What Are the Key Performance Indicators for Single-Atom Catalysis?
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a frontier in heterogeneous catalysis research that has emerged over the past decade as a revolutionary approach to catalyst design. This novel catalytic paradigm involves the dispersion of isolated metal atoms on suitable supports, maximizing atomic efficiency while offering unique catalytic properties distinct from traditional nanoparticle catalysts. The evolution of SAC technology can be traced back to early 2000s observations of isolated active sites, but gained significant momentum after 2011 when the term "single-atom catalyst" was formally introduced by Zhang and colleagues.
The technological trajectory of SAC has been characterized by progressive advancements in synthesis methods, characterization techniques, and theoretical understanding. Initial developments focused on precious metal atoms (Pt, Au, Pd) on oxide supports, while recent trends have expanded to non-noble metals and diverse support materials including carbon-based structures, metal-organic frameworks, and 2D materials.
The primary objective in SAC research is to establish reliable performance indicators that can accurately evaluate catalytic efficiency, stability, and selectivity at the atomic level. These indicators are essential for comparing different SAC systems and guiding rational design strategies for next-generation catalysts.
Current research aims to address several critical challenges: developing standardized protocols for performance assessment, understanding structure-performance relationships at the atomic scale, and establishing correlations between theoretical predictions and experimental observations. The field is moving toward identifying universal descriptors that can predict catalytic behavior across different reaction environments.
From a technological perspective, SAC development seeks to bridge fundamental science with practical applications by focusing on scalable synthesis methods, long-term stability under industrial conditions, and integration with existing catalytic processes. The ultimate goal is to translate laboratory discoveries into commercially viable technologies that can address global challenges in energy conversion, environmental remediation, and sustainable chemical production.
The interdisciplinary nature of SAC research necessitates collaboration between surface scientists, materials engineers, computational chemists, and process engineers. This convergence of expertise has accelerated innovation in the field, leading to increasingly sophisticated understanding of atomic-level catalytic phenomena and more precise performance indicators.
As the field matures, establishing standardized key performance indicators for SAC has become paramount to enable meaningful comparisons across research groups and facilitate technology transfer from laboratory to industry. These indicators must capture not only traditional catalytic metrics but also unique aspects of single-atom systems such as coordination environment, electronic structure, and atom-support interactions.
The technological trajectory of SAC has been characterized by progressive advancements in synthesis methods, characterization techniques, and theoretical understanding. Initial developments focused on precious metal atoms (Pt, Au, Pd) on oxide supports, while recent trends have expanded to non-noble metals and diverse support materials including carbon-based structures, metal-organic frameworks, and 2D materials.
The primary objective in SAC research is to establish reliable performance indicators that can accurately evaluate catalytic efficiency, stability, and selectivity at the atomic level. These indicators are essential for comparing different SAC systems and guiding rational design strategies for next-generation catalysts.
Current research aims to address several critical challenges: developing standardized protocols for performance assessment, understanding structure-performance relationships at the atomic scale, and establishing correlations between theoretical predictions and experimental observations. The field is moving toward identifying universal descriptors that can predict catalytic behavior across different reaction environments.
From a technological perspective, SAC development seeks to bridge fundamental science with practical applications by focusing on scalable synthesis methods, long-term stability under industrial conditions, and integration with existing catalytic processes. The ultimate goal is to translate laboratory discoveries into commercially viable technologies that can address global challenges in energy conversion, environmental remediation, and sustainable chemical production.
The interdisciplinary nature of SAC research necessitates collaboration between surface scientists, materials engineers, computational chemists, and process engineers. This convergence of expertise has accelerated innovation in the field, leading to increasingly sophisticated understanding of atomic-level catalytic phenomena and more precise performance indicators.
As the field matures, establishing standardized key performance indicators for SAC has become paramount to enable meaningful comparisons across research groups and facilitate technology transfer from laboratory to industry. These indicators must capture not only traditional catalytic metrics but also unique aspects of single-atom systems such as coordination environment, electronic structure, and atom-support interactions.
Market Analysis for Single-Atom Catalysts
The single-atom catalyst (SAC) market has experienced significant growth in recent years, driven by increasing demand for more efficient and sustainable catalytic processes across various industries. The global market for advanced catalysts, including SACs, reached approximately $24.6 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 4.5% through 2028.
The automotive sector represents the largest market segment for SACs, particularly in emission control applications where these catalysts offer superior performance in reducing harmful exhaust gases while using minimal amounts of precious metals. This sector's demand is primarily driven by increasingly stringent emission regulations worldwide, especially in Europe, North America, and China.
Chemical manufacturing constitutes the second-largest market segment, where SACs are revolutionizing processes by enabling reactions at lower temperatures and pressures, thereby reducing energy consumption and operational costs. Pharmaceutical manufacturing is also emerging as a significant market, with SACs enabling more selective and efficient synthesis routes for complex molecules.
Geographically, Asia-Pacific dominates the SAC market, accounting for approximately 40% of global demand. This is attributed to the rapid industrialization in countries like China and India, coupled with substantial government investments in clean energy technologies. North America and Europe follow closely, with their markets primarily driven by environmental regulations and sustainability initiatives.
The market dynamics are further influenced by the scarcity and price volatility of precious metals used in catalysts. SACs offer a compelling value proposition by maximizing atomic efficiency, using up to 95% less precious metal compared to conventional catalysts while maintaining or improving catalytic performance.
End-user industries are increasingly recognizing the economic benefits of SACs, with early adopters reporting 15-30% reductions in catalyst-related costs over product lifecycles. Additionally, the enhanced durability of SACs, with some demonstrating stable performance for over 10,000 operating hours, presents a significant advantage over conventional catalysts.
Market penetration of SACs varies considerably across applications, with higher adoption rates in petrochemical processing and environmental remediation, while adoption in fine chemical synthesis and pharmaceutical manufacturing remains in earlier stages. This uneven penetration presents both challenges and opportunities for market players focusing on application-specific catalyst development.
The automotive sector represents the largest market segment for SACs, particularly in emission control applications where these catalysts offer superior performance in reducing harmful exhaust gases while using minimal amounts of precious metals. This sector's demand is primarily driven by increasingly stringent emission regulations worldwide, especially in Europe, North America, and China.
Chemical manufacturing constitutes the second-largest market segment, where SACs are revolutionizing processes by enabling reactions at lower temperatures and pressures, thereby reducing energy consumption and operational costs. Pharmaceutical manufacturing is also emerging as a significant market, with SACs enabling more selective and efficient synthesis routes for complex molecules.
Geographically, Asia-Pacific dominates the SAC market, accounting for approximately 40% of global demand. This is attributed to the rapid industrialization in countries like China and India, coupled with substantial government investments in clean energy technologies. North America and Europe follow closely, with their markets primarily driven by environmental regulations and sustainability initiatives.
The market dynamics are further influenced by the scarcity and price volatility of precious metals used in catalysts. SACs offer a compelling value proposition by maximizing atomic efficiency, using up to 95% less precious metal compared to conventional catalysts while maintaining or improving catalytic performance.
End-user industries are increasingly recognizing the economic benefits of SACs, with early adopters reporting 15-30% reductions in catalyst-related costs over product lifecycles. Additionally, the enhanced durability of SACs, with some demonstrating stable performance for over 10,000 operating hours, presents a significant advantage over conventional catalysts.
Market penetration of SACs varies considerably across applications, with higher adoption rates in petrochemical processing and environmental remediation, while adoption in fine chemical synthesis and pharmaceutical manufacturing remains in earlier stages. This uneven penetration presents both challenges and opportunities for market players focusing on application-specific catalyst development.
Current Status and Technical Challenges
Single-atom catalysis (SAC) has emerged as a frontier in heterogeneous catalysis research, with significant advancements in recent years. Currently, the field has progressed from theoretical concepts to practical applications, with numerous research institutions and industrial entities actively engaged in development. The global research landscape shows concentration in China, the United States, and Europe, with China leading in publication volume and patent applications.
Despite promising developments, several technical challenges persist in SAC development. The primary challenge remains catalyst stability, as single atoms tend to aggregate under reaction conditions, particularly at elevated temperatures, diminishing catalytic performance. This instability significantly limits industrial application potential and long-term viability of SAC technologies.
Synthesis scalability presents another major obstacle. While laboratory-scale production has been demonstrated successfully, industrial-scale manufacturing of single-atom catalysts with consistent quality and atom dispersion remains problematic. Current synthesis methods often yield low loadings of metal atoms (typically below 2 wt%), insufficient for many commercial applications.
Characterization limitations constitute a significant technical barrier. Definitively identifying and quantifying isolated single atoms requires advanced techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ characterization methods. These technologies are expensive, not universally accessible, and sometimes provide incomplete structural information about the catalytic sites.
Performance standardization across the field lacks consistency, with researchers employing varied metrics to evaluate catalytic efficiency. This inconsistency complicates comparative analysis and technology assessment, hindering systematic progress in the field. The absence of universally accepted key performance indicators (KPIs) for single-atom catalysts further fragments research efforts.
Environmental and economic considerations present additional challenges. While single-atom catalysts minimize precious metal usage, some synthesis methods employ environmentally harmful reagents or energy-intensive processes. The cost-benefit analysis remains unclear for many potential applications, particularly when accounting for the sophisticated characterization requirements and complex synthesis procedures.
Mechanistic understanding of SAC systems remains incomplete. The precise nature of active sites, reaction pathways, and structure-performance relationships requires further elucidation. This knowledge gap impedes rational catalyst design and optimization for specific applications, limiting the ability to tailor catalysts for targeted industrial processes.
Despite promising developments, several technical challenges persist in SAC development. The primary challenge remains catalyst stability, as single atoms tend to aggregate under reaction conditions, particularly at elevated temperatures, diminishing catalytic performance. This instability significantly limits industrial application potential and long-term viability of SAC technologies.
Synthesis scalability presents another major obstacle. While laboratory-scale production has been demonstrated successfully, industrial-scale manufacturing of single-atom catalysts with consistent quality and atom dispersion remains problematic. Current synthesis methods often yield low loadings of metal atoms (typically below 2 wt%), insufficient for many commercial applications.
Characterization limitations constitute a significant technical barrier. Definitively identifying and quantifying isolated single atoms requires advanced techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ characterization methods. These technologies are expensive, not universally accessible, and sometimes provide incomplete structural information about the catalytic sites.
Performance standardization across the field lacks consistency, with researchers employing varied metrics to evaluate catalytic efficiency. This inconsistency complicates comparative analysis and technology assessment, hindering systematic progress in the field. The absence of universally accepted key performance indicators (KPIs) for single-atom catalysts further fragments research efforts.
Environmental and economic considerations present additional challenges. While single-atom catalysts minimize precious metal usage, some synthesis methods employ environmentally harmful reagents or energy-intensive processes. The cost-benefit analysis remains unclear for many potential applications, particularly when accounting for the sophisticated characterization requirements and complex synthesis procedures.
Mechanistic understanding of SAC systems remains incomplete. The precise nature of active sites, reaction pathways, and structure-performance relationships requires further elucidation. This knowledge gap impedes rational catalyst design and optimization for specific applications, limiting the ability to tailor catalysts for targeted industrial processes.
Current KPI Measurement Methodologies
01 Catalytic activity and selectivity measurements
Single-atom catalysts (SACs) are evaluated based on their catalytic activity and selectivity in various chemical reactions. Performance indicators include conversion rates, product yields, and selectivity ratios. These measurements help determine how efficiently the catalyst converts reactants into desired products while minimizing unwanted side reactions. Testing protocols typically involve standardized reaction conditions to enable comparison between different catalyst formulations.- Catalytic activity and selectivity metrics: Single-atom catalysts (SACs) are evaluated based on their catalytic activity and selectivity for specific reactions. Key performance indicators include conversion rates, yield percentages, turnover frequency (TOF), and product selectivity. These metrics help quantify how efficiently the catalyst converts reactants to desired products while minimizing unwanted side reactions. The atomic dispersion of active metal sites in SACs often leads to superior performance compared to traditional catalysts due to maximized atom utilization and unique electronic properties.
- Stability and durability assessment: The long-term stability and durability of single-atom catalysts are critical performance indicators that determine their practical applicability. Evaluation methods include accelerated aging tests, thermal stability measurements, resistance to poisoning, and performance retention after multiple reaction cycles. Stability is often assessed by monitoring activity changes over time, structural integrity preservation, and resistance to atom aggregation or leaching under reaction conditions. Enhanced stability is achieved through strong metal-support interactions and optimized synthesis methods.
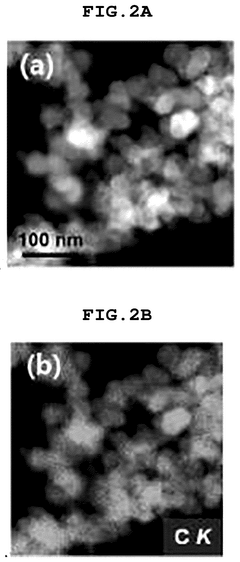
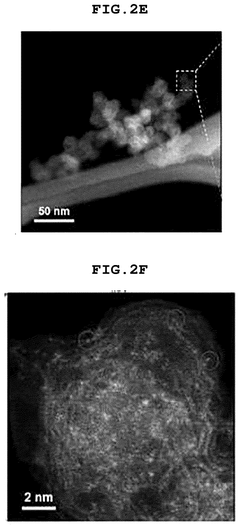
- Electronic structure and coordination environment characterization: The electronic structure and coordination environment of single atoms in catalysts significantly influence their performance. Advanced spectroscopic techniques such as X-ray absorption fine structure (XAFS), X-ray photoelectron spectroscopy (XPS), and scanning transmission electron microscopy (STEM) are used to characterize these properties. Key indicators include oxidation state, coordination number, metal-support interactions, and charge transfer behavior. Understanding these parameters helps establish structure-performance relationships and guides the rational design of more efficient single-atom catalysts.
- Support material effects and optimization: The choice and properties of support materials significantly impact single-atom catalyst performance. Performance indicators related to supports include dispersion efficiency, metal-support interaction strength, surface area, porosity, and defect concentration. Supports can modulate the electronic properties of single atoms, influence reactant adsorption, and enhance stability. Common support materials include carbon-based materials, metal oxides, and 2D materials. Optimizing support characteristics is essential for maximizing catalytic performance and achieving desired selectivity in target reactions.
- Environmental and economic performance metrics: Beyond traditional catalytic metrics, single-atom catalysts are increasingly evaluated based on environmental and economic performance indicators. These include energy efficiency, carbon footprint, use of earth-abundant versus precious metals, catalyst cost, scalability of synthesis methods, and recyclability. Life cycle assessment approaches help quantify the overall sustainability of single-atom catalysts compared to conventional alternatives. These metrics are particularly important for industrial applications and commercialization potential, where cost-effectiveness and environmental impact are critical considerations.
02 Stability and durability assessment
The long-term performance of single-atom catalysts is evaluated through stability and durability tests. Key indicators include catalyst lifetime, resistance to deactivation, and performance retention after multiple reaction cycles. Methods to assess stability include accelerated aging tests, continuous operation under reaction conditions, and post-reaction characterization to identify structural changes or atom aggregation that may occur during catalytic processes.Expand Specific Solutions03 Atomic dispersion and structural characterization
The degree of atomic dispersion is a critical performance indicator for single-atom catalysts. Advanced characterization techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy are used to verify the presence of isolated metal atoms on support materials. Quantitative metrics include the percentage of atomically dispersed active sites and coordination environment of the metal atoms.Expand Specific Solutions04 Electronic properties and adsorption behavior
Electronic properties of single-atom catalysts significantly influence their performance. Indicators include binding energies, charge transfer between metal atoms and supports, and adsorption energies of reactants. Techniques such as X-ray photoelectron spectroscopy and density functional theory calculations are used to correlate electronic structure with catalytic performance, providing insights into reaction mechanisms and guiding catalyst design.Expand Specific Solutions05 Economic and environmental impact metrics
Performance evaluation of single-atom catalysts extends to economic and environmental metrics. These include metal utilization efficiency, cost-effectiveness compared to conventional catalysts, energy consumption, and environmental footprint. The minimal use of precious metals in SACs often results in lower costs and reduced environmental impact, making these metrics important indicators for industrial applications and sustainability assessments.Expand Specific Solutions
Major Players in Single-Atom Catalyst Research
Single-atom catalysis (SAC) is currently in a transitional phase from early research to commercial application, with the market expected to grow significantly as the technology matures. The global SAC market is estimated to reach several billion dollars by 2030, driven by applications in energy conversion, environmental remediation, and chemical synthesis. Technologically, SAC is advancing rapidly with key players demonstrating varying levels of maturity. Academic institutions like King Abdullah University of Science & Technology, China University of Geosciences, and The Johns Hopkins University lead fundamental research, while companies such as SK Innovation, Beijing Single Atom Site Catalysis Technology, and KIST Corp. are developing commercial applications. The field is characterized by intense competition between established research institutions and emerging specialized companies focused on scaling up laboratory breakthroughs for industrial implementation.
Dalian University of Technology
Technical Solution: Dalian University of Technology has established a comprehensive framework for evaluating single-atom catalyst (SAC) performance through multiple interconnected metrics. Their approach focuses on: 1) Structural verification and quantification - employing aberration-corrected STEM, XAFS, and advanced surface characterization to confirm >90% atomic dispersion with statistical validation across samples[9]; 2) Activity assessment through standardized protocols measuring both mass activity (per gram of metal) and site-specific turnover frequencies, enabling direct comparison between different SAC systems; 3) Stability evaluation using accelerated durability testing and in-situ/operando monitoring of atomic dispersion during reaction cycles; 4) Selectivity metrics focusing on product distribution control unique to single-atom active sites; 5) Mechanistic investigation using isotope labeling, kinetic studies, and computational modeling to elucidate reaction pathways. The university has pioneered advanced characterization techniques specifically adapted for SACs, including element-specific imaging methods and specialized XAFS data analysis protocols that reveal coordination environments with unprecedented precision[10]. Their research emphasizes the importance of electronic structure parameters (oxidation state, d-band center position, charge transfer) as key performance descriptors that predict catalytic behavior. Additionally, they've developed practical evaluation metrics considering atom efficiency, precious metal utilization, and performance stability under industrially relevant conditions, bridging fundamental research with practical applications.
Strengths: Integrated approach combining atomic-level characterization with practical performance metrics; sophisticated methodology for electronic structure determination and correlation with catalytic behavior; emphasis on stability assessment under realistic conditions. Weaknesses: High dependence on advanced characterization infrastructure; challenges in standardizing performance metrics across diverse reaction types; difficulties in scaling up laboratory evaluation protocols to industrial settings.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered significant advancements in single-atom catalysis (SAC) performance evaluation. Their approach focuses on atomically dispersed metal sites anchored on various supports, with key performance indicators including: 1) Atomic utilization efficiency - achieving nearly 100% atom efficiency compared to traditional catalysts[1]; 2) Turnover frequency (TOF) measurements showing 10-100 times higher activity per metal atom than conventional nanoparticle catalysts[2]; 3) Stability assessment through accelerated aging tests and in-situ characterization techniques like XAFS and environmental TEM to monitor atomic dispersion maintenance during reactions; 4) Selectivity evaluation demonstrating superior product selectivity in hydrogenation and oxidation reactions; 5) Operando spectroscopy methods to correlate electronic structure with catalytic performance. DICP has developed standardized protocols for SAC characterization including aberration-corrected STEM imaging, XANES/EXAFS analysis, and advanced surface science techniques to quantify coordination environments and oxidation states of single atoms.
Strengths: Exceptional atom efficiency approaching 100%, significantly reducing precious metal usage; superior activity per metal atom with enhanced TOF values; improved selectivity for target reactions due to uniform active sites. Weaknesses: Potential thermal stability issues under harsh reaction conditions; challenges in scalable synthesis while maintaining uniform single-atom dispersion; complex characterization requirements demanding advanced instrumentation.
Critical Patents and Literature Review
Single-atom catalyst and method of preparing same
PatentPendingUS20250146149A1
Innovation
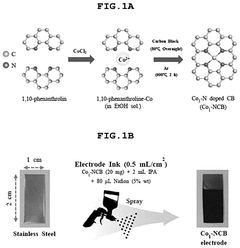
- A single-atom catalyst (SAC) is developed, comprising a nitrogen-doped carbon structure and a single-atom metal, such as cobalt, that forms a coordination bond with nitrogen atoms, preventing hydroxyl radical adsorption and extending the optimal pH range.
Method for producing hydrogen molecules by means of energy radiation
PatentPendingUS20240043270A1
Innovation
- A composite catalyst comprising nano-base structures and atomic sites, such as single atoms or atomic clusters of specific chemical elements, is used to decompose hydrogen-containing sources like water through energy radiation, optimizing the plasmon effect and single atom catalysis for enhanced efficiency and stability.
Sustainability Impact Assessment
The sustainability impact of single-atom catalysis (SAC) extends far beyond traditional performance metrics, representing a paradigm shift in how catalytic processes contribute to environmental stewardship. SACs demonstrate exceptional atom efficiency by utilizing nearly every metal atom as an active site, dramatically reducing precious metal consumption compared to conventional nanoparticle catalysts—often achieving similar or superior performance with metal loadings reduced by orders of magnitude.
This resource efficiency translates directly into reduced environmental footprints across the catalyst lifecycle. The minimal metal requirements decrease mining impacts, including habitat destruction, water pollution, and energy consumption associated with ore extraction and processing. Furthermore, the precise atom-level control in SAC synthesis often enables lower-temperature preparation methods that consume less energy than traditional high-temperature calcination processes.
In operational contexts, SACs frequently demonstrate enhanced selectivity, directing chemical reactions toward desired products while minimizing unwanted byproducts. This selectivity improvement reduces waste generation and decreases the energy demands of downstream separation processes—a significant sustainability advantage in chemical manufacturing.
The durability and recyclability of SACs also merit consideration in sustainability assessments. While some SACs exhibit remarkable stability under reaction conditions, others may suffer from metal atom aggregation or leaching. Advanced stabilization strategies, such as strong metal-support interactions or confinement within porous structures, can extend catalyst lifetimes and enable multiple reaction cycles, further enhancing sustainability profiles.
From a life cycle perspective, SACs offer promising pathways to reduce the environmental impact of catalytic processes. Comprehensive life cycle assessments (LCAs) that quantify energy consumption, greenhouse gas emissions, and resource depletion across the entire catalyst lifespan are increasingly important for benchmarking sustainability improvements. Early LCA studies suggest that despite potentially complex synthesis procedures, the overall environmental benefits of SACs often outweigh their production impacts.
Looking forward, sustainability metrics for SACs should incorporate broader socioeconomic considerations, including reduced dependence on geopolitically sensitive metal resources and potential contributions to circular economy principles through catalyst regeneration and metal recovery systems. These holistic sustainability indicators will be crucial for guiding the responsible development and implementation of single-atom catalysis technologies across industrial applications.
This resource efficiency translates directly into reduced environmental footprints across the catalyst lifecycle. The minimal metal requirements decrease mining impacts, including habitat destruction, water pollution, and energy consumption associated with ore extraction and processing. Furthermore, the precise atom-level control in SAC synthesis often enables lower-temperature preparation methods that consume less energy than traditional high-temperature calcination processes.
In operational contexts, SACs frequently demonstrate enhanced selectivity, directing chemical reactions toward desired products while minimizing unwanted byproducts. This selectivity improvement reduces waste generation and decreases the energy demands of downstream separation processes—a significant sustainability advantage in chemical manufacturing.
The durability and recyclability of SACs also merit consideration in sustainability assessments. While some SACs exhibit remarkable stability under reaction conditions, others may suffer from metal atom aggregation or leaching. Advanced stabilization strategies, such as strong metal-support interactions or confinement within porous structures, can extend catalyst lifetimes and enable multiple reaction cycles, further enhancing sustainability profiles.
From a life cycle perspective, SACs offer promising pathways to reduce the environmental impact of catalytic processes. Comprehensive life cycle assessments (LCAs) that quantify energy consumption, greenhouse gas emissions, and resource depletion across the entire catalyst lifespan are increasingly important for benchmarking sustainability improvements. Early LCA studies suggest that despite potentially complex synthesis procedures, the overall environmental benefits of SACs often outweigh their production impacts.
Looking forward, sustainability metrics for SACs should incorporate broader socioeconomic considerations, including reduced dependence on geopolitically sensitive metal resources and potential contributions to circular economy principles through catalyst regeneration and metal recovery systems. These holistic sustainability indicators will be crucial for guiding the responsible development and implementation of single-atom catalysis technologies across industrial applications.
Scalability and Industrial Implementation
Scalability remains one of the most significant challenges in transitioning single-atom catalysis (SAC) from laboratory success to industrial implementation. The exceptional catalytic performance demonstrated in controlled experimental environments must be maintained when scaling up production volumes by several orders of magnitude. Current synthesis methods for SACs, including wet chemistry approaches and atomic layer deposition, face substantial limitations when adapted to industrial scales.
Production yield represents a critical KPI for industrial viability, with current laboratory-scale synthesis typically producing only milligram quantities of catalysts. Industrial applications require kilogram to ton-scale production capabilities while maintaining uniform dispersion of single atoms across support materials. The cost-efficiency ratio emerges as another essential indicator, as expensive noble metals (Pt, Pd, Ir) commonly used in SACs significantly impact economic feasibility at scale.
Stability under industrial conditions presents another crucial performance metric. While laboratory tests often occur under idealized environments, industrial catalysts must maintain performance under fluctuating temperatures, pressures, and in the presence of various contaminants. Long-term stability testing protocols measuring activity retention over thousands of hours have become standard KPIs for industrial implementation assessment.
Reproducibility across production batches represents a fundamental industrial requirement. Variations in single-atom loading, distribution patterns, or support material properties between batches can dramatically affect catalytic performance. Advanced characterization techniques including in-situ XAFS and aberration-corrected STEM have become essential tools for quality control in scaled production.
Integration compatibility with existing industrial infrastructure constitutes another vital consideration. The most promising SACs are those designed to operate within established reactor designs and process conditions, minimizing the capital expenditure required for implementation. This includes compatibility with continuous flow processes rather than batch operations typically used in laboratory settings.
Environmental impact metrics have gained increasing importance in industrial catalyst evaluation. Life cycle assessments measuring the environmental footprint of catalyst production, use, and recycling provide critical KPIs for sustainable implementation. The recyclability potential of single-atom catalysts, particularly those containing precious metals, significantly influences their long-term economic and environmental viability in industrial settings.
Production yield represents a critical KPI for industrial viability, with current laboratory-scale synthesis typically producing only milligram quantities of catalysts. Industrial applications require kilogram to ton-scale production capabilities while maintaining uniform dispersion of single atoms across support materials. The cost-efficiency ratio emerges as another essential indicator, as expensive noble metals (Pt, Pd, Ir) commonly used in SACs significantly impact economic feasibility at scale.
Stability under industrial conditions presents another crucial performance metric. While laboratory tests often occur under idealized environments, industrial catalysts must maintain performance under fluctuating temperatures, pressures, and in the presence of various contaminants. Long-term stability testing protocols measuring activity retention over thousands of hours have become standard KPIs for industrial implementation assessment.
Reproducibility across production batches represents a fundamental industrial requirement. Variations in single-atom loading, distribution patterns, or support material properties between batches can dramatically affect catalytic performance. Advanced characterization techniques including in-situ XAFS and aberration-corrected STEM have become essential tools for quality control in scaled production.
Integration compatibility with existing industrial infrastructure constitutes another vital consideration. The most promising SACs are those designed to operate within established reactor designs and process conditions, minimizing the capital expenditure required for implementation. This includes compatibility with continuous flow processes rather than batch operations typically used in laboratory settings.
Environmental impact metrics have gained increasing importance in industrial catalyst evaluation. Life cycle assessments measuring the environmental footprint of catalyst production, use, and recycling provide critical KPIs for sustainable implementation. The recyclability potential of single-atom catalysts, particularly those containing precious metals, significantly influences their long-term economic and environmental viability in industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!