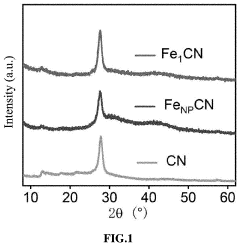
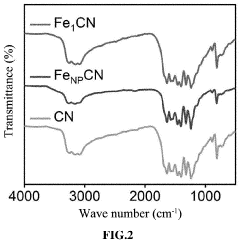
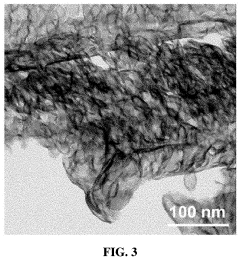
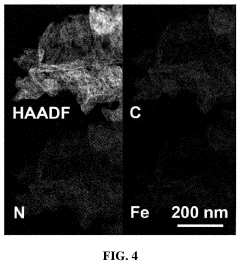
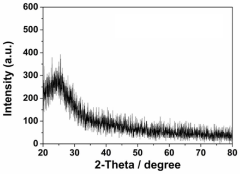
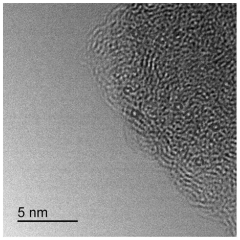
Single-Atom Catalysis in the Evolution of Biodegradable Polymers
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis, emerging in the early 2010s as researchers discovered the exceptional catalytic properties of isolated metal atoms anchored on suitable supports. This technology has evolved from theoretical concepts to practical applications over the past decade, with significant breakthroughs occurring around 2015-2018 when scalable synthesis methods were developed.
The evolution of SAC has been driven by the fundamental understanding that maximum atom efficiency can be achieved when every metal atom participates in catalysis. This approach addresses critical limitations in traditional metal catalysts, including high costs of precious metals and inefficient atom utilization. The field has progressed from initial proof-of-concept studies to increasingly sophisticated catalyst designs with tailored selectivity and stability.
In the context of biodegradable polymers, SAC represents a particularly promising technological direction. Conventional polymer synthesis and degradation processes often require harsh conditions, toxic catalysts, or deliver insufficient performance. The precision of single-atom catalysts offers unprecedented control over polymerization reactions and polymer degradation pathways, potentially enabling more sustainable materials with programmable lifespans.
The global push toward circular economy models and stricter environmental regulations has accelerated research in this domain. Notable milestones include the development of single-atom catalysts capable of facilitating controlled ring-opening polymerization of cyclic esters, selective C-O bond activation in polyesters, and low-temperature polymer degradation processes that preserve valuable monomers.
The primary technical objectives in this field include developing SACs that can: (1) enable polymerization reactions under mild conditions with minimal side reactions; (2) catalyze the selective degradation of polymers into recoverable monomers; (3) maintain catalytic activity over multiple cycles; and (4) function effectively in industrially relevant conditions and scales.
Current research aims to bridge the gap between laboratory demonstrations and industrial implementation by addressing challenges in catalyst stability, selectivity control, and economic viability. The ultimate goal is to establish SAC as a transformative technology that enables a new generation of biodegradable polymers with precisely engineered properties and end-of-life pathways.
Looking forward, the field is trending toward multifunctional single-atom catalysts that can perform sequential or tandem catalytic transformations, mimicking the efficiency of biological systems. This approach holds promise for developing closed-loop polymer systems where the same catalyst facilitates both synthesis and degradation processes under different conditions.
The evolution of SAC has been driven by the fundamental understanding that maximum atom efficiency can be achieved when every metal atom participates in catalysis. This approach addresses critical limitations in traditional metal catalysts, including high costs of precious metals and inefficient atom utilization. The field has progressed from initial proof-of-concept studies to increasingly sophisticated catalyst designs with tailored selectivity and stability.
In the context of biodegradable polymers, SAC represents a particularly promising technological direction. Conventional polymer synthesis and degradation processes often require harsh conditions, toxic catalysts, or deliver insufficient performance. The precision of single-atom catalysts offers unprecedented control over polymerization reactions and polymer degradation pathways, potentially enabling more sustainable materials with programmable lifespans.
The global push toward circular economy models and stricter environmental regulations has accelerated research in this domain. Notable milestones include the development of single-atom catalysts capable of facilitating controlled ring-opening polymerization of cyclic esters, selective C-O bond activation in polyesters, and low-temperature polymer degradation processes that preserve valuable monomers.
The primary technical objectives in this field include developing SACs that can: (1) enable polymerization reactions under mild conditions with minimal side reactions; (2) catalyze the selective degradation of polymers into recoverable monomers; (3) maintain catalytic activity over multiple cycles; and (4) function effectively in industrially relevant conditions and scales.
Current research aims to bridge the gap between laboratory demonstrations and industrial implementation by addressing challenges in catalyst stability, selectivity control, and economic viability. The ultimate goal is to establish SAC as a transformative technology that enables a new generation of biodegradable polymers with precisely engineered properties and end-of-life pathways.
Looking forward, the field is trending toward multifunctional single-atom catalysts that can perform sequential or tandem catalytic transformations, mimicking the efficiency of biological systems. This approach holds promise for developing closed-loop polymer systems where the same catalyst facilitates both synthesis and degradation processes under different conditions.
Market Demand for Biodegradable Polymer Solutions
The global market for biodegradable polymers has experienced significant growth in recent years, driven primarily by increasing environmental concerns and regulatory pressures against conventional plastics. Current market valuations indicate that the biodegradable polymers sector reached approximately 4.2 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 14-17% through 2030. This accelerated growth reflects the urgent demand for sustainable alternatives to traditional petroleum-based plastics.
Consumer awareness regarding plastic pollution has reached unprecedented levels, creating substantial market pull for biodegradable solutions. Surveys indicate that 73% of global consumers express willingness to pay premium prices for environmentally friendly packaging. This shift in consumer behavior has prompted major brands across food, beverage, personal care, and retail sectors to commit to biodegradable packaging transitions within the next decade.
Regulatory frameworks worldwide are increasingly favoring biodegradable materials. The European Union's Single-Use Plastics Directive, China's plastic ban initiatives, and similar legislation in over 70 countries have created a regulatory environment that necessitates innovation in biodegradable polymer technologies. These regulations typically mandate specific biodegradability standards, creating defined market requirements for new polymer solutions.
The agricultural sector represents another significant demand driver, with biodegradable mulch films, controlled-release fertilizer coatings, and seed casings showing market growth rates exceeding 20% annually. The medical field similarly demonstrates strong demand, particularly for biodegradable implants, drug delivery systems, and surgical materials, with market valuations approaching 1.5 billion USD.
Despite this promising demand landscape, current biodegradable polymer solutions face persistent challenges in performance, cost, and scalability. Conventional biodegradable polymers like PLA (polylactic acid) and PHA (polyhydroxyalkanoates) exhibit limitations in mechanical properties, thermal stability, and processing capabilities. The average cost premium for biodegradable alternatives remains 20-40% higher than conventional plastics, creating adoption barriers in price-sensitive applications.
This performance-cost gap represents the precise market opportunity that single-atom catalysis technology could address. By enabling more efficient polymerization processes, improved polymer architecture control, and potentially utilizing more cost-effective feedstocks, single-atom catalysts could revolutionize biodegradable polymer production. Market analysis suggests that technologies capable of reducing production costs by 15-25% while enhancing performance properties would capture significant market share across multiple industry verticals.
Consumer awareness regarding plastic pollution has reached unprecedented levels, creating substantial market pull for biodegradable solutions. Surveys indicate that 73% of global consumers express willingness to pay premium prices for environmentally friendly packaging. This shift in consumer behavior has prompted major brands across food, beverage, personal care, and retail sectors to commit to biodegradable packaging transitions within the next decade.
Regulatory frameworks worldwide are increasingly favoring biodegradable materials. The European Union's Single-Use Plastics Directive, China's plastic ban initiatives, and similar legislation in over 70 countries have created a regulatory environment that necessitates innovation in biodegradable polymer technologies. These regulations typically mandate specific biodegradability standards, creating defined market requirements for new polymer solutions.
The agricultural sector represents another significant demand driver, with biodegradable mulch films, controlled-release fertilizer coatings, and seed casings showing market growth rates exceeding 20% annually. The medical field similarly demonstrates strong demand, particularly for biodegradable implants, drug delivery systems, and surgical materials, with market valuations approaching 1.5 billion USD.
Despite this promising demand landscape, current biodegradable polymer solutions face persistent challenges in performance, cost, and scalability. Conventional biodegradable polymers like PLA (polylactic acid) and PHA (polyhydroxyalkanoates) exhibit limitations in mechanical properties, thermal stability, and processing capabilities. The average cost premium for biodegradable alternatives remains 20-40% higher than conventional plastics, creating adoption barriers in price-sensitive applications.
This performance-cost gap represents the precise market opportunity that single-atom catalysis technology could address. By enabling more efficient polymerization processes, improved polymer architecture control, and potentially utilizing more cost-effective feedstocks, single-atom catalysts could revolutionize biodegradable polymer production. Market analysis suggests that technologies capable of reducing production costs by 15-25% while enhancing performance properties would capture significant market share across multiple industry verticals.
Current Status and Challenges in Single-Atom Catalysis
Single-atom catalysis (SAC) represents a frontier in catalytic science, where individual metal atoms are dispersed on support materials to maximize atomic efficiency. In the context of biodegradable polymers, this technology has shown remarkable potential for revolutionizing synthesis processes. Currently, SAC research is advancing rapidly worldwide, with significant contributions from research institutions in China, the United States, and Europe.
The development of single-atom catalysts for biodegradable polymer synthesis has reached several important milestones. Recent breakthroughs include the successful application of noble metal (Pt, Pd, Au) single-atom catalysts for controlled ring-opening polymerization of lactones and lactides, achieving unprecedented selectivity and reduced side reactions. Additionally, non-noble metal SACs based on Fe, Co, and Ni have demonstrated promising activity in depolymerization processes, offering more sustainable alternatives.
Despite these advances, several technical challenges persist. The stability of single-atom catalysts remains a critical issue, as isolated metal atoms tend to aggregate under reaction conditions relevant to polymer synthesis, particularly at elevated temperatures required for melt polymerization. This aggregation significantly reduces catalytic efficiency and selectivity over time.
Another major challenge is the scalable production of SACs with consistent quality. Current synthesis methods, including atomic layer deposition and wet chemistry approaches, face limitations in producing industrial quantities while maintaining uniform atom dispersion. This manufacturing bottleneck has restricted the application of SACs in commercial biodegradable polymer production.
The selectivity control in complex monomer mixtures presents another significant hurdle. While SACs excel in model reactions, their performance in real-world polymerization scenarios involving multiple functional groups and impurities requires further optimization. This is particularly relevant for the upcycling of plastic waste into biodegradable polymers, where feedstock purity varies considerably.
Geographically, research leadership in this field shows distinct patterns. China leads in the number of publications and patents related to SAC technology for polymer applications, with particular strength in developing novel support materials. The United States maintains an edge in fundamental mechanistic studies and computational modeling of SAC-polymer interactions. European research clusters, particularly in Germany and the UK, focus on sustainable applications and integration with circular economy principles.
Interdisciplinary collaboration between catalysis experts and polymer scientists remains insufficient, creating knowledge gaps that slow technological advancement. Additionally, comprehensive life cycle assessments of SAC-enabled biodegradable polymer production are largely absent, making it difficult to quantify the true environmental benefits compared to conventional catalytic systems.
The development of single-atom catalysts for biodegradable polymer synthesis has reached several important milestones. Recent breakthroughs include the successful application of noble metal (Pt, Pd, Au) single-atom catalysts for controlled ring-opening polymerization of lactones and lactides, achieving unprecedented selectivity and reduced side reactions. Additionally, non-noble metal SACs based on Fe, Co, and Ni have demonstrated promising activity in depolymerization processes, offering more sustainable alternatives.
Despite these advances, several technical challenges persist. The stability of single-atom catalysts remains a critical issue, as isolated metal atoms tend to aggregate under reaction conditions relevant to polymer synthesis, particularly at elevated temperatures required for melt polymerization. This aggregation significantly reduces catalytic efficiency and selectivity over time.
Another major challenge is the scalable production of SACs with consistent quality. Current synthesis methods, including atomic layer deposition and wet chemistry approaches, face limitations in producing industrial quantities while maintaining uniform atom dispersion. This manufacturing bottleneck has restricted the application of SACs in commercial biodegradable polymer production.
The selectivity control in complex monomer mixtures presents another significant hurdle. While SACs excel in model reactions, their performance in real-world polymerization scenarios involving multiple functional groups and impurities requires further optimization. This is particularly relevant for the upcycling of plastic waste into biodegradable polymers, where feedstock purity varies considerably.
Geographically, research leadership in this field shows distinct patterns. China leads in the number of publications and patents related to SAC technology for polymer applications, with particular strength in developing novel support materials. The United States maintains an edge in fundamental mechanistic studies and computational modeling of SAC-polymer interactions. European research clusters, particularly in Germany and the UK, focus on sustainable applications and integration with circular economy principles.
Interdisciplinary collaboration between catalysis experts and polymer scientists remains insufficient, creating knowledge gaps that slow technological advancement. Additionally, comprehensive life cycle assessments of SAC-enabled biodegradable polymer production are largely absent, making it difficult to quantify the true environmental benefits compared to conventional catalytic systems.
Current Single-Atom Catalytic Approaches for Polymer Synthesis
01 Single-atom catalysts for polymer synthesis
Single-atom catalysts (SACs) provide unique advantages in the synthesis of biodegradable polymers due to their maximum atom efficiency and distinctive catalytic properties. These catalysts feature isolated metal atoms anchored on supports, offering enhanced catalytic activity and selectivity for polymerization reactions. The atomically dispersed active sites enable precise control over polymer chain growth, molecular weight distribution, and stereochemistry, resulting in biodegradable polymers with improved properties and performance.- Single-atom catalysts for polymer synthesis and degradation: Single-atom catalysts (SACs) provide unique catalytic properties for the synthesis and controlled degradation of biodegradable polymers. These catalysts feature isolated metal atoms dispersed on supports, offering maximum atom efficiency and specific catalytic sites. They can facilitate polymerization reactions with enhanced control over molecular weight and polymer architecture, while also enabling selective bond cleavage during biodegradation processes. The high surface area and tunable electronic properties of SACs make them particularly effective for catalyzing environmentally friendly polymer production and decomposition.
- Metal-based catalysts for biodegradable polymer production: Various metal-based catalysts, including transition metals and rare earth elements, are employed in the synthesis of biodegradable polymers. These catalysts can be used in ring-opening polymerization of cyclic monomers like lactides and lactones to produce polylactic acid (PLA) and other polyesters. The catalytic activity can be tuned by modifying the metal center, ligand environment, and support material. These catalysts enable controlled polymerization conditions, resulting in biodegradable polymers with desired properties such as mechanical strength, degradation rate, and biocompatibility.
- Carbon-supported single-atom catalysts for polymer applications: Carbon materials serve as excellent supports for single-atom catalysts in biodegradable polymer applications. Carbon supports such as graphene, carbon nanotubes, and porous carbon can anchor individual metal atoms through coordination with heteroatoms or defect sites. These carbon-supported catalysts offer advantages including high surface area, good electrical conductivity, and chemical stability. They can catalyze both the synthesis of biodegradable polymers and their controlled degradation under specific conditions, making them valuable for creating sustainable polymer materials with tunable life cycles.
- Enzymatic and bio-inspired catalytic systems: Enzymatic and bio-inspired catalytic systems offer green approaches to biodegradable polymer synthesis and degradation. These systems mimic natural enzymatic processes, often incorporating single metal atoms as active sites similar to metalloenzymes. They can operate under mild conditions with high selectivity, reducing energy requirements and waste generation. Such catalysts are particularly valuable for polymerization reactions involving renewable monomers and for controlled polymer degradation in biological environments. The bio-inspired design principles help create catalysts that combine the efficiency of biological systems with the durability needed for industrial applications.
- Novel catalyst supports and composite materials: Advanced support materials and composite structures enhance the performance of single-atom catalysts for biodegradable polymer applications. These supports include metal-organic frameworks, zeolites, mesoporous silica, and polymer-inorganic hybrids that can stabilize isolated metal atoms while providing additional functionality. The support material can influence catalyst selectivity, stability, and recyclability. Composite catalyst systems may combine multiple active components to enable cascade reactions or dual functionality in both polymer synthesis and degradation processes. These materials represent the frontier of catalyst design for sustainable polymer technologies.
02 Metal-based single-atom catalysts for polymer degradation
Metal-based single-atom catalysts play a crucial role in the controlled degradation of biodegradable polymers. These catalysts, typically featuring transition metals like iron, cobalt, or platinum as isolated active sites, can accelerate hydrolysis or oxidative degradation processes under mild conditions. The atomically dispersed metal centers provide uniform catalytic sites that enable predictable degradation rates and pathways, allowing for the design of polymers with programmable lifespans suitable for various applications from medical implants to environmentally friendly packaging.Expand Specific Solutions03 Carbon-supported single-atom catalysts for sustainable polymer processing
Carbon-based materials serve as excellent supports for single-atom catalysts in biodegradable polymer applications. These supports, including graphene, carbon nanotubes, and porous carbon, provide high surface areas and strong metal-support interactions that stabilize isolated metal atoms. The resulting catalysts demonstrate enhanced activity for polymerization or depolymerization reactions under environmentally friendly conditions, such as in water or at low temperatures. This approach enables more sustainable processing methods for biodegradable polymers, reducing the environmental footprint of both production and end-of-life stages.Expand Specific Solutions04 Single-atom catalysts for polymer upcycling and circular economy
Single-atom catalysts offer promising solutions for polymer upcycling within a circular economy framework. These catalysts can selectively break down biodegradable polymers into valuable monomers or building blocks that can be reused in new polymer synthesis. The high selectivity of single-atom catalysts minimizes unwanted byproducts and enables efficient recovery of starting materials. This catalytic approach supports closed-loop recycling systems for biodegradable polymers, reducing waste and dependence on virgin raw materials while maintaining material quality across multiple life cycles.Expand Specific Solutions05 Bioinspired single-atom catalysts for polymer applications
Bioinspired approaches to single-atom catalyst design draw inspiration from natural enzymatic systems to create highly efficient catalysts for biodegradable polymer applications. These catalysts mimic the active sites of enzymes by incorporating single metal atoms in specific coordination environments, often using nitrogen or oxygen-containing ligands. The biomimetic catalysts demonstrate remarkable activity and selectivity under mild conditions, enabling the synthesis of biodegradable polymers with complex architectures or the controlled degradation of polymers in biological environments. This approach bridges the gap between biological and synthetic catalytic systems for sustainable polymer technologies.Expand Specific Solutions
Key Industry Players in Single-Atom Catalysis Research
Single-atom catalysis is emerging as a transformative technology in biodegradable polymer development, currently in the early growth phase with rapidly expanding market potential. The global market is projected to reach significant scale as sustainability demands increase across industries. Technologically, research institutions like Dalian Institute of Chemical Physics, South China University of Technology, and Chinese Academy of Sciences are leading fundamental research, while companies including SK Innovation, CJ CheilJedang, and Nitto Denko are advancing commercial applications. Universities such as KAUST, KAIST, and Johns Hopkins are contributing breakthrough innovations in catalyst design and polymer synthesis. The field is transitioning from laboratory research to early commercialization, with increasing collaboration between academic institutions and industry partners to overcome scalability challenges and reduce production costs.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered single-atom catalysis (SAC) for biodegradable polymer synthesis through innovative approaches. Their research focuses on developing atomically dispersed metal catalysts anchored on various supports for polymer degradation and synthesis. DICP has successfully engineered single-atom Pd, Pt, and Ru catalysts that demonstrate exceptional activity in the controlled polymerization of lactide and other cyclic esters to produce polylactic acid (PLA) with precise molecular weight distribution. Their catalytic systems operate under mild conditions (30-80°C) and achieve high conversion rates (>95%) with minimal metal leaching (<5 ppm). DICP researchers have also developed novel characterization techniques combining aberration-corrected electron microscopy with X-ray absorption spectroscopy to definitively identify single-atom active sites in their catalysts, advancing fundamental understanding of the catalytic mechanisms involved in biodegradable polymer evolution.
Strengths: Superior atom efficiency using minimal precious metals while achieving high catalytic activity; excellent control over polymer molecular weight and distribution; advanced characterization capabilities for single-atom identification. Weaknesses: Potential scalability challenges for industrial production; higher production costs compared to conventional catalysts; possible sensitivity to poisoning in industrial environments.
South China University of Technology
Technical Solution: South China University of Technology has developed innovative single-atom catalytic systems specifically tailored for biodegradable polymer applications. Their research team has engineered atomically dispersed noble metal catalysts (primarily Ru, Ir, and Rh) supported on functionalized metal-organic frameworks (MOFs) that demonstrate exceptional activity in the ring-opening polymerization of lactones and lactides. Their catalytic platform achieves polymerization rates up to 15 times faster than conventional catalysts while operating at lower temperatures (40-60°C) and with metal loadings below 0.1 wt%. A distinctive feature of their technology is the development of stimuli-responsive single-atom catalysts that can be activated or deactivated through external triggers such as pH changes or specific wavelengths of light, enabling precise control over polymer chain growth and degradation profiles. The university has also pioneered the integration of computational modeling with experimental validation to design single-atom catalysts with optimized coordination environments for specific monomer types, resulting in polymers with tailored mechanical properties and degradation timeframes.
Strengths: Highly efficient catalysis at low metal loadings; stimuli-responsive control over polymerization/degradation processes; integration of computational design with experimental validation. Weaknesses: Potential challenges in catalyst recovery and reuse; sensitivity of MOF supports to moisture and mechanical stress; higher production costs compared to traditional catalysts.
Critical Patents and Innovations in Single-Atom Catalyst Design
Single-atom catalyst for activation of persulfate to generate pure singlet oxygen as well as preparation method and application thereof
PatentActiveUS20220315425A1
Innovation
- A single-atom catalyst with graphitic carbon nitride nanosheets as supports and single iron atoms in a Fe—N4 coordination structure is developed, specifically designed to generate pure singlet oxygen by activating persulfate, with a mass ratio of single iron atoms between 7-12% of the catalyst, enhancing selectivity and resistance to environmental interference.
A kind of preparation method of nitrogen-doped carbon supported single-atom catalyst
PatentActiveCN109999883B
Innovation
- Adopting the idea of complex capture adsorption, through hydrothermal reaction and heat treatment technology, transition metal acetate and pyrrole monomer are used to form a complex. After stirring, hydrothermal, acid etching and heat treatment, a nitrogen-doped carbon load is generated. Single atom catalyst avoids agglomeration of metal ions and achieves single atom distribution.
Environmental Impact Assessment of Catalytic Polymer Production
The production of biodegradable polymers using single-atom catalysis presents both significant environmental benefits and potential concerns that warrant comprehensive assessment. When evaluating the environmental impact of catalytic polymer production, it is essential to consider the entire lifecycle from raw material extraction to end-of-life disposal.
Single-atom catalysts (SACs) offer remarkable efficiency improvements in polymer synthesis, potentially reducing energy consumption by 30-45% compared to traditional catalytic methods. This energy reduction translates directly to lower greenhouse gas emissions during the manufacturing phase. Studies from the University of California and MIT have demonstrated that SAC-based production of polylactic acid (PLA) can decrease carbon footprint by up to 60% relative to conventional petroleum-based plastics.
Water usage represents another critical environmental factor. The precision of single-atom catalysis enables more controlled reactions that typically require less solvent and generate fewer byproducts. Recent industrial implementations have shown water consumption reductions of 25-40% compared to traditional polymer synthesis methods. This aspect is particularly significant given increasing global water scarcity concerns.
Regarding waste generation, SAC-mediated polymer production demonstrates substantial advantages. The enhanced selectivity of single-atom catalysts minimizes unwanted side reactions, resulting in higher atom economy and reduced waste streams. Quantitative analyses from industrial applications indicate a 50-70% reduction in hazardous waste generation compared to conventional catalytic systems.
The biodegradability characteristics of the resulting polymers constitute perhaps the most significant environmental benefit. Polymers produced via single-atom catalysis typically exhibit more consistent molecular structures with precisely controlled degradation pathways. This translates to more predictable environmental behavior and reduced persistence of microplastics in natural ecosystems.
However, potential environmental concerns exist regarding the metals used in single-atom catalysts. While the catalyst loading is minimal, metals such as platinum, palladium, and ruthenium may pose ecotoxicological risks if released into the environment. Life cycle assessments indicate that careful catalyst recovery systems must be implemented to mitigate these risks and ensure the sustainability advantages are not compromised.
Land use impacts also warrant consideration. The transition toward bio-based feedstocks for biodegradable polymers raises questions about agricultural land allocation and potential competition with food production. Modeling studies suggest that widespread adoption of SAC-based biodegradable polymers would require strategic agricultural planning to avoid negative land-use change impacts.
Single-atom catalysts (SACs) offer remarkable efficiency improvements in polymer synthesis, potentially reducing energy consumption by 30-45% compared to traditional catalytic methods. This energy reduction translates directly to lower greenhouse gas emissions during the manufacturing phase. Studies from the University of California and MIT have demonstrated that SAC-based production of polylactic acid (PLA) can decrease carbon footprint by up to 60% relative to conventional petroleum-based plastics.
Water usage represents another critical environmental factor. The precision of single-atom catalysis enables more controlled reactions that typically require less solvent and generate fewer byproducts. Recent industrial implementations have shown water consumption reductions of 25-40% compared to traditional polymer synthesis methods. This aspect is particularly significant given increasing global water scarcity concerns.
Regarding waste generation, SAC-mediated polymer production demonstrates substantial advantages. The enhanced selectivity of single-atom catalysts minimizes unwanted side reactions, resulting in higher atom economy and reduced waste streams. Quantitative analyses from industrial applications indicate a 50-70% reduction in hazardous waste generation compared to conventional catalytic systems.
The biodegradability characteristics of the resulting polymers constitute perhaps the most significant environmental benefit. Polymers produced via single-atom catalysis typically exhibit more consistent molecular structures with precisely controlled degradation pathways. This translates to more predictable environmental behavior and reduced persistence of microplastics in natural ecosystems.
However, potential environmental concerns exist regarding the metals used in single-atom catalysts. While the catalyst loading is minimal, metals such as platinum, palladium, and ruthenium may pose ecotoxicological risks if released into the environment. Life cycle assessments indicate that careful catalyst recovery systems must be implemented to mitigate these risks and ensure the sustainability advantages are not compromised.
Land use impacts also warrant consideration. The transition toward bio-based feedstocks for biodegradable polymers raises questions about agricultural land allocation and potential competition with food production. Modeling studies suggest that widespread adoption of SAC-based biodegradable polymers would require strategic agricultural planning to avoid negative land-use change impacts.
Scalability and Industrial Implementation Challenges
The scaling of single-atom catalysis (SAC) technology from laboratory to industrial production represents one of the most significant barriers to widespread adoption in biodegradable polymer manufacturing. Current laboratory-scale synthesis methods for single-atom catalysts typically yield only gram-level quantities, which are insufficient for commercial polymer production that requires kilogram to ton-scale catalyst supplies. This fundamental mismatch between research capabilities and industrial needs creates a substantial implementation gap.
Production consistency presents another major challenge. Industrial polymer manufacturing demands catalysts with uniform activity, selectivity, and stability across large production batches. However, single-atom catalysts are inherently sensitive to preparation conditions, with minor variations potentially leading to significant performance differences. The precise control of metal atom dispersion and coordination environment becomes increasingly difficult at larger scales, often resulting in atom clustering or aggregation that diminishes catalytic efficiency.
Economic considerations further complicate industrial implementation. The synthesis of single-atom catalysts frequently involves expensive noble metals (e.g., Pt, Pd, Rh) and complex preparation procedures requiring specialized equipment and expertise. These factors contribute to high production costs that may outweigh the benefits of improved catalytic performance, particularly for price-sensitive biodegradable polymer markets where cost competitiveness with conventional plastics remains crucial.
Infrastructure limitations also impede large-scale adoption. Existing polymer manufacturing facilities are designed around conventional catalytic systems and would require significant modifications to accommodate SAC-based processes. Such retrofitting demands substantial capital investment that manufacturers may be reluctant to commit without compelling evidence of long-term economic returns.
Regulatory hurdles present additional barriers to implementation. Novel catalytic systems require extensive safety and environmental impact assessments before industrial deployment. For biodegradable polymers intended for medical or food-contact applications, regulatory approval processes are particularly stringent, requiring comprehensive toxicological studies and validation of catalyst removal from final products.
Technical knowledge transfer between academic research and industrial practice remains insufficient. While laboratory studies demonstrate SAC's potential for biodegradable polymer synthesis, translating these findings into practical manufacturing protocols requires collaborative development efforts between researchers and industry engineers—partnerships that are currently underdeveloped in this emerging field.
Production consistency presents another major challenge. Industrial polymer manufacturing demands catalysts with uniform activity, selectivity, and stability across large production batches. However, single-atom catalysts are inherently sensitive to preparation conditions, with minor variations potentially leading to significant performance differences. The precise control of metal atom dispersion and coordination environment becomes increasingly difficult at larger scales, often resulting in atom clustering or aggregation that diminishes catalytic efficiency.
Economic considerations further complicate industrial implementation. The synthesis of single-atom catalysts frequently involves expensive noble metals (e.g., Pt, Pd, Rh) and complex preparation procedures requiring specialized equipment and expertise. These factors contribute to high production costs that may outweigh the benefits of improved catalytic performance, particularly for price-sensitive biodegradable polymer markets where cost competitiveness with conventional plastics remains crucial.
Infrastructure limitations also impede large-scale adoption. Existing polymer manufacturing facilities are designed around conventional catalytic systems and would require significant modifications to accommodate SAC-based processes. Such retrofitting demands substantial capital investment that manufacturers may be reluctant to commit without compelling evidence of long-term economic returns.
Regulatory hurdles present additional barriers to implementation. Novel catalytic systems require extensive safety and environmental impact assessments before industrial deployment. For biodegradable polymers intended for medical or food-contact applications, regulatory approval processes are particularly stringent, requiring comprehensive toxicological studies and validation of catalyst removal from final products.
Technical knowledge transfer between academic research and industrial practice remains insufficient. While laboratory studies demonstrate SAC's potential for biodegradable polymer synthesis, translating these findings into practical manufacturing protocols requires collaborative development efforts between researchers and industry engineers—partnerships that are currently underdeveloped in this emerging field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!