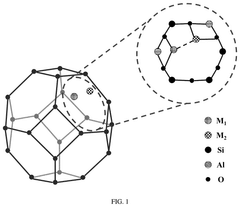
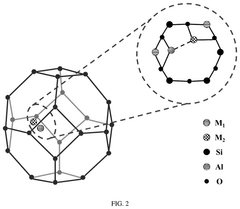
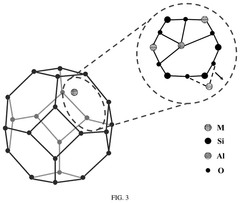
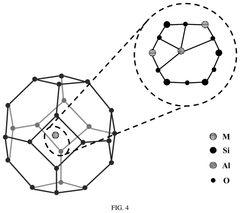
Understanding Industrial Standards for Single-Atom Catalysis
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a frontier in heterogeneous catalysis that has evolved significantly over the past decade. This revolutionary approach involves dispersing individual metal atoms on suitable supports, maximizing atomic efficiency while delivering exceptional catalytic performance. The concept emerged in the early 2000s but gained substantial momentum after 2011 when Zhang and colleagues published their seminal work on single-atom Pt catalysts, demonstrating unprecedented activity for CO oxidation.
The technological evolution of SAC has progressed through several distinct phases: initial theoretical predictions, experimental verification, methodological development for synthesis and characterization, and most recently, practical applications in industrial processes. This progression reflects the maturation of SAC from an academic curiosity to a potentially transformative industrial technology with applications spanning energy conversion, environmental remediation, and fine chemical synthesis.
Current industrial interest in SAC stems from its potential to address critical challenges in sustainability and resource utilization. With precious metal resources becoming increasingly scarce and expensive, the atom-efficient nature of SAC offers significant economic advantages. Additionally, the unique electronic properties of isolated metal atoms often enable novel reaction pathways with improved selectivity compared to traditional nanoparticle catalysts.
The primary technical objectives in SAC development include establishing reliable and scalable synthesis methods, improving catalyst stability under realistic operating conditions, and developing standardized characterization protocols. These objectives align with broader industrial goals of reducing precious metal usage, enhancing catalytic efficiency, and enabling more sustainable chemical processes.
Despite its promise, SAC faces significant standardization challenges. The field currently lacks unified protocols for catalyst preparation, characterization, and performance evaluation. This absence of standardization complicates cross-study comparisons and technology transfer from laboratory to industrial settings. Establishing industrial standards for SAC represents a critical step toward commercial viability.
The scope of standardization efforts must encompass multiple dimensions: synthesis parameters (including support selection, metal loading, and activation procedures), characterization techniques (with emphasis on confirming true atomic dispersion), and performance metrics (activity, selectivity, stability, and regeneration capabilities). These standards must balance scientific rigor with practical industrial considerations such as scalability, reproducibility, and cost-effectiveness.
Looking forward, the trajectory of SAC development points toward integration with complementary technologies such as computational modeling, in-situ characterization, and automated synthesis platforms. These synergistic approaches will accelerate the transition from fundamental understanding to practical implementation, ultimately establishing SAC as a mainstream industrial technology with transformative potential across multiple sectors.
The technological evolution of SAC has progressed through several distinct phases: initial theoretical predictions, experimental verification, methodological development for synthesis and characterization, and most recently, practical applications in industrial processes. This progression reflects the maturation of SAC from an academic curiosity to a potentially transformative industrial technology with applications spanning energy conversion, environmental remediation, and fine chemical synthesis.
Current industrial interest in SAC stems from its potential to address critical challenges in sustainability and resource utilization. With precious metal resources becoming increasingly scarce and expensive, the atom-efficient nature of SAC offers significant economic advantages. Additionally, the unique electronic properties of isolated metal atoms often enable novel reaction pathways with improved selectivity compared to traditional nanoparticle catalysts.
The primary technical objectives in SAC development include establishing reliable and scalable synthesis methods, improving catalyst stability under realistic operating conditions, and developing standardized characterization protocols. These objectives align with broader industrial goals of reducing precious metal usage, enhancing catalytic efficiency, and enabling more sustainable chemical processes.
Despite its promise, SAC faces significant standardization challenges. The field currently lacks unified protocols for catalyst preparation, characterization, and performance evaluation. This absence of standardization complicates cross-study comparisons and technology transfer from laboratory to industrial settings. Establishing industrial standards for SAC represents a critical step toward commercial viability.
The scope of standardization efforts must encompass multiple dimensions: synthesis parameters (including support selection, metal loading, and activation procedures), characterization techniques (with emphasis on confirming true atomic dispersion), and performance metrics (activity, selectivity, stability, and regeneration capabilities). These standards must balance scientific rigor with practical industrial considerations such as scalability, reproducibility, and cost-effectiveness.
Looking forward, the trajectory of SAC development points toward integration with complementary technologies such as computational modeling, in-situ characterization, and automated synthesis platforms. These synergistic approaches will accelerate the transition from fundamental understanding to practical implementation, ultimately establishing SAC as a mainstream industrial technology with transformative potential across multiple sectors.
Market Applications and Demand Analysis
The market for single-atom catalysis (SAC) technologies has witnessed significant growth in recent years, driven by increasing demands for sustainable and efficient catalytic processes across multiple industries. The global catalyst market, valued at approximately $33.5 billion in 2022, is projected to reach $47.9 billion by 2030, with single-atom catalysts representing one of the fastest-growing segments due to their superior atom efficiency and selectivity.
The automotive sector constitutes a primary market for SAC applications, particularly in emission control systems. With stringent environmental regulations like Euro 7 and China VI standards imposing tighter limits on vehicle emissions, manufacturers are actively seeking advanced catalytic solutions that utilize minimal amounts of precious metals while maintaining high conversion efficiencies. Single-atom catalysts offer up to 95% reduction in precious metal usage compared to conventional catalysts, presenting substantial cost advantages.
The petrochemical industry represents another significant market for SAC technologies, where catalytic processes account for over 85% of all chemical manufacturing processes. The demand for more selective catalysts that minimize unwanted by-products and operate at lower temperatures aligns perfectly with the capabilities of single-atom catalysts. Market research indicates that implementation of SAC technologies could potentially reduce energy consumption in certain petrochemical processes by 20-30%.
Renewable energy applications, particularly in hydrogen production and fuel cell technologies, constitute a rapidly expanding market segment for SAC. The global green hydrogen market is expected to grow at a CAGR of 54% between 2023 and 2030, creating substantial demand for efficient electrocatalysts. Single-atom catalysts have demonstrated superior performance in hydrogen evolution reactions, oxygen reduction reactions, and CO2 reduction, positioning them as critical components in the clean energy transition.
The pharmaceutical industry is increasingly exploring SAC technologies for fine chemical synthesis, where high selectivity and mild reaction conditions are essential. Market analysis suggests that approximately 40% of pharmaceutical manufacturing processes could benefit from advanced catalytic technologies, with SAC offering particular advantages in asymmetric synthesis and C-H activation reactions.
Environmental remediation represents an emerging application area, with growing demand for catalysts capable of degrading persistent organic pollutants and converting harmful emissions. The global environmental catalysts market is projected to grow at 8.2% annually through 2028, with water treatment and air purification applications driving significant portions of this growth.
Despite these promising market opportunities, widespread industrial adoption faces challenges related to standardization, scalability, and long-term stability under real-world operating conditions. Market surveys indicate that approximately 65% of potential industrial users cite the lack of established performance standards and certification protocols as a significant barrier to adoption.
The automotive sector constitutes a primary market for SAC applications, particularly in emission control systems. With stringent environmental regulations like Euro 7 and China VI standards imposing tighter limits on vehicle emissions, manufacturers are actively seeking advanced catalytic solutions that utilize minimal amounts of precious metals while maintaining high conversion efficiencies. Single-atom catalysts offer up to 95% reduction in precious metal usage compared to conventional catalysts, presenting substantial cost advantages.
The petrochemical industry represents another significant market for SAC technologies, where catalytic processes account for over 85% of all chemical manufacturing processes. The demand for more selective catalysts that minimize unwanted by-products and operate at lower temperatures aligns perfectly with the capabilities of single-atom catalysts. Market research indicates that implementation of SAC technologies could potentially reduce energy consumption in certain petrochemical processes by 20-30%.
Renewable energy applications, particularly in hydrogen production and fuel cell technologies, constitute a rapidly expanding market segment for SAC. The global green hydrogen market is expected to grow at a CAGR of 54% between 2023 and 2030, creating substantial demand for efficient electrocatalysts. Single-atom catalysts have demonstrated superior performance in hydrogen evolution reactions, oxygen reduction reactions, and CO2 reduction, positioning them as critical components in the clean energy transition.
The pharmaceutical industry is increasingly exploring SAC technologies for fine chemical synthesis, where high selectivity and mild reaction conditions are essential. Market analysis suggests that approximately 40% of pharmaceutical manufacturing processes could benefit from advanced catalytic technologies, with SAC offering particular advantages in asymmetric synthesis and C-H activation reactions.
Environmental remediation represents an emerging application area, with growing demand for catalysts capable of degrading persistent organic pollutants and converting harmful emissions. The global environmental catalysts market is projected to grow at 8.2% annually through 2028, with water treatment and air purification applications driving significant portions of this growth.
Despite these promising market opportunities, widespread industrial adoption faces challenges related to standardization, scalability, and long-term stability under real-world operating conditions. Market surveys indicate that approximately 65% of potential industrial users cite the lack of established performance standards and certification protocols as a significant barrier to adoption.
Global Research Status and Technical Barriers
Single-atom catalysis (SAC) has emerged as a frontier research area in heterogeneous catalysis globally, with significant advancements occurring across different regions. The United States leads in fundamental research, with institutions like Stanford University and MIT pioneering atomic-level characterization techniques. Their work has established critical parameters for single-atom dispersion and stability verification. The European Union, particularly Germany and France, has focused on standardizing characterization protocols, with the European Catalysis Research Institute developing unified testing frameworks that have become reference points for industrial implementation.
China has made remarkable progress in scaling up SAC production, with the Chinese Academy of Sciences establishing pilot-scale synthesis protocols that maintain atomic dispersion at larger volumes. These protocols have addressed key industrial challenges in maintaining catalyst uniformity during scale-up processes.
Despite these advancements, significant technical barriers persist in the standardization of SAC. The primary challenge remains the development of universally accepted characterization methods that can reliably confirm single-atom dispersion across different support materials. Current techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy often yield inconsistent results when applied to diverse catalyst systems.
Another major barrier is the lack of standardized stability metrics. Single-atom catalysts frequently suffer from atom aggregation under industrial conditions, yet no unified protocol exists to evaluate long-term stability across different reaction environments. This hampers industrial adoption as companies cannot reliably compare performance claims from different research groups or manufacturers.
The absence of standardized synthesis protocols presents another significant obstacle. Current laboratory-scale methods often employ highly controlled conditions that are difficult to replicate in industrial settings. The transition from milligram to kilogram production introduces variables that affect atomic dispersion, requiring new standardization approaches for quality control during manufacturing.
Regulatory frameworks also lag behind technological development. Most existing industrial catalyst standards were developed for conventional nanoparticle catalysts and fail to address the unique properties and performance metrics of single-atom systems. This regulatory gap creates uncertainty for companies seeking to commercialize SAC technologies and slows market penetration of these advanced materials.
China has made remarkable progress in scaling up SAC production, with the Chinese Academy of Sciences establishing pilot-scale synthesis protocols that maintain atomic dispersion at larger volumes. These protocols have addressed key industrial challenges in maintaining catalyst uniformity during scale-up processes.
Despite these advancements, significant technical barriers persist in the standardization of SAC. The primary challenge remains the development of universally accepted characterization methods that can reliably confirm single-atom dispersion across different support materials. Current techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy often yield inconsistent results when applied to diverse catalyst systems.
Another major barrier is the lack of standardized stability metrics. Single-atom catalysts frequently suffer from atom aggregation under industrial conditions, yet no unified protocol exists to evaluate long-term stability across different reaction environments. This hampers industrial adoption as companies cannot reliably compare performance claims from different research groups or manufacturers.
The absence of standardized synthesis protocols presents another significant obstacle. Current laboratory-scale methods often employ highly controlled conditions that are difficult to replicate in industrial settings. The transition from milligram to kilogram production introduces variables that affect atomic dispersion, requiring new standardization approaches for quality control during manufacturing.
Regulatory frameworks also lag behind technological development. Most existing industrial catalyst standards were developed for conventional nanoparticle catalysts and fail to address the unique properties and performance metrics of single-atom systems. This regulatory gap creates uncertainty for companies seeking to commercialize SAC technologies and slows market penetration of these advanced materials.
Current Standardization Approaches and Methodologies
01 Metal-based single-atom catalysts
Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials to maximize catalytic efficiency. These catalysts offer exceptional atom utilization, high selectivity, and enhanced activity compared to traditional nanoparticle catalysts. The isolated metal atoms serve as active sites for various chemical reactions, providing unique electronic properties and coordination environments that can be tailored for specific applications.- Metal-based single-atom catalysts: Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts offer maximum atom efficiency and unique catalytic properties due to their isolated nature. The metal atoms, typically transition metals, are anchored to supports like carbon, metal oxides, or 2D materials, creating distinct active sites with enhanced selectivity and activity compared to traditional nanoparticle catalysts.
- Support materials for single-atom catalysts: The choice of support material plays a crucial role in stabilizing single atoms and influencing their catalytic performance. Various supports including carbon-based materials (graphene, carbon nanotubes), metal oxides (TiO2, ZnO), zeolites, and MOFs (Metal-Organic Frameworks) are used to anchor single atoms. The support not only prevents aggregation of metal atoms but also participates in the catalytic process through metal-support interactions, affecting electron transfer and adsorption properties.
- Synthesis methods for single-atom catalysts: Various synthesis approaches have been developed to prepare single-atom catalysts with high metal dispersion and stability. These include atomic layer deposition, wet chemistry methods (impregnation, co-precipitation), high-temperature atom trapping, and defect engineering. Advanced techniques like spatial confinement strategies and coordination-based synthesis are employed to achieve precise control over the atomic structure and prevent aggregation during catalytic reactions.
- Applications in energy conversion and environmental remediation: Single-atom catalysts demonstrate exceptional performance in various energy and environmental applications. They are particularly effective in electrochemical processes like water splitting, oxygen reduction reaction, and CO2 reduction, offering higher activity and selectivity than conventional catalysts. In environmental remediation, these catalysts excel at converting pollutants through oxidation or reduction reactions. Their high atom efficiency makes them economically attractive for industrial applications where precious metals are typically used.
- Characterization and theoretical studies of single-atom catalysts: Advanced characterization techniques and theoretical modeling are essential for understanding the structure-performance relationship of single-atom catalysts. Methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy provide atomic-level insights into the catalyst structure. Computational approaches including density functional theory calculations help elucidate reaction mechanisms, predict catalytic behavior, and guide the rational design of more efficient single-atom catalysts with tailored properties.
02 Support materials for single-atom catalysts
The choice of support material plays a crucial role in stabilizing single atoms and preventing aggregation during catalytic reactions. Common supports include carbon-based materials (graphene, carbon nanotubes), metal oxides, and metal-organic frameworks (MOFs). These supports not only anchor the single atoms but also influence their electronic structure and catalytic performance through metal-support interactions. The design of appropriate support materials enables the creation of stable and efficient single-atom catalysts for various applications.Expand Specific Solutions03 Synthesis methods for single-atom catalysts
Various synthesis strategies have been developed to prepare single-atom catalysts with high metal dispersion and stability. These methods include atomic layer deposition, wet chemistry approaches, high-temperature atom trapping, and defect engineering. The synthesis typically involves precise control of metal loading, careful selection of precursors, and optimization of treatment conditions to achieve isolated single atoms rather than nanoparticles or clusters. Advanced characterization techniques are essential to confirm the single-atom nature of the prepared catalysts.Expand Specific Solutions04 Applications in energy conversion and environmental remediation
Single-atom catalysts demonstrate exceptional performance in energy-related applications such as hydrogen evolution, oxygen reduction, CO2 reduction, and water splitting. They also show promise in environmental remediation processes including pollutant degradation and emission control. The high atom efficiency and tunable electronic properties of single-atom catalysts make them particularly valuable for addressing energy and environmental challenges, offering improved activity and selectivity compared to conventional catalysts.Expand Specific Solutions05 Theoretical understanding and computational design
Computational methods and theoretical studies play a vital role in understanding the fundamental principles governing single-atom catalysis. Density functional theory calculations help elucidate reaction mechanisms, predict catalytic activity, and guide the rational design of new single-atom catalysts. These theoretical approaches provide insights into the electronic structure, coordination environment, and metal-support interactions that determine catalytic performance, enabling the development of catalysts with optimized properties for specific reactions.Expand Specific Solutions
Leading Research Institutions and Industrial Players
Single-atom catalysis is emerging as a transformative field in heterogeneous catalysis, currently transitioning from early research to commercial development. The market is experiencing rapid growth, projected to reach significant scale as industrial applications expand across energy, chemicals, and environmental sectors. Technologically, research institutions lead development with KIST Corp., Dalian Institute of Chemical Physics, and Beijing Single Atom Site Catalysis Technology demonstrating advanced capabilities. Major chemical corporations including LG Chem, China Petroleum & Chemical Corp., and Hanwha Chemical are increasingly investing in commercialization efforts. Academic-industrial partnerships are accelerating, with universities like King Abdullah University of Science & Technology and Sun Yat-Sen University collaborating with industry to bridge fundamental research and practical applications, though standardization remains a challenge for widespread industrial adoption.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE-CAS) has developed comprehensive industrial standards for single-atom catalysis focusing on scalable manufacturing processes. Their approach emphasizes standardized protocols for mass production of single-atom catalysts (SACs) through controlled pyrolysis and precise metal loading techniques. IPE-CAS has established quality control standards for industrial SAC production, including quantitative metrics for atom utilization efficiency, dispersion verification, and catalyst uniformity. Their industrial standards include detailed specifications for support material preparation, metal precursor selection, and post-synthesis treatments to ensure consistent performance. IPE-CAS has also pioneered standardized testing frameworks for evaluating SAC stability under industrial conditions, including high-temperature resistance, poison tolerance, and mechanical strength parameters critical for industrial implementation. Their standards incorporate life cycle assessment protocols specifically designed for SAC technologies to evaluate environmental impacts and sustainability metrics.
Strengths: Strong focus on scalable production methods applicable to industrial settings; comprehensive quality control standards; integration of sustainability metrics into standardization efforts. Weaknesses: Some standards may be overly specific to certain catalyst types or applications; implementation costs for full standardization can be prohibitive for smaller manufacturers.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered significant advancements in single-atom catalysis (SAC) industrial standards. Their approach focuses on developing atomically dispersed metal catalysts on various supports with precise control over the coordination environment. DICP has established standardized protocols for SAC synthesis, including atomic layer deposition and wet chemistry methods that ensure reproducible single-atom dispersion. Their characterization standards incorporate advanced techniques like aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ/operando methodologies to verify single-atom dispersion and understand reaction mechanisms. DICP has also developed benchmark testing protocols for evaluating SAC performance in industrially relevant reactions such as CO oxidation, water-gas shift, and hydrogenation processes, with standardized metrics for activity, selectivity, and stability assessment.
Strengths: Strong expertise in fundamental SAC research with direct industrial applications; comprehensive characterization capabilities; established protocols for quality control in SAC production. Weaknesses: Some standardization efforts remain primarily academic rather than fully adopted by industry; scaling up production while maintaining single-atom dispersion remains challenging.
Key Patents and Scientific Breakthroughs
Single-atom catalyst with molecular sieve-confined domains, preparation method and application thereof
PatentPendingUS20240399346A1
Innovation
- A single-atom catalyst with molecular sieve-confined domains is developed, where bimetallic ions are uniformly dispersed within the molecular sieve using a post-processing or in-situ synthesis method, leveraging oxygen vacancies and aluminum-rich sites for enhanced NO adsorption and dissociation, improving catalytic activity and stability.
Single-atom catalyst for activation of persulfate to generate pure singlet oxygen as well as preparation method and application thereof
PatentActiveUS11629052B2
Innovation
- A single-atom catalyst with graphitic carbon nitride nanosheets as supports and single iron atoms in a Fe—N4 coordination structure is developed, specifically designed to activate persulfate and generate pure singlet oxygen, enhancing selectivity and resistance to environmental interference.
Regulatory Framework and Compliance Requirements
The regulatory landscape for single-atom catalysis (SAC) is evolving rapidly as this emerging technology gains industrial significance. Currently, there is no unified global standard specifically addressing SAC, requiring manufacturers and researchers to navigate a complex web of existing chemical, environmental, and industrial regulations. In the United States, the Environmental Protection Agency (EPA) regulates catalytic materials under the Toxic Substances Control Act (TSCA), with specific provisions for nanomaterials that may apply to SAC technologies. Similarly, the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation encompasses novel catalytic materials, requiring extensive safety documentation and risk assessments.
For industrial implementation, ISO standards such as ISO/TC 229 for nanotechnologies provide partial guidance, though specific SAC protocols remain under development. The International Union of Pure and Applied Chemistry (IUPAC) has established preliminary nomenclature and characterization guidelines that serve as de facto standards in scientific literature. These frameworks address atomic dispersion verification, catalyst stability assessment, and performance benchmarking methodologies.
Safety compliance represents a critical regulatory component for SAC deployment. Occupational Safety and Health Administration (OSHA) guidelines in the US and similar bodies internationally mandate specific handling protocols for atomically dispersed precious metals and their precursors. These requirements include specialized containment systems, monitoring equipment, and worker training programs tailored to the unique properties of single-atom catalysts.
Intellectual property considerations add another layer of complexity to the regulatory framework. Patent offices worldwide are developing specialized examination guidelines for SAC innovations, focusing on atomic-scale characterization evidence and reproducibility documentation. The World Intellectual Property Organization (WIPO) has initiated efforts to harmonize these approaches across jurisdictions.
Environmental compliance for SAC technologies centers on lifecycle assessment requirements. Regulatory bodies increasingly demand cradle-to-grave analysis of environmental impacts, including resource extraction, synthesis processes, operational emissions, and end-of-life recovery of precious metals. The International Organization for Standardization's ISO 14040 series provides the methodological framework for these assessments, though industry-specific adaptations for SAC remain in development.
Quality assurance standards represent the final regulatory pillar, with Good Manufacturing Practice (GMP) guidelines being adapted for atomic-precision manufacturing. These standards emphasize statistical process control, advanced characterization techniques, and validation protocols that can demonstrate consistent atomic dispersion across production batches.
For industrial implementation, ISO standards such as ISO/TC 229 for nanotechnologies provide partial guidance, though specific SAC protocols remain under development. The International Union of Pure and Applied Chemistry (IUPAC) has established preliminary nomenclature and characterization guidelines that serve as de facto standards in scientific literature. These frameworks address atomic dispersion verification, catalyst stability assessment, and performance benchmarking methodologies.
Safety compliance represents a critical regulatory component for SAC deployment. Occupational Safety and Health Administration (OSHA) guidelines in the US and similar bodies internationally mandate specific handling protocols for atomically dispersed precious metals and their precursors. These requirements include specialized containment systems, monitoring equipment, and worker training programs tailored to the unique properties of single-atom catalysts.
Intellectual property considerations add another layer of complexity to the regulatory framework. Patent offices worldwide are developing specialized examination guidelines for SAC innovations, focusing on atomic-scale characterization evidence and reproducibility documentation. The World Intellectual Property Organization (WIPO) has initiated efforts to harmonize these approaches across jurisdictions.
Environmental compliance for SAC technologies centers on lifecycle assessment requirements. Regulatory bodies increasingly demand cradle-to-grave analysis of environmental impacts, including resource extraction, synthesis processes, operational emissions, and end-of-life recovery of precious metals. The International Organization for Standardization's ISO 14040 series provides the methodological framework for these assessments, though industry-specific adaptations for SAC remain in development.
Quality assurance standards represent the final regulatory pillar, with Good Manufacturing Practice (GMP) guidelines being adapted for atomic-precision manufacturing. These standards emphasize statistical process control, advanced characterization techniques, and validation protocols that can demonstrate consistent atomic dispersion across production batches.
Sustainability Impact and Green Chemistry Applications
Single-atom catalysis represents a significant advancement in sustainable chemistry, offering unprecedented atom efficiency and selectivity that directly supports green chemistry principles. By utilizing individual metal atoms dispersed on supports, these catalysts minimize material waste and reduce the consumption of precious metals by up to 95% compared to traditional nanoparticle catalysts. This efficiency translates to substantial environmental benefits, including reduced mining impacts and decreased energy requirements in catalyst production.
The environmental footprint of single-atom catalysts (SACs) extends beyond resource conservation. These catalysts demonstrate superior performance in critical environmental applications such as CO2 reduction, nitrogen fixation, and water purification. For instance, iron-based SACs have shown remarkable ability to convert CO2 to value-added chemicals at near-ambient conditions, potentially transforming a greenhouse gas into useful products while operating at lower temperatures than conventional processes.
In the context of green chemistry's twelve principles, SACs excel in multiple dimensions. They enable atom economy through their structure, prevent waste through their high selectivity, and often operate under milder conditions that reduce energy consumption. The precision of single-atom active sites frequently eliminates the need for hazardous reagents and solvents, further enhancing their sustainability profile.
Industrial implementation of SACs is increasingly aligned with circular economy concepts. The recyclability of these catalysts, particularly when anchored on recoverable supports, creates closed-loop systems that minimize waste generation. Several companies have reported catalyst recovery rates exceeding 90%, significantly reducing the lifecycle environmental impact of catalytic processes.
Regulatory frameworks worldwide are beginning to recognize the sustainability advantages of SACs. The European Chemical Agency has highlighted single-atom catalysis as a preferred technology in its sustainable chemistry roadmap, while China's recent five-year plan explicitly promotes SAC development for environmental remediation applications. These policy endorsements are accelerating industrial adoption.
Economic analyses demonstrate that despite higher initial development costs, the long-term sustainability benefits of SACs often translate to favorable total cost of ownership. Life cycle assessments conducted by leading chemical manufacturers indicate that processes utilizing SACs can reduce environmental impact indicators by 30-60% compared to conventional catalytic approaches, particularly in pharmaceutical and fine chemical production where selectivity is paramount.
The environmental footprint of single-atom catalysts (SACs) extends beyond resource conservation. These catalysts demonstrate superior performance in critical environmental applications such as CO2 reduction, nitrogen fixation, and water purification. For instance, iron-based SACs have shown remarkable ability to convert CO2 to value-added chemicals at near-ambient conditions, potentially transforming a greenhouse gas into useful products while operating at lower temperatures than conventional processes.
In the context of green chemistry's twelve principles, SACs excel in multiple dimensions. They enable atom economy through their structure, prevent waste through their high selectivity, and often operate under milder conditions that reduce energy consumption. The precision of single-atom active sites frequently eliminates the need for hazardous reagents and solvents, further enhancing their sustainability profile.
Industrial implementation of SACs is increasingly aligned with circular economy concepts. The recyclability of these catalysts, particularly when anchored on recoverable supports, creates closed-loop systems that minimize waste generation. Several companies have reported catalyst recovery rates exceeding 90%, significantly reducing the lifecycle environmental impact of catalytic processes.
Regulatory frameworks worldwide are beginning to recognize the sustainability advantages of SACs. The European Chemical Agency has highlighted single-atom catalysis as a preferred technology in its sustainable chemistry roadmap, while China's recent five-year plan explicitly promotes SAC development for environmental remediation applications. These policy endorsements are accelerating industrial adoption.
Economic analyses demonstrate that despite higher initial development costs, the long-term sustainability benefits of SACs often translate to favorable total cost of ownership. Life cycle assessments conducted by leading chemical manufacturers indicate that processes utilizing SACs can reduce environmental impact indicators by 30-60% compared to conventional catalytic approaches, particularly in pharmaceutical and fine chemical production where selectivity is paramount.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!