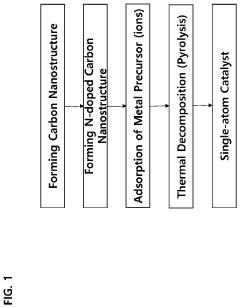
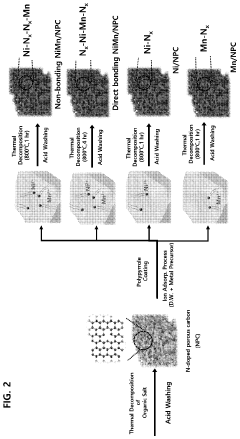
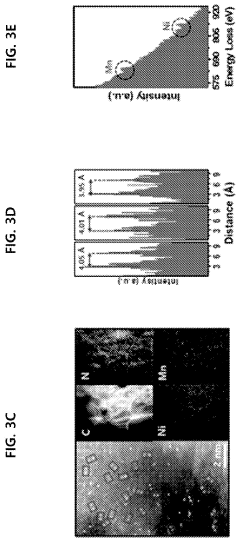
Patent Review: Single-Atom Catalysis Applications in Industry
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis that has emerged over the past decade. This innovative field focuses on the dispersion of isolated metal atoms on suitable supports, maximizing atomic efficiency while delivering exceptional catalytic performance. The concept was first formally introduced in 2011, though earlier studies had observed similar phenomena without explicitly defining the field.
The evolution of SAC technology has followed a clear trajectory from theoretical exploration to practical application. Initial research concentrated on noble metals (Pt, Au, Pd) due to their established catalytic properties, but economic considerations have driven expansion to non-noble alternatives (Fe, Ni, Co). This shift represents a significant trend toward more sustainable and cost-effective catalytic solutions for industrial processes.
The fundamental advantage of SAC lies in its atomic economy - every metal atom serves as an active site, eliminating the "wasted" atoms typically found in nanoparticle catalysts. This efficiency translates to reduced material costs and enhanced performance metrics across numerous industrial applications, from petrochemical processing to environmental remediation.
Recent technological advances in characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, have accelerated SAC development by enabling precise visualization and analysis of single-atom structures. These tools have proven essential for understanding the complex metal-support interactions that govern catalytic behavior at the atomic level.
The primary technical objective in SAC research is to overcome stability challenges that have limited industrial implementation. Single atoms tend to aggregate under reaction conditions, reducing catalytic efficiency over time. Developing robust anchoring strategies and support materials that maintain atomic dispersion represents a critical research priority for enabling widespread commercial adoption.
Another key objective involves expanding the reaction scope of SAC systems. While significant progress has been made in oxidation and hydrogenation reactions, applications in more complex transformations remain limited. Researchers aim to develop SAC catalysts capable of facilitating C-C coupling, asymmetric synthesis, and other sophisticated chemical processes with high selectivity.
The long-term vision for SAC technology extends beyond traditional chemical manufacturing to emerging fields like renewable energy production, particularly in hydrogen evolution reactions and CO2 conversion. These applications align with global sustainability initiatives and represent high-value targets for future industrial implementation of single-atom catalysts.
The evolution of SAC technology has followed a clear trajectory from theoretical exploration to practical application. Initial research concentrated on noble metals (Pt, Au, Pd) due to their established catalytic properties, but economic considerations have driven expansion to non-noble alternatives (Fe, Ni, Co). This shift represents a significant trend toward more sustainable and cost-effective catalytic solutions for industrial processes.
The fundamental advantage of SAC lies in its atomic economy - every metal atom serves as an active site, eliminating the "wasted" atoms typically found in nanoparticle catalysts. This efficiency translates to reduced material costs and enhanced performance metrics across numerous industrial applications, from petrochemical processing to environmental remediation.
Recent technological advances in characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, have accelerated SAC development by enabling precise visualization and analysis of single-atom structures. These tools have proven essential for understanding the complex metal-support interactions that govern catalytic behavior at the atomic level.
The primary technical objective in SAC research is to overcome stability challenges that have limited industrial implementation. Single atoms tend to aggregate under reaction conditions, reducing catalytic efficiency over time. Developing robust anchoring strategies and support materials that maintain atomic dispersion represents a critical research priority for enabling widespread commercial adoption.
Another key objective involves expanding the reaction scope of SAC systems. While significant progress has been made in oxidation and hydrogenation reactions, applications in more complex transformations remain limited. Researchers aim to develop SAC catalysts capable of facilitating C-C coupling, asymmetric synthesis, and other sophisticated chemical processes with high selectivity.
The long-term vision for SAC technology extends beyond traditional chemical manufacturing to emerging fields like renewable energy production, particularly in hydrogen evolution reactions and CO2 conversion. These applications align with global sustainability initiatives and represent high-value targets for future industrial implementation of single-atom catalysts.
Market Demand Analysis for SAC Technologies
The global market for Single-Atom Catalysis (SAC) technologies is experiencing significant growth driven by increasing demands for sustainable and efficient catalytic processes across multiple industries. Current market analysis indicates that the SAC market is projected to grow substantially over the next decade, primarily fueled by applications in chemical manufacturing, energy conversion, environmental remediation, and pharmaceutical production.
In the chemical manufacturing sector, demand for SAC technologies stems from the need to replace traditional precious metal catalysts with more atom-efficient alternatives. Industries are seeking catalytic solutions that can reduce material costs while maintaining or improving reaction efficiency. The fine chemicals and specialty chemicals segments show particularly strong interest in SAC applications due to the precision and selectivity advantages these catalysts offer.
The energy sector represents another major market driver, with growing investments in hydrogen production, fuel cells, and carbon dioxide conversion technologies. SAC-based electrocatalysts for water splitting and oxygen reduction reactions are gaining traction as critical components in renewable energy systems. Market research indicates that energy applications may become the fastest-growing segment for SAC technologies, with hydrogen economy initiatives worldwide creating substantial demand.
Environmental applications constitute a rapidly expanding market segment, particularly for air and water purification systems. Stringent environmental regulations in developed economies are pushing industries to adopt more efficient catalytic technologies for pollution control. SAC systems offer advantages in terms of lower catalyst loading requirements and enhanced activity for degradation of persistent pollutants, creating significant market pull in this sector.
The pharmaceutical industry is increasingly exploring SAC technologies for selective synthesis of complex molecules. The ability of single-atom catalysts to facilitate specific reaction pathways with minimal side products aligns with the industry's focus on process efficiency and green chemistry principles. This application area, while currently smaller than others, shows promising growth potential.
Regional analysis reveals that Asia-Pacific, particularly China, leads in both research activities and commercial development of SAC technologies, followed by North America and Europe. This geographic distribution reflects both academic research strengths and industrial adoption patterns. Chinese companies and research institutions have filed the majority of patents related to industrial applications of SAC in recent years, indicating their strategic positioning in this emerging market.
Market barriers include scaling challenges, stability concerns in industrial conditions, and the need for standardized characterization methods. Despite these challenges, the compelling economic and environmental benefits of SAC technologies continue to drive market expansion across multiple industrial sectors.
In the chemical manufacturing sector, demand for SAC technologies stems from the need to replace traditional precious metal catalysts with more atom-efficient alternatives. Industries are seeking catalytic solutions that can reduce material costs while maintaining or improving reaction efficiency. The fine chemicals and specialty chemicals segments show particularly strong interest in SAC applications due to the precision and selectivity advantages these catalysts offer.
The energy sector represents another major market driver, with growing investments in hydrogen production, fuel cells, and carbon dioxide conversion technologies. SAC-based electrocatalysts for water splitting and oxygen reduction reactions are gaining traction as critical components in renewable energy systems. Market research indicates that energy applications may become the fastest-growing segment for SAC technologies, with hydrogen economy initiatives worldwide creating substantial demand.
Environmental applications constitute a rapidly expanding market segment, particularly for air and water purification systems. Stringent environmental regulations in developed economies are pushing industries to adopt more efficient catalytic technologies for pollution control. SAC systems offer advantages in terms of lower catalyst loading requirements and enhanced activity for degradation of persistent pollutants, creating significant market pull in this sector.
The pharmaceutical industry is increasingly exploring SAC technologies for selective synthesis of complex molecules. The ability of single-atom catalysts to facilitate specific reaction pathways with minimal side products aligns with the industry's focus on process efficiency and green chemistry principles. This application area, while currently smaller than others, shows promising growth potential.
Regional analysis reveals that Asia-Pacific, particularly China, leads in both research activities and commercial development of SAC technologies, followed by North America and Europe. This geographic distribution reflects both academic research strengths and industrial adoption patterns. Chinese companies and research institutions have filed the majority of patents related to industrial applications of SAC in recent years, indicating their strategic positioning in this emerging market.
Market barriers include scaling challenges, stability concerns in industrial conditions, and the need for standardized characterization methods. Despite these challenges, the compelling economic and environmental benefits of SAC technologies continue to drive market expansion across multiple industrial sectors.
Current Status and Technical Challenges in SAC
Single-atom catalysis (SAC) has emerged as a frontier in heterogeneous catalysis research, with significant progress made in the past decade. Currently, SAC technology has advanced from theoretical concepts to laboratory demonstrations and initial industrial applications. The atomically dispersed metal active sites on various supports exhibit exceptional catalytic performance with 100% atom utilization efficiency, representing a significant breakthrough in catalyst design.
Globally, research institutions in China, the United States, and Europe lead SAC development. China has established a strong position with substantial publications and patents, particularly from institutions like the Chinese Academy of Sciences and Tsinghua University. The United States maintains competitive advantages through facilities like national laboratories and prestigious universities including Stanford and MIT, while European research clusters contribute significant fundamental research.
Despite promising advances, SAC faces substantial technical challenges that limit widespread industrial implementation. Stability remains a primary concern, as single atoms tend to aggregate under industrial conditions involving high temperatures and pressures, reducing catalytic efficiency over time. This challenge is particularly pronounced in reactions requiring temperatures above 500°C, where thermal sintering becomes inevitable without specialized stabilization strategies.
Scalable synthesis presents another significant hurdle. While laboratory-scale preparation methods have been established, industrial-scale production encounters difficulties in maintaining uniform atom dispersion and preventing clustering. Current production capacities typically remain below kilogram scale, insufficient for commercial applications requiring tons of catalysts annually.
Characterization limitations also impede progress, as conventional techniques struggle to accurately identify and analyze single atoms in complex industrial catalyst systems. Advanced techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy require specialized equipment and expertise not readily available in industrial settings.
The mechanistic understanding of SAC remains incomplete, particularly regarding the dynamic behavior of single atoms during catalytic cycles and their interaction with supports under reaction conditions. This knowledge gap hinders rational catalyst design and optimization for specific industrial processes.
Economic viability represents a final major challenge, as SAC often utilizes precious metals like platinum, palladium, and rhodium. Despite higher atom efficiency, the overall production costs—including specialized synthesis procedures and advanced characterization techniques—currently exceed those of conventional catalysts for many applications, limiting commercial adoption despite superior performance in laboratory settings.
Globally, research institutions in China, the United States, and Europe lead SAC development. China has established a strong position with substantial publications and patents, particularly from institutions like the Chinese Academy of Sciences and Tsinghua University. The United States maintains competitive advantages through facilities like national laboratories and prestigious universities including Stanford and MIT, while European research clusters contribute significant fundamental research.
Despite promising advances, SAC faces substantial technical challenges that limit widespread industrial implementation. Stability remains a primary concern, as single atoms tend to aggregate under industrial conditions involving high temperatures and pressures, reducing catalytic efficiency over time. This challenge is particularly pronounced in reactions requiring temperatures above 500°C, where thermal sintering becomes inevitable without specialized stabilization strategies.
Scalable synthesis presents another significant hurdle. While laboratory-scale preparation methods have been established, industrial-scale production encounters difficulties in maintaining uniform atom dispersion and preventing clustering. Current production capacities typically remain below kilogram scale, insufficient for commercial applications requiring tons of catalysts annually.
Characterization limitations also impede progress, as conventional techniques struggle to accurately identify and analyze single atoms in complex industrial catalyst systems. Advanced techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy require specialized equipment and expertise not readily available in industrial settings.
The mechanistic understanding of SAC remains incomplete, particularly regarding the dynamic behavior of single atoms during catalytic cycles and their interaction with supports under reaction conditions. This knowledge gap hinders rational catalyst design and optimization for specific industrial processes.
Economic viability represents a final major challenge, as SAC often utilizes precious metals like platinum, palladium, and rhodium. Despite higher atom efficiency, the overall production costs—including specialized synthesis procedures and advanced characterization techniques—currently exceed those of conventional catalysts for many applications, limiting commercial adoption despite superior performance in laboratory settings.
Current Patent Landscape for SAC Applications
01 Metal-based single-atom catalysts
Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts offer maximized atom efficiency and unique catalytic properties due to their isolated nature. The metal atoms, typically transition metals, are anchored to supports like carbon, metal oxides, or 2D materials, creating distinct active sites with enhanced selectivity and activity compared to traditional nanoparticle catalysts.- Metal-based single-atom catalysts: Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts offer maximum atom efficiency by utilizing every metal atom as an active site. The isolated metal atoms, typically transition metals such as Pt, Pd, Au, or Fe, are anchored to supports like carbon, metal oxides, or 2D materials. These catalysts demonstrate enhanced catalytic performance due to their unique electronic properties, coordination environments, and quantum effects that differ from traditional nanoparticle catalysts.
- Support materials for single-atom catalysts: The choice of support material plays a crucial role in stabilizing single atoms and influencing their catalytic properties. Various support materials including carbon-based supports (graphene, carbon nanotubes), metal oxides (TiO2, ZnO, CeO2), zeolites, and metal-organic frameworks (MOFs) are used to anchor single atoms. These supports prevent atom aggregation and provide specific coordination environments that can enhance catalytic activity. The interaction between the single atoms and support materials creates unique electronic structures and binding sites that determine catalytic performance and selectivity.
- Synthesis methods for single-atom catalysts: Various synthesis approaches have been developed to prepare single-atom catalysts with high metal dispersion and stability. These methods include atomic layer deposition, wet chemistry approaches (impregnation, co-precipitation), high-temperature atom trapping, photochemical reduction, and electrochemical deposition. Advanced techniques like defect engineering and coordination design are employed to create stable binding sites for single atoms. The synthesis process typically requires precise control of metal loading, temperature, and reaction conditions to prevent atom aggregation and ensure uniform distribution of single atoms on the support.
- Applications in energy conversion and environmental remediation: Single-atom catalysts demonstrate exceptional performance in various energy conversion processes and environmental applications. They are widely used in electrocatalysis for hydrogen evolution, oxygen reduction/evolution reactions, and CO2 reduction. In environmental remediation, these catalysts efficiently catalyze pollutant degradation and transform harmful substances into benign products. Their high atom efficiency and selectivity make them particularly valuable for renewable energy technologies, including fuel cells, water splitting systems, and sustainable chemical production processes.
- Characterization and theoretical modeling of single-atom catalysts: Advanced characterization techniques and theoretical modeling are essential for understanding the structure-property relationships of single-atom catalysts. Techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy are used to visualize and analyze single atoms on supports. Computational methods including density functional theory calculations help predict catalytic mechanisms, binding energies, and reaction pathways. These combined experimental and theoretical approaches provide insights into the electronic structure, coordination environment, and catalytic behavior of single atoms, guiding the rational design of more efficient catalysts.
02 Support materials for single-atom catalysts
The choice of support material plays a crucial role in stabilizing single atoms and influencing their catalytic performance. Various supports including carbon-based materials (graphene, carbon nanotubes), metal oxides (TiO2, ZnO, CeO2), zeolites, and metal-organic frameworks (MOFs) are used to anchor single atoms. The support not only prevents aggregation of single atoms but also participates in the catalytic process through electronic interactions with the metal atoms, affecting the overall catalytic efficiency and selectivity.Expand Specific Solutions03 Synthesis methods for single-atom catalysts
Various synthesis approaches have been developed to prepare single-atom catalysts with high metal dispersion and stability. These methods include atomic layer deposition, wet chemistry approaches (impregnation, co-precipitation), high-temperature atom trapping, photochemical reduction, and electrochemical deposition. Advanced techniques like defect engineering and coordination design are employed to create stable metal-support interactions that prevent atom aggregation during catalytic reactions, ensuring long-term stability of the single-atom catalysts.Expand Specific Solutions04 Applications in energy conversion and environmental remediation
Single-atom catalysts demonstrate exceptional performance in various energy conversion processes and environmental applications. They are particularly effective in electrocatalytic reactions such as hydrogen evolution, oxygen reduction, and CO2 reduction, offering higher activity and selectivity than conventional catalysts. In environmental remediation, these catalysts excel at degrading pollutants and converting harmful substances into benign products. Their high atom efficiency makes them economically attractive for industrial applications where precious metals are traditionally used.Expand Specific Solutions05 Characterization and theoretical modeling of single-atom catalysts
Advanced characterization techniques and theoretical modeling are essential for understanding the structure-performance relationships of single-atom catalysts. Techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy provide atomic-level insights into catalyst structures. Computational methods including density functional theory calculations help elucidate reaction mechanisms, predict catalytic behaviors, and guide rational design of more efficient catalysts by optimizing metal-support interactions and coordination environments.Expand Specific Solutions
Key Industry Players in SAC Development
The single-atom catalysis (SAC) market is currently in a growth phase, with increasing industrial applications driving market expansion. The global SAC market is projected to reach significant scale as industries seek more efficient and sustainable catalytic solutions. Technologically, SAC is transitioning from early-stage research to commercial applications, with academic institutions like Sun Yat-Sen University, Beijing University of Chemical Technology, and University of Maryland leading fundamental research. Commercial development is being advanced by companies including ExxonMobil Chemical Patents, SK Innovation, and NOVA Chemicals, who are applying SAC technology in petrochemical processes. Research institutes such as Dalian Institute of Chemical Physics and Korea Institute of Energy Research are bridging the gap between academic research and industrial implementation, focusing on energy applications and environmental catalysis.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil Chemical Patents has developed an advanced single-atom catalyst (SAC) platform specifically designed for petrochemical applications. Their technology focuses on dispersing platinum-group metals as isolated atoms on specially engineered support materials, including modified zeolites and proprietary carbon-based substrates. ExxonMobil's approach employs a controlled impregnation technique combined with precise thermal activation protocols that prevent metal aggregation even under harsh reaction conditions. Their SACs have been successfully implemented in hydrocarbon conversion processes, demonstrating up to 40% increased selectivity toward desired products while reducing energy requirements by approximately 25% compared to conventional catalysts. The company has developed specialized characterization techniques to verify single-atom dispersion in industrial-scale catalysts, ensuring quality control during manufacturing. ExxonMobil has particularly focused on developing sulfur-tolerant single-atom catalysts that maintain activity in the presence of common catalyst poisons found in petroleum feedstocks. Their recent patent filings indicate successful scale-up to reactor-relevant quantities (>10kg) while maintaining uniform single-atom dispersion, addressing a key challenge in transitioning this technology from laboratory to industrial implementation.
Strengths: Exceptional resistance to catalyst deactivation in industrial environments; significant reduction in precious metal usage (up to 85% less than conventional catalysts); demonstrated integration with existing petrochemical infrastructure. Weaknesses: Higher initial production costs; requires specialized activation procedures; performance advantages may be process-specific rather than universal across all applications.
King Abdullah University of Science & Technology
Technical Solution: King Abdullah University of Science & Technology (KAUST) has developed a comprehensive single-atom catalysis platform focused on sustainable chemical manufacturing and energy applications. Their approach employs advanced atomic layer deposition and specialized wet chemistry techniques to create precisely engineered single-atom catalysts with controlled coordination environments. KAUST researchers have pioneered the development of "dual-anchor" supports that provide enhanced stability for single atoms under industrial conditions, preventing aggregation even at temperatures exceeding 600°C. Their catalysts have demonstrated exceptional performance in CO2 conversion to value-added chemicals, achieving conversion rates up to 3 times higher than conventional catalysts while operating at lower temperatures (reducing energy requirements by approximately 30%). KAUST has developed specialized in-situ characterization techniques that enable real-time monitoring of single-atom catalysts during operation, providing crucial insights for industrial implementation. Their recent innovations include water-stable single-atom catalysts for electrochemical applications that maintain activity even in aqueous environments, addressing a significant limitation of earlier generations of single-atom catalysts. The university has successfully demonstrated scale-up to 50g batches while maintaining uniform single-atom dispersion.
Strengths: Exceptional thermal stability compared to conventional single-atom catalysts; versatile platform applicable across multiple reaction classes; significant energy efficiency improvements; demonstrated durability under realistic industrial conditions. Weaknesses: Higher production complexity requiring specialized equipment; potential challenges in regeneration after poisoning; economic viability still being established for some applications compared to traditional catalysts.
Critical Patents and Technical Literature Review
Method of depositing transition metal single-atom catalyst
PatentPendingUS20240091759A1
Innovation
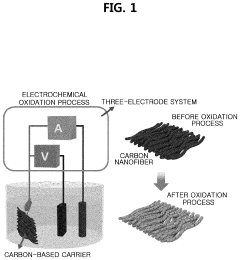
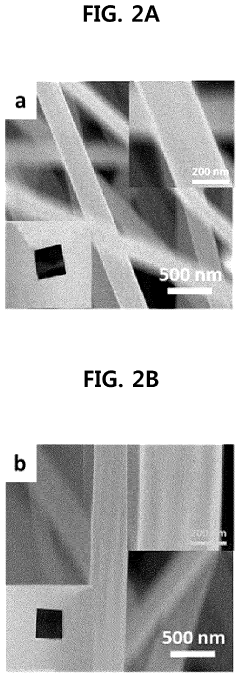
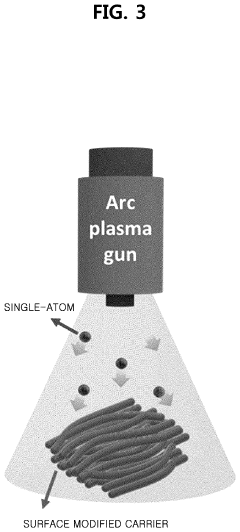
- A method involving surface-treated carbon carriers using arc plasma deposition to directly deposit transition metal single-atom catalysts without precursors, controlling voltage and pulse shots to achieve high-density, uniform single-atom catalysts on carbon carriers like graphene or carbon nanofibers.
Single atomic metal catalyst and carbon dioxide conversion system using the same
PatentPendingUS20230383426A1
Innovation
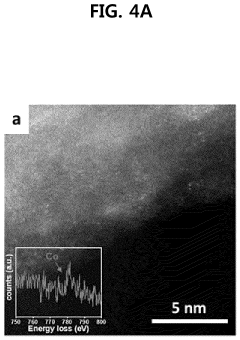
- A nitrogen-doped carbon nanostructure loaded with indirectly linked nickel (Ni) and manganese (Mn) single atoms as a bimetallic catalyst, which enhances carbon dioxide conversion activity and selectivity for carbon monoxide at lower overpotential.
Scalability and Manufacturing Considerations
The transition from laboratory-scale single-atom catalysis (SAC) to industrial applications presents significant manufacturing challenges that must be addressed. Current synthesis methods for SACs, including wet chemistry approaches, atomic layer deposition, and high-temperature atom trapping, demonstrate excellent control at small scales but face substantial hurdles when scaled to industrial volumes. The primary challenge lies in maintaining atomic dispersion while increasing production quantity, as atoms naturally tend to aggregate into clusters or nanoparticles when synthesis parameters change during scale-up.
Production economics represent another critical consideration. Laboratory-scale synthesis often utilizes expensive precursors and energy-intensive processes that become economically prohibitive at industrial scales. For instance, the use of precious metals like platinum and palladium as single-atom catalysts requires development of recovery and recycling systems to make large-scale applications financially viable. Additionally, the specialized equipment needed for precise atom deposition significantly increases capital expenditure for manufacturing facilities.
Quality control and characterization present unique challenges for SAC manufacturing. Unlike conventional catalysts, single-atom catalysts require atomic-level verification techniques such as aberration-corrected electron microscopy and X-ray absorption spectroscopy. Implementing these sophisticated analytical methods in production environments necessitates development of streamlined, high-throughput characterization protocols that can maintain quality assurance without becoming production bottlenecks.
Stability during manufacturing and subsequent application represents another significant hurdle. Single atoms anchored to support materials can detach or migrate under industrial reaction conditions, particularly at elevated temperatures or in harsh chemical environments. Engineering supports with stronger metal-support interactions and developing stabilization strategies that prevent atom migration during both manufacturing and operation are essential for commercial viability.
Recent advances in continuous flow manufacturing show promise for addressing some scalability issues. Companies like Johnson Matthey and BASF have begun adapting microreactor technologies for controlled synthesis of advanced catalytic materials. These approaches allow for precise control of reaction parameters while increasing throughput, potentially bridging the gap between laboratory precision and industrial scale requirements for single-atom catalysts.
Environmental considerations must also factor into manufacturing strategies. Green chemistry principles should guide process development, with emphasis on reducing solvent use, minimizing waste generation, and lowering energy consumption. Life cycle assessments of single-atom catalyst manufacturing processes indicate potential for significant environmental benefits compared to traditional catalysts, provided that sustainable manufacturing methods are implemented from the outset.
Production economics represent another critical consideration. Laboratory-scale synthesis often utilizes expensive precursors and energy-intensive processes that become economically prohibitive at industrial scales. For instance, the use of precious metals like platinum and palladium as single-atom catalysts requires development of recovery and recycling systems to make large-scale applications financially viable. Additionally, the specialized equipment needed for precise atom deposition significantly increases capital expenditure for manufacturing facilities.
Quality control and characterization present unique challenges for SAC manufacturing. Unlike conventional catalysts, single-atom catalysts require atomic-level verification techniques such as aberration-corrected electron microscopy and X-ray absorption spectroscopy. Implementing these sophisticated analytical methods in production environments necessitates development of streamlined, high-throughput characterization protocols that can maintain quality assurance without becoming production bottlenecks.
Stability during manufacturing and subsequent application represents another significant hurdle. Single atoms anchored to support materials can detach or migrate under industrial reaction conditions, particularly at elevated temperatures or in harsh chemical environments. Engineering supports with stronger metal-support interactions and developing stabilization strategies that prevent atom migration during both manufacturing and operation are essential for commercial viability.
Recent advances in continuous flow manufacturing show promise for addressing some scalability issues. Companies like Johnson Matthey and BASF have begun adapting microreactor technologies for controlled synthesis of advanced catalytic materials. These approaches allow for precise control of reaction parameters while increasing throughput, potentially bridging the gap between laboratory precision and industrial scale requirements for single-atom catalysts.
Environmental considerations must also factor into manufacturing strategies. Green chemistry principles should guide process development, with emphasis on reducing solvent use, minimizing waste generation, and lowering energy consumption. Life cycle assessments of single-atom catalyst manufacturing processes indicate potential for significant environmental benefits compared to traditional catalysts, provided that sustainable manufacturing methods are implemented from the outset.
Environmental Impact and Sustainability Assessment
Single-atom catalysis represents a significant advancement in sustainable industrial processes, offering unprecedented atom efficiency by utilizing individual metal atoms as catalytic sites. The environmental impact of this technology is overwhelmingly positive when compared to traditional catalytic systems that rely on bulk or nanoparticle catalysts.
The primary environmental benefit stems from the dramatic reduction in precious metal usage. Single-atom catalysts (SACs) can achieve comparable or superior catalytic performance while using only a fraction of the metal content—often reducing requirements by 90-95%. This directly addresses critical resource depletion concerns, particularly for platinum group metals and rare earth elements that face severe supply constraints and environmentally destructive mining practices.
Energy efficiency represents another crucial environmental advantage. SACs frequently demonstrate lower activation energy requirements for catalytic reactions, enabling processes to operate at reduced temperatures and pressures. Industrial implementation data indicates potential energy savings of 15-30% across various applications, with corresponding reductions in greenhouse gas emissions from power generation.
Waste reduction capabilities of SACs are equally impressive. Their enhanced selectivity minimizes unwanted by-products in chemical synthesis, reducing the environmental footprint associated with separation processes and waste treatment. In pharmaceutical manufacturing applications, SACs have demonstrated up to 40% reduction in E-factor (environmental factor) metrics that quantify waste generation.
The lifecycle assessment of SAC technologies reveals significant sustainability improvements. When properly designed, SACs show extended operational lifetimes compared to conventional catalysts, reducing the frequency of catalyst replacement and associated environmental impacts. Recent studies indicate that properly stabilized SACs can maintain activity for thousands of reaction cycles without significant degradation.
However, environmental challenges remain. The synthesis of SACs often requires specialized precursors and complex preparation methods that may involve energy-intensive processes or hazardous chemicals. Additionally, the long-term environmental fate and potential toxicity of dispersed single atoms after catalyst deactivation require further investigation to ensure complete lifecycle sustainability.
The recyclability of spent SACs presents both opportunities and challenges. While the minimal metal content reduces recovery incentives from an economic perspective, the development of efficient recovery technologies remains essential for closing material loops and preventing potential environmental contamination from ultrafine metal species.
The primary environmental benefit stems from the dramatic reduction in precious metal usage. Single-atom catalysts (SACs) can achieve comparable or superior catalytic performance while using only a fraction of the metal content—often reducing requirements by 90-95%. This directly addresses critical resource depletion concerns, particularly for platinum group metals and rare earth elements that face severe supply constraints and environmentally destructive mining practices.
Energy efficiency represents another crucial environmental advantage. SACs frequently demonstrate lower activation energy requirements for catalytic reactions, enabling processes to operate at reduced temperatures and pressures. Industrial implementation data indicates potential energy savings of 15-30% across various applications, with corresponding reductions in greenhouse gas emissions from power generation.
Waste reduction capabilities of SACs are equally impressive. Their enhanced selectivity minimizes unwanted by-products in chemical synthesis, reducing the environmental footprint associated with separation processes and waste treatment. In pharmaceutical manufacturing applications, SACs have demonstrated up to 40% reduction in E-factor (environmental factor) metrics that quantify waste generation.
The lifecycle assessment of SAC technologies reveals significant sustainability improvements. When properly designed, SACs show extended operational lifetimes compared to conventional catalysts, reducing the frequency of catalyst replacement and associated environmental impacts. Recent studies indicate that properly stabilized SACs can maintain activity for thousands of reaction cycles without significant degradation.
However, environmental challenges remain. The synthesis of SACs often requires specialized precursors and complex preparation methods that may involve energy-intensive processes or hazardous chemicals. Additionally, the long-term environmental fate and potential toxicity of dispersed single atoms after catalyst deactivation require further investigation to ensure complete lifecycle sustainability.
The recyclability of spent SACs presents both opportunities and challenges. While the minimal metal content reduces recovery incentives from an economic perspective, the development of efficient recovery technologies remains essential for closing material loops and preventing potential environmental contamination from ultrafine metal species.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!