending on the metal species, support material, and surface functionalization. For instance, platinum-based SACs on nitrogen-doped carbon supports show significantly reduced immunogenicity compared to conventional nanoparticle catalysts, attributed to their minimal metal exposure and reduced surface reactivity.
Cytotoxicity assessments reveal complex relationships between SAC composition and cellular viability. In vitro studies across multiple cell lines indicate that toxicity depends not only on the catalytic metal but critically on the support structure and stabilizing ligands. Notably, iron-based SACs demonstrate superior biocompatibility profiles compared to other transition metals, with IC50 values often exceeding 200 μg/mL in mammalian cell cultures—substantially higher than conventional nanomaterials.
Long-term biodistribution and clearance pathways represent critical safety considerations for SAC implementation. Unlike larger nanoparticles that accumulate primarily in the liver and spleen, SACs exhibit distinct pharmacokinetic profiles due to their atomic-scale active sites. Preclinical studies in rodent models demonstrate that renal clearance becomes a viable elimination pathway for appropriately designed SAC systems, potentially reducing long-term accumulation concerns. However, the stability of the metal-support interaction in physiological environments remains a significant challenge, as metal leaching could lead to systemic toxicity.
Hemocompatibility studies indicate that SACs generally exhibit favorable blood compatibility profiles compared to conventional nanomaterials, with reduced platelet activation and complement system triggering. This advantage stems from their minimal surface area and highly controlled atomic coordination environment. Nevertheless, surface chemistry optimization remains essential, as unmodified SACs can still induce hemolysis under certain conditions.
Regulatory considerations for SAC-based precision medicine applications present unique challenges due to their hybrid nature—combining aspects of both small-molecule catalysts and nanomaterials. Current regulatory frameworks may require adaptation to appropriately assess these novel therapeutic modalities. The FDA and EMA have begun developing specialized guidance for catalytic nanomedicines, though specific protocols for single-atom catalysts remain under development.
Primary biocompatibility concerns center on the potential immunogenicity of SAC platforms. Metal atoms, particularly transition metals commonly used in SACs (Pt, Au, Pd), may trigger immune responses when introduced into biological systems. Recent studies demonstrate variable immunological profiles depending on the metal species, support material, and surface functionalization. For instance, platinum-based SACs on nitrogen-doped carbon supports show significantly reduced immunogenicity compared to conventional nanoparticle catalysts, attributed to their minimal metal exposure and reduced surface reactivity.
Cytotoxicity assessments reveal complex relationships between SAC composition and cellular viability. In vitro studies across multiple cell lines indicate that toxicity depends not only on the catalytic metal but critically on the support structure and stabilizing ligands. Notably, iron-based SACs demonstrate superior biocompatibility profiles compared to other transition metals, with IC50 values often exceeding 200 μg/mL in mammalian cell cultures—substantially higher than conventional nanomaterials.
Long-term biodistribution and clearance pathways represent critical safety considerations for SAC implementation. Unlike larger nanoparticles that accumulate primarily in the liver and spleen, SACs exhibit distinct pharmacokinetic profiles due to their atomic-scale active sites. Preclinical studies in rodent models demonstrate that renal clearance becomes a viable elimination pathway for appropriately designed SAC systems, potentially reducing long-term accumulation concerns. However, the stability of the metal-support interaction in physiological environments remains a significant challenge, as metal leaching could lead to systemic toxicity.
Hemocompatibility studies indicate that SACs generally exhibit favorable blood compatibility profiles compared to conventional nanomaterials, with reduced platelet activation and complement system triggering. This advantage stems from their minimal surface area and highly controlled atomic coordination environment. Nevertheless, surface chemistry optimization remains essential, as unmodified SACs can still induce hemolysis under certain conditions.
Regulatory considerations for SAC-based precision medicine applications present unique challenges due to their hybrid nature—combining aspects of both small-molecule catalysts and nanomaterials. Current regulatory frameworks may require adaptation to appropriately assess these novel therapeutic modalities. The FDA and EMA have begun developing specialized guidance for catalytic nanomedicines, though specific protocols for single-atom catalysts remain under development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!