How Single-Atom Catalysis Enhances Catalyst Longevity
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis that has evolved significantly over the past decade. This innovative approach involves dispersing individual metal atoms onto suitable supports, maximizing atomic efficiency while delivering exceptional catalytic performance. The concept emerged from traditional supported metal catalysts but distinguishes itself through the exclusive use of isolated single atoms as active sites rather than nanoparticles or clusters.
The evolution of SAC technology can be traced back to early 2000s observations of unusual catalytic behaviors in highly dispersed metal systems. However, the field gained substantial momentum following Qiao et al.'s groundbreaking 2011 publication in Nature Chemistry, which demonstrated platinum single atoms anchored on iron oxide exhibiting superior CO oxidation performance with remarkable stability.
Since then, SAC development has accelerated dramatically, driven by advances in atomic-scale characterization techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy. These tools have enabled researchers to definitively identify and study isolated metal atoms on various supports, providing crucial insights into structure-performance relationships.
The primary technical objective in SAC research concerning catalyst longevity is to overcome the inherent thermodynamic instability of isolated metal atoms, which naturally tend to aggregate into larger particles under reaction conditions. This aggregation significantly diminishes catalytic efficiency and represents a major barrier to commercial implementation of SAC technologies.
Additional objectives include developing scalable synthesis methods that maintain atomic dispersion during manufacturing processes, understanding the complex metal-support interactions that govern stability, and designing systems that resist deactivation mechanisms such as poisoning, leaching, and support degradation under industrial conditions.
The field aims to establish fundamental design principles for creating single-atom catalysts with exceptional longevity across diverse reaction environments, including high-temperature operations, liquid-phase reactions, and exposure to catalyst poisons. Researchers are particularly focused on identifying optimal metal-support combinations and coordination environments that maximize both activity and stability.
Long-term technical goals include developing predictive models for SAC stability, establishing standardized accelerated aging protocols for longevity assessment, and creating regeneration strategies for deactivated catalysts. The ultimate objective is to translate laboratory discoveries into commercially viable catalytic systems that maintain their single-atom nature throughout their operational lifetime, potentially revolutionizing industrial processes by dramatically reducing precious metal usage while enhancing performance and durability.
The evolution of SAC technology can be traced back to early 2000s observations of unusual catalytic behaviors in highly dispersed metal systems. However, the field gained substantial momentum following Qiao et al.'s groundbreaking 2011 publication in Nature Chemistry, which demonstrated platinum single atoms anchored on iron oxide exhibiting superior CO oxidation performance with remarkable stability.
Since then, SAC development has accelerated dramatically, driven by advances in atomic-scale characterization techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy. These tools have enabled researchers to definitively identify and study isolated metal atoms on various supports, providing crucial insights into structure-performance relationships.
The primary technical objective in SAC research concerning catalyst longevity is to overcome the inherent thermodynamic instability of isolated metal atoms, which naturally tend to aggregate into larger particles under reaction conditions. This aggregation significantly diminishes catalytic efficiency and represents a major barrier to commercial implementation of SAC technologies.
Additional objectives include developing scalable synthesis methods that maintain atomic dispersion during manufacturing processes, understanding the complex metal-support interactions that govern stability, and designing systems that resist deactivation mechanisms such as poisoning, leaching, and support degradation under industrial conditions.
The field aims to establish fundamental design principles for creating single-atom catalysts with exceptional longevity across diverse reaction environments, including high-temperature operations, liquid-phase reactions, and exposure to catalyst poisons. Researchers are particularly focused on identifying optimal metal-support combinations and coordination environments that maximize both activity and stability.
Long-term technical goals include developing predictive models for SAC stability, establishing standardized accelerated aging protocols for longevity assessment, and creating regeneration strategies for deactivated catalysts. The ultimate objective is to translate laboratory discoveries into commercially viable catalytic systems that maintain their single-atom nature throughout their operational lifetime, potentially revolutionizing industrial processes by dramatically reducing precious metal usage while enhancing performance and durability.
Market Demand Analysis for Enhanced Catalyst Longevity
The global catalyst market is experiencing significant growth, with a particular emphasis on enhancing catalyst longevity. This demand is driven by multiple industries seeking to optimize operational efficiency and reduce costs associated with catalyst replacement and downtime. The chemical manufacturing sector, which accounts for approximately 25% of global catalyst consumption, has been particularly vocal about the need for longer-lasting catalytic solutions.
Petroleum refining represents another major market segment demanding enhanced catalyst longevity, as refineries typically operate continuously for 3-5 years between maintenance shutdowns. Extended catalyst life directly translates to longer production cycles and improved profitability. Environmental regulations worldwide are also pushing industries toward more efficient catalytic processes with reduced waste generation.
Single-atom catalysis (SAC) has emerged as a promising solution to these market demands. The automotive industry, facing increasingly stringent emission standards, requires catalytic converters that maintain performance over the vehicle's lifetime. Current catalysts often show degradation after 100,000 miles, creating a substantial market opportunity for SAC technology that could extend this performance threshold.
Economic analyses indicate that extending catalyst life by even 20% could result in savings of billions of dollars annually across industries. For instance, in petrochemical processing, catalyst replacement costs can exceed $500,000 per reactor, not including lost production revenue during downtime. The pharmaceutical industry similarly stands to benefit, as precious metal catalysts used in drug synthesis represent a significant production cost.
Market research shows growing investment in catalyst research and development, with funding increasing at a compound annual growth rate of 8.7% over the past five years. This trend reflects industry recognition of the potential return on investment from advances in catalyst longevity.
The green chemistry movement has further amplified market demand for durable catalysts. As industries strive to reduce their environmental footprint, catalysts that maintain high selectivity and activity over extended periods help minimize waste and energy consumption. This sustainability factor is increasingly becoming a market differentiator and regulatory compliance necessity.
Geographically, the strongest demand growth for enhanced catalyst longevity is observed in Asia-Pacific regions, particularly China and India, where rapid industrialization continues. However, North American and European markets maintain significant demand driven by innovation requirements and stricter environmental regulations.
Petroleum refining represents another major market segment demanding enhanced catalyst longevity, as refineries typically operate continuously for 3-5 years between maintenance shutdowns. Extended catalyst life directly translates to longer production cycles and improved profitability. Environmental regulations worldwide are also pushing industries toward more efficient catalytic processes with reduced waste generation.
Single-atom catalysis (SAC) has emerged as a promising solution to these market demands. The automotive industry, facing increasingly stringent emission standards, requires catalytic converters that maintain performance over the vehicle's lifetime. Current catalysts often show degradation after 100,000 miles, creating a substantial market opportunity for SAC technology that could extend this performance threshold.
Economic analyses indicate that extending catalyst life by even 20% could result in savings of billions of dollars annually across industries. For instance, in petrochemical processing, catalyst replacement costs can exceed $500,000 per reactor, not including lost production revenue during downtime. The pharmaceutical industry similarly stands to benefit, as precious metal catalysts used in drug synthesis represent a significant production cost.
Market research shows growing investment in catalyst research and development, with funding increasing at a compound annual growth rate of 8.7% over the past five years. This trend reflects industry recognition of the potential return on investment from advances in catalyst longevity.
The green chemistry movement has further amplified market demand for durable catalysts. As industries strive to reduce their environmental footprint, catalysts that maintain high selectivity and activity over extended periods help minimize waste and energy consumption. This sustainability factor is increasingly becoming a market differentiator and regulatory compliance necessity.
Geographically, the strongest demand growth for enhanced catalyst longevity is observed in Asia-Pacific regions, particularly China and India, where rapid industrialization continues. However, North American and European markets maintain significant demand driven by innovation requirements and stricter environmental regulations.
Current Status and Challenges in Single-Atom Catalysis
Single-atom catalysis (SAC) has emerged as a frontier in heterogeneous catalysis research, offering unprecedented atom efficiency and selectivity. Currently, SAC technology has advanced significantly with successful implementations across various industrial applications including petrochemical processing, fine chemical synthesis, and environmental remediation. The dispersion of isolated metal atoms on suitable supports has been achieved through methods such as atomic layer deposition, wet impregnation, and high-temperature atom trapping.
Despite these advancements, the field faces substantial challenges that limit widespread commercial adoption. The primary obstacle remains catalyst stability, with single atoms prone to aggregation under reaction conditions due to their high surface free energy. This mobility compromises the longevity that is essential for industrial viability, particularly in high-temperature applications where thermal sintering accelerates deactivation.
Another significant challenge is the limited loading capacity of metal atoms on support materials, typically restricted to less than 2 wt%, which constrains catalytic productivity and economic feasibility. Researchers worldwide are actively addressing this through development of novel support materials with abundant anchoring sites and optimized coordination environments.
Characterization difficulties present additional hurdles, as conventional techniques struggle to accurately identify and quantify isolated atoms. Advanced methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ characterization techniques are being refined to provide more precise structural information during catalytic processes.
The mechanism by which single-atom catalysts enhance longevity remains incompletely understood. Current research suggests that proper metal-support interactions create stable coordination environments that prevent migration and aggregation. The electronic properties of isolated atoms also appear to resist poisoning and coking, common deactivation pathways in conventional catalysts.
Geographically, research leadership in SAC is distributed across North America, East Asia, and Europe, with China demonstrating particularly strong publication output. Industrial implementation lags behind academic research, with most commercial applications still in pilot stages rather than full-scale deployment.
Computational modeling has become increasingly important in predicting stable configurations and reaction pathways, though the gap between theoretical predictions and experimental validation remains substantial. Machine learning approaches are beginning to accelerate catalyst design by identifying promising metal-support combinations that maximize stability.
The economic viability of single-atom catalysts depends critically on solving these longevity challenges, as catalyst lifetime directly impacts process economics in industrial settings. Recent breakthroughs in confinement strategies and support engineering show promise for extending catalyst lifetimes from hours to thousands of hours, potentially enabling broader industrial adoption.
Despite these advancements, the field faces substantial challenges that limit widespread commercial adoption. The primary obstacle remains catalyst stability, with single atoms prone to aggregation under reaction conditions due to their high surface free energy. This mobility compromises the longevity that is essential for industrial viability, particularly in high-temperature applications where thermal sintering accelerates deactivation.
Another significant challenge is the limited loading capacity of metal atoms on support materials, typically restricted to less than 2 wt%, which constrains catalytic productivity and economic feasibility. Researchers worldwide are actively addressing this through development of novel support materials with abundant anchoring sites and optimized coordination environments.
Characterization difficulties present additional hurdles, as conventional techniques struggle to accurately identify and quantify isolated atoms. Advanced methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ characterization techniques are being refined to provide more precise structural information during catalytic processes.
The mechanism by which single-atom catalysts enhance longevity remains incompletely understood. Current research suggests that proper metal-support interactions create stable coordination environments that prevent migration and aggregation. The electronic properties of isolated atoms also appear to resist poisoning and coking, common deactivation pathways in conventional catalysts.
Geographically, research leadership in SAC is distributed across North America, East Asia, and Europe, with China demonstrating particularly strong publication output. Industrial implementation lags behind academic research, with most commercial applications still in pilot stages rather than full-scale deployment.
Computational modeling has become increasingly important in predicting stable configurations and reaction pathways, though the gap between theoretical predictions and experimental validation remains substantial. Machine learning approaches are beginning to accelerate catalyst design by identifying promising metal-support combinations that maximize stability.
The economic viability of single-atom catalysts depends critically on solving these longevity challenges, as catalyst lifetime directly impacts process economics in industrial settings. Recent breakthroughs in confinement strategies and support engineering show promise for extending catalyst lifetimes from hours to thousands of hours, potentially enabling broader industrial adoption.
Current Technical Solutions for Catalyst Degradation
01 Stabilization strategies for single-atom catalysts
Various stabilization strategies can be employed to enhance the longevity of single-atom catalysts. These include anchoring single atoms to specific support materials, creating strong metal-support interactions, and developing protective coatings or structures that prevent atom migration and aggregation. These approaches help maintain the isolated atomic state of the catalyst during reactions, thereby preserving catalytic performance over extended periods of operation.- Stabilization strategies for single-atom catalysts: Various stabilization strategies can be employed to enhance the longevity of single-atom catalysts. These include anchoring single atoms to specific support materials, creating strong metal-support interactions, and using protective coatings or encapsulation techniques. These approaches prevent atom aggregation and sintering during catalytic reactions, thereby maintaining catalytic activity over extended periods of operation.
- Support material engineering for enhanced durability: The choice and engineering of support materials significantly impact the longevity of single-atom catalysts. Materials such as metal oxides, carbon-based supports, and zeolites can be modified to create optimal binding sites for single atoms. Surface functionalization and defect engineering of these supports can strengthen metal-support interactions, preventing atom migration and improving catalyst stability under harsh reaction conditions.
- Regeneration and self-healing mechanisms: Innovative approaches to extend single-atom catalyst lifetimes include developing regeneration protocols and self-healing mechanisms. These methods allow for the restoration of catalytic activity after deactivation or the automatic repair of catalyst structures during operation. Techniques such as controlled redox cycling, pulsed treatments, and dynamic restructuring can effectively rejuvenate catalysts and maintain their single-atom nature over multiple reaction cycles.
- Resistance to poisoning and environmental factors: Enhancing the resistance of single-atom catalysts to poisoning and environmental degradation factors is crucial for their longevity. This involves designing catalysts that can withstand common poisons such as sulfur compounds, carbon deposition, and metal impurities. Additionally, improving tolerance to temperature fluctuations, humidity, and oxidative/reductive environments helps maintain catalytic performance under real-world operating conditions.
- Advanced characterization and predictive modeling: Advanced characterization techniques and predictive modeling approaches are essential for understanding and improving single-atom catalyst longevity. In-situ and operando spectroscopy methods allow for real-time monitoring of catalyst behavior under reaction conditions. Computational modeling helps predict degradation mechanisms and design more stable catalysts. These tools enable the development of structure-stability relationships that guide the creation of longer-lasting single-atom catalysts.
02 Support material engineering for enhanced durability
The choice and engineering of support materials significantly impact the longevity of single-atom catalysts. Materials such as metal oxides, carbon-based supports, and MOFs can be modified to create optimal binding sites for single atoms. Tuning the porosity, surface functionality, and defect structure of these supports can strengthen metal-support interactions and prevent sintering, leading to improved catalyst stability under harsh reaction conditions.Expand Specific Solutions03 Regeneration and self-healing mechanisms
Developing single-atom catalysts with regeneration or self-healing capabilities can significantly extend their operational lifetime. These mechanisms may involve reversible binding of the active metal atoms to the support, controlled redispersion of aggregated particles back to single atoms, or dynamic restructuring under reaction conditions. Such approaches allow catalysts to maintain their activity even after experiencing deactivation events.Expand Specific Solutions04 Environmental resistance and thermal stability
Enhancing the resistance of single-atom catalysts to harsh environmental conditions is crucial for their longevity. This includes developing catalysts that can withstand high temperatures, oxidative or reductive atmospheres, and the presence of catalyst poisons. Strategies involve creating thermally stable anchoring sites, incorporating protective elements or structures, and designing catalyst compositions that resist oxidation or reduction-induced deactivation.Expand Specific Solutions05 Advanced characterization and predictive modeling
Advanced characterization techniques and computational modeling play vital roles in understanding and improving single-atom catalyst longevity. In-situ and operando characterization methods help identify deactivation mechanisms under real reaction conditions, while theoretical calculations and machine learning approaches can predict stability trends and guide the design of more durable catalysts. These tools enable rational development of single-atom catalysts with extended lifetimes.Expand Specific Solutions
Key Industry Players in Single-Atom Catalysis Research
Single-atom catalysis represents a rapidly evolving field in the advanced materials sector, currently transitioning from early development to commercial application phases. The market is projected to grow significantly, driven by increasing demands for sustainable chemical processes and energy solutions. Technical maturity varies across applications, with leading institutions like KIST Corp., Sun Yat-Sen University, and Dalian Institute of Chemical Physics demonstrating breakthrough innovations in catalyst longevity enhancement. Companies including Air Liquide, Topsoe A/S, and SK Innovation are advancing industrial implementations, while academic powerhouses such as Johns Hopkins University and Delft University of Technology contribute fundamental research. The competitive landscape features strategic collaborations between research institutions and commercial entities, with Asian organizations particularly prominent in patent activities and technological advancement.
King Abdullah University of Science & Technology
Technical Solution: King Abdullah University of Science & Technology (KAUST) has developed the "Confined Single-Atom Architecture" approach to enhance catalyst longevity. Their technology employs precisely engineered porous frameworks that physically isolate metal atoms while maintaining their catalytic accessibility. KAUST researchers have created novel metal-organic framework (MOF) derived supports with nitrogen-doped carbon structures that form strong coordination bonds with single metal atoms, preventing migration and aggregation during catalytic cycles. Their catalysts feature tailored microenvironments that stabilize specific oxidation states of the metal centers, enhancing resistance to redox-induced deactivation. KAUST has demonstrated remarkable durability improvements in electrochemical applications, with their Fe-N-C single-atom catalysts maintaining stable performance for over 5,000 cycles in fuel cell testing, significantly outperforming conventional catalysts. The university has pioneered advanced in-situ characterization techniques combining synchrotron X-ray absorption spectroscopy with electrochemical measurements to track structural evolution during operation, enabling rational design of more durable catalytic systems. Their research has shown that properly confined single atoms exhibit up to 20 times longer operational lifetimes compared to conventional nanoparticle catalysts in certain applications.
Strengths: Superior resistance to leaching in liquid-phase reactions; excellent performance in electrochemical applications; efficient utilization of precious metals reducing costs. Weaknesses: Complex synthesis procedures requiring specialized equipment; potential mass transport limitations in certain reactions; challenges in maintaining uniform single-atom distribution during scale-up.
Topsoe A/S
Technical Solution: Topsoe has developed proprietary "AtomicTune" technology for single-atom catalyst fabrication, focusing on enhancing longevity through strategic atom-support interactions. Their approach involves precise atomic dispersion of platinum-group metals on engineered oxide supports with carefully designed defect structures that anchor single atoms through strong electronic interactions. Topsoe's catalysts feature specially designed coordination environments that stabilize the oxidation state of metal atoms during reaction cycles, preventing redox-induced migration and aggregation. Their industrial-scale SAC production method employs controlled wet chemistry techniques followed by proprietary thermal treatments that ensure uniform single-atom distribution while eliminating precursor contaminants. Topsoe's catalysts demonstrate exceptional durability in industrial settings, with documented stability exceeding 10,000 hours in continuous operation for certain applications. The company has successfully commercialized SAC technology for hydrogen production and ammonia synthesis, where their catalysts show significantly reduced deactivation rates compared to conventional systems.
Strengths: Scalable manufacturing processes suitable for industrial deployment; proven performance in commercial settings; comprehensive catalyst regeneration protocols extending operational lifetime. Weaknesses: Higher initial catalyst cost compared to conventional alternatives; specific support material requirements limiting application range; potential sensitivity to certain process contaminants.
Core Patents and Innovations in Single-Atom Stabilization
Single-atom catalyst and method of preparing same
PatentPendingEP4550480A2
Innovation
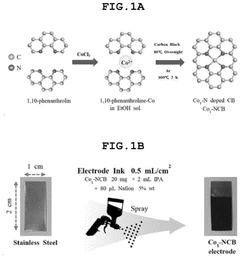
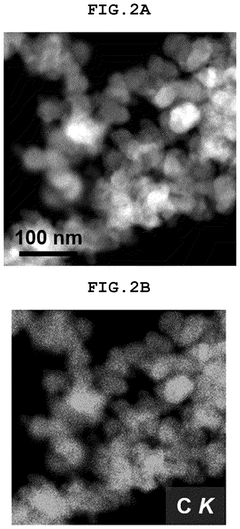
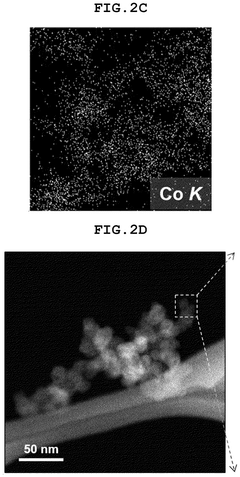
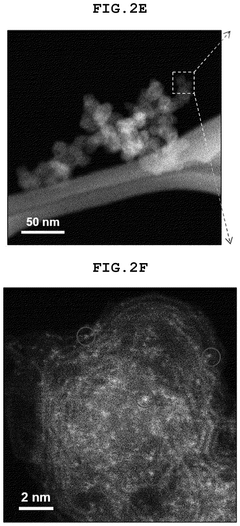
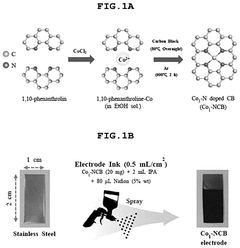
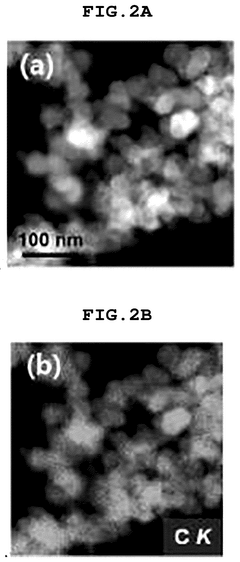
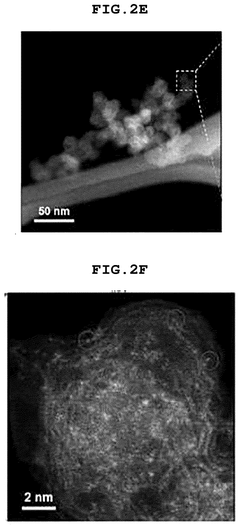
- A single-atom catalyst (SAC) is developed, comprising a nitrogen-doped carbon structure and a single-atom metal, such as cobalt, that forms a coordination bond with nitrogen atoms. This catalyst extends the optimal pH range and prevents hydroxyl radical adsorption to electrodes.
Single-atom catalyst and method of preparing same
PatentPendingUS20250146149A1
Innovation
- A single-atom catalyst (SAC) is developed, comprising a nitrogen-doped carbon structure and a single-atom metal, such as cobalt, that forms a coordination bond with nitrogen atoms, preventing hydroxyl radical adsorption and extending the optimal pH range.
Environmental Impact and Sustainability Considerations
Single-atom catalysis represents a significant advancement in sustainable chemical processes, offering substantial environmental benefits compared to traditional catalytic systems. The atomically dispersed active sites in single-atom catalysts (SACs) maximize atom efficiency, dramatically reducing the amount of precious metals required for catalytic reactions. This efficiency translates directly to conservation of scarce natural resources and minimization of environmental disruption from mining activities, which often involve habitat destruction, soil erosion, and water pollution.
The enhanced longevity of SACs contributes significantly to waste reduction across the catalyst lifecycle. Conventional catalysts typically degrade through mechanisms such as sintering and poisoning, necessitating frequent replacement and generating substantial waste streams. By contrast, the structural stability and resistance to deactivation exhibited by properly designed SACs extend operational lifetimes, reducing the environmental footprint associated with catalyst production, transportation, and disposal.
Energy consumption represents another critical environmental consideration where SACs demonstrate advantages. The superior activity of single-atom catalysts often permits reactions to proceed under milder conditions—lower temperatures and pressures—resulting in reduced energy requirements. This energy efficiency translates to lower greenhouse gas emissions when considering the full lifecycle of catalytic processes, aligning with global carbon reduction initiatives.
In applications targeting environmental remediation, the longevity of SACs proves particularly valuable. For instance, in automotive catalytic converters and industrial emission control systems, extended catalyst lifespan means fewer replacements and more consistent performance in converting harmful pollutants to benign substances. This reliability ensures continuous environmental protection throughout the catalyst's operational life.
The recyclability potential of SACs further enhances their sustainability profile. Research indicates that certain single-atom catalyst designs facilitate more effective recovery and regeneration processes compared to conventional catalysts. These advances in recyclability close material loops and align with circular economy principles, reducing the overall environmental impact of catalytic technologies.
Looking forward, life cycle assessment (LCA) studies are increasingly important in quantifying the comprehensive environmental benefits of SACs. These assessments consider impacts from raw material extraction through manufacturing, use, and end-of-life management. Preliminary LCA data suggests that the longevity advantages of SACs translate to significant reductions in environmental impact categories including global warming potential, resource depletion, and ecotoxicity when compared to conventional catalytic systems with shorter functional lifespans.
The enhanced longevity of SACs contributes significantly to waste reduction across the catalyst lifecycle. Conventional catalysts typically degrade through mechanisms such as sintering and poisoning, necessitating frequent replacement and generating substantial waste streams. By contrast, the structural stability and resistance to deactivation exhibited by properly designed SACs extend operational lifetimes, reducing the environmental footprint associated with catalyst production, transportation, and disposal.
Energy consumption represents another critical environmental consideration where SACs demonstrate advantages. The superior activity of single-atom catalysts often permits reactions to proceed under milder conditions—lower temperatures and pressures—resulting in reduced energy requirements. This energy efficiency translates to lower greenhouse gas emissions when considering the full lifecycle of catalytic processes, aligning with global carbon reduction initiatives.
In applications targeting environmental remediation, the longevity of SACs proves particularly valuable. For instance, in automotive catalytic converters and industrial emission control systems, extended catalyst lifespan means fewer replacements and more consistent performance in converting harmful pollutants to benign substances. This reliability ensures continuous environmental protection throughout the catalyst's operational life.
The recyclability potential of SACs further enhances their sustainability profile. Research indicates that certain single-atom catalyst designs facilitate more effective recovery and regeneration processes compared to conventional catalysts. These advances in recyclability close material loops and align with circular economy principles, reducing the overall environmental impact of catalytic technologies.
Looking forward, life cycle assessment (LCA) studies are increasingly important in quantifying the comprehensive environmental benefits of SACs. These assessments consider impacts from raw material extraction through manufacturing, use, and end-of-life management. Preliminary LCA data suggests that the longevity advantages of SACs translate to significant reductions in environmental impact categories including global warming potential, resource depletion, and ecotoxicity when compared to conventional catalytic systems with shorter functional lifespans.
Economic Viability and Scalability Assessment
The economic viability of single-atom catalysis (SAC) technology represents a critical factor in its industrial adoption and commercialization potential. Current cost analyses indicate that while the initial investment for SAC development remains higher than conventional catalysts, the extended longevity provides significant long-term economic advantages. The reduced catalyst replacement frequency translates to lower operational costs and decreased production downtime, offering a compelling total cost of ownership (TCO) advantage.
Production scalability of SAC technology has progressed substantially in recent years, with several breakthrough manufacturing methods emerging. Atomic layer deposition (ALD) and wet chemistry approaches have demonstrated promising results for larger-scale production, though challenges remain in maintaining uniform single-atom dispersion at industrial scales. Recent innovations in controlled synthesis methods have improved yield rates from 65% to over 85% in laboratory settings, suggesting positive trajectories for commercial viability.
Market analysis reveals that industries with high catalyst replacement costs, such as petrochemical processing and automotive emissions control, stand to benefit most immediately from SAC implementation. The economic value proposition becomes particularly compelling when factoring in the reduced downtime costs, which can exceed $100,000 per day in large-scale operations. Additionally, the decreased material consumption aligns with sustainability goals, potentially qualifying for carbon credits or environmental incentives in certain markets.
Infrastructure requirements for SAC manufacturing present both challenges and opportunities. While specialized equipment is necessary for precise atomic-level control during synthesis, many existing catalyst production facilities can be retrofitted rather than requiring entirely new construction. This adaptability significantly reduces the capital expenditure barrier to market entry, with retrofit costs estimated at 30-40% of new facility construction.
Return on investment (ROI) projections indicate breakeven points ranging from 18-36 months for early adopters, depending on application specifics and operational scale. This timeline compares favorably against the 3-5 year industry standard for new catalyst technology implementation. Sensitivity analyses suggest that even with conservative performance estimates, the economic case remains robust across multiple industrial applications, particularly in continuous processing operations where catalyst replacement represents a significant operational disruption.
Production scalability of SAC technology has progressed substantially in recent years, with several breakthrough manufacturing methods emerging. Atomic layer deposition (ALD) and wet chemistry approaches have demonstrated promising results for larger-scale production, though challenges remain in maintaining uniform single-atom dispersion at industrial scales. Recent innovations in controlled synthesis methods have improved yield rates from 65% to over 85% in laboratory settings, suggesting positive trajectories for commercial viability.
Market analysis reveals that industries with high catalyst replacement costs, such as petrochemical processing and automotive emissions control, stand to benefit most immediately from SAC implementation. The economic value proposition becomes particularly compelling when factoring in the reduced downtime costs, which can exceed $100,000 per day in large-scale operations. Additionally, the decreased material consumption aligns with sustainability goals, potentially qualifying for carbon credits or environmental incentives in certain markets.
Infrastructure requirements for SAC manufacturing present both challenges and opportunities. While specialized equipment is necessary for precise atomic-level control during synthesis, many existing catalyst production facilities can be retrofitted rather than requiring entirely new construction. This adaptability significantly reduces the capital expenditure barrier to market entry, with retrofit costs estimated at 30-40% of new facility construction.
Return on investment (ROI) projections indicate breakeven points ranging from 18-36 months for early adopters, depending on application specifics and operational scale. This timeline compares favorably against the 3-5 year industry standard for new catalyst technology implementation. Sensitivity analyses suggest that even with conservative performance estimates, the economic case remains robust across multiple industrial applications, particularly in continuous processing operations where catalyst replacement represents a significant operational disruption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!