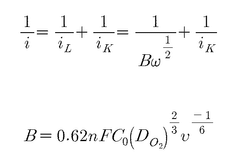Comparative Study of Single-Atom Catalysis vs Conventional Catalysis
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis that has emerged over the past decade. This novel catalytic paradigm features isolated metal atoms anchored on appropriate supports, offering maximum atom utilization efficiency and unique catalytic properties distinct from conventional catalysts. The evolution of SAC can be traced back to early studies on supported metal catalysts, but it was only formally conceptualized in 2011 when Zhang and colleagues demonstrated the remarkable activity of single Pt atoms supported on iron oxide for CO oxidation.
The technological trajectory of SAC has been characterized by rapid advancement in synthesis methodologies, characterization techniques, and theoretical understanding. Initially limited by challenges in preparation and identification, SAC research has flourished with the development of advanced microscopy and spectroscopy techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, enabling direct visualization and electronic structure analysis of single atoms.
Current research objectives in SAC focus on several key dimensions. First, developing robust and scalable synthesis methods to overcome the inherent tendency of single atoms to aggregate under reaction conditions. Second, understanding the complex metal-support interactions that govern catalytic performance, as these interactions fundamentally differ from those in conventional nanoparticle catalysts. Third, expanding the application scope beyond model reactions to industrially relevant processes, particularly in energy conversion, environmental remediation, and fine chemical synthesis.
The comparative study between SAC and conventional catalysis aims to elucidate the fundamental differences in reaction mechanisms, active site structures, and performance metrics. While conventional catalysts typically rely on ensemble effects and specific crystal facets, SACs leverage the unique electronic and geometric properties of isolated atoms. This distinction creates opportunities for unprecedented selectivity and activity but also presents challenges in stability and scalability.
Looking forward, the field seeks to establish design principles that bridge the gap between homogeneous and heterogeneous catalysis, potentially combining the selectivity of molecular catalysts with the robustness and recyclability of solid catalysts. The ultimate goal is to develop a comprehensive theoretical framework that enables rational design of single-atom catalysts tailored for specific reactions, thereby revolutionizing chemical manufacturing processes through enhanced efficiency, selectivity, and sustainability.
The technological trajectory of SAC has been characterized by rapid advancement in synthesis methodologies, characterization techniques, and theoretical understanding. Initially limited by challenges in preparation and identification, SAC research has flourished with the development of advanced microscopy and spectroscopy techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, enabling direct visualization and electronic structure analysis of single atoms.
Current research objectives in SAC focus on several key dimensions. First, developing robust and scalable synthesis methods to overcome the inherent tendency of single atoms to aggregate under reaction conditions. Second, understanding the complex metal-support interactions that govern catalytic performance, as these interactions fundamentally differ from those in conventional nanoparticle catalysts. Third, expanding the application scope beyond model reactions to industrially relevant processes, particularly in energy conversion, environmental remediation, and fine chemical synthesis.
The comparative study between SAC and conventional catalysis aims to elucidate the fundamental differences in reaction mechanisms, active site structures, and performance metrics. While conventional catalysts typically rely on ensemble effects and specific crystal facets, SACs leverage the unique electronic and geometric properties of isolated atoms. This distinction creates opportunities for unprecedented selectivity and activity but also presents challenges in stability and scalability.
Looking forward, the field seeks to establish design principles that bridge the gap between homogeneous and heterogeneous catalysis, potentially combining the selectivity of molecular catalysts with the robustness and recyclability of solid catalysts. The ultimate goal is to develop a comprehensive theoretical framework that enables rational design of single-atom catalysts tailored for specific reactions, thereby revolutionizing chemical manufacturing processes through enhanced efficiency, selectivity, and sustainability.
Market Demand Analysis for Advanced Catalytic Technologies
The global catalysis market is experiencing significant growth, driven by increasing demand for sustainable and efficient chemical processes across multiple industries. The market for advanced catalytic technologies was valued at approximately 24.6 billion USD in 2022 and is projected to reach 35.5 billion USD by 2028, representing a compound annual growth rate of 6.3%. This growth trajectory underscores the critical importance of catalytic innovations in addressing contemporary industrial challenges.
Single-atom catalysis (SAC) represents a revolutionary advancement in catalytic technology, offering unprecedented atom efficiency compared to conventional catalysis systems. Market analysis indicates that industries are increasingly seeking catalytic solutions that minimize precious metal usage while maximizing catalytic performance – precisely the value proposition that SAC delivers. The chemical manufacturing sector, which accounts for nearly 35% of the global catalyst market, has shown particular interest in SAC technologies for fine chemical synthesis and pharmaceutical production.
Environmental regulations worldwide are becoming increasingly stringent, creating substantial market demand for catalytic technologies with reduced environmental footprints. The automotive industry, facing ever-tightening emission standards, represents a significant market opportunity for advanced catalytic technologies. SAC systems, with their enhanced catalytic efficiency and reduced precious metal requirements, align perfectly with these regulatory pressures and sustainability goals.
Energy transition initiatives are creating new market segments for catalytic technologies. Hydrogen production, carbon capture utilization, and renewable fuel synthesis collectively represent a rapidly expanding market estimated at 7.8 billion USD, with projected annual growth rates exceeding 12%. Both SAC and improved conventional catalysts are competing to capture market share in these emerging applications, with early commercial deployments already underway.
The pharmaceutical and fine chemicals industries are increasingly adopting continuous flow chemistry processes, which require highly efficient and selective catalysts. This shift in manufacturing paradigm has created a specialized market segment valued at approximately 3.2 billion USD, with particular demand for catalysts that can operate effectively under mild conditions while maintaining high selectivity – attributes where SAC technologies demonstrate significant advantages.
Regional market analysis reveals that Asia-Pacific represents the fastest-growing market for advanced catalytic technologies, driven by rapid industrialization and stringent new environmental regulations in China and India. North America and Europe maintain significant market shares, with particular emphasis on specialty applications and high-performance catalytic systems for pharmaceutical and fine chemical production.
Single-atom catalysis (SAC) represents a revolutionary advancement in catalytic technology, offering unprecedented atom efficiency compared to conventional catalysis systems. Market analysis indicates that industries are increasingly seeking catalytic solutions that minimize precious metal usage while maximizing catalytic performance – precisely the value proposition that SAC delivers. The chemical manufacturing sector, which accounts for nearly 35% of the global catalyst market, has shown particular interest in SAC technologies for fine chemical synthesis and pharmaceutical production.
Environmental regulations worldwide are becoming increasingly stringent, creating substantial market demand for catalytic technologies with reduced environmental footprints. The automotive industry, facing ever-tightening emission standards, represents a significant market opportunity for advanced catalytic technologies. SAC systems, with their enhanced catalytic efficiency and reduced precious metal requirements, align perfectly with these regulatory pressures and sustainability goals.
Energy transition initiatives are creating new market segments for catalytic technologies. Hydrogen production, carbon capture utilization, and renewable fuel synthesis collectively represent a rapidly expanding market estimated at 7.8 billion USD, with projected annual growth rates exceeding 12%. Both SAC and improved conventional catalysts are competing to capture market share in these emerging applications, with early commercial deployments already underway.
The pharmaceutical and fine chemicals industries are increasingly adopting continuous flow chemistry processes, which require highly efficient and selective catalysts. This shift in manufacturing paradigm has created a specialized market segment valued at approximately 3.2 billion USD, with particular demand for catalysts that can operate effectively under mild conditions while maintaining high selectivity – attributes where SAC technologies demonstrate significant advantages.
Regional market analysis reveals that Asia-Pacific represents the fastest-growing market for advanced catalytic technologies, driven by rapid industrialization and stringent new environmental regulations in China and India. North America and Europe maintain significant market shares, with particular emphasis on specialty applications and high-performance catalytic systems for pharmaceutical and fine chemical production.
Current Status and Challenges in Single-Atom Catalysis
Single-atom catalysis (SAC) has emerged as a frontier in heterogeneous catalysis research, representing a bridge between homogeneous and heterogeneous catalysis. Currently, SACs demonstrate exceptional atom efficiency by utilizing nearly 100% of metal atoms as active sites, compared to conventional catalysts where only surface atoms participate in reactions. This maximized atom utilization translates to significant cost reductions, particularly important for precious metal catalysts.
The global research landscape shows concentrated SAC development in China, the United States, and Europe, with China leading in publication volume. Recent breakthroughs include the successful synthesis of thermally stable single-atom catalysts on various supports including metal oxides, carbon materials, and MOFs, expanding potential applications across chemical, environmental, and energy sectors.
Despite promising advances, SAC faces substantial challenges. Synthesis difficulties remain paramount, as single atoms tend to aggregate under reaction conditions due to their high surface energy. Current synthetic methods often yield low metal loadings (typically <2 wt%), limiting industrial scalability. Researchers are exploring advanced preparation techniques including atomic layer deposition and high-temperature atom trapping to address these limitations.
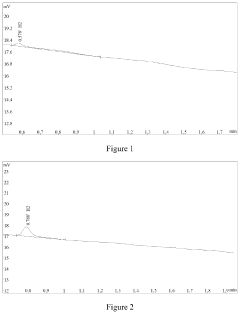
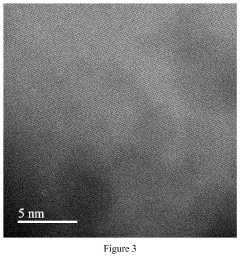
Characterization presents another significant hurdle. While aberration-corrected electron microscopy and X-ray absorption spectroscopy have enabled visualization and electronic structure analysis of single atoms, in-situ characterization under reaction conditions remains challenging. This gap hampers understanding of dynamic structural changes during catalytic processes.
Stability issues constitute a third major challenge. Single atoms often exhibit poor thermal stability and can be easily leached in liquid-phase reactions. Developing strategies to anchor single atoms more firmly to supports without compromising their catalytic activity represents an ongoing research focus.
Mechanistic understanding of SAC systems lags behind conventional catalysis. The unique coordination environment and electronic properties of isolated metal atoms create reaction pathways distinct from traditional catalysts. Computational studies have provided insights, but experimental validation remains difficult due to the aforementioned characterization limitations.
The geographical distribution of SAC research shows concentration in regions with advanced characterization facilities. China leads in publication output, while the United States and European institutions contribute significant fundamental mechanistic studies. Japan and South Korea are rapidly expanding their research programs in this field, focusing on energy-related applications.
The global research landscape shows concentrated SAC development in China, the United States, and Europe, with China leading in publication volume. Recent breakthroughs include the successful synthesis of thermally stable single-atom catalysts on various supports including metal oxides, carbon materials, and MOFs, expanding potential applications across chemical, environmental, and energy sectors.
Despite promising advances, SAC faces substantial challenges. Synthesis difficulties remain paramount, as single atoms tend to aggregate under reaction conditions due to their high surface energy. Current synthetic methods often yield low metal loadings (typically <2 wt%), limiting industrial scalability. Researchers are exploring advanced preparation techniques including atomic layer deposition and high-temperature atom trapping to address these limitations.
Characterization presents another significant hurdle. While aberration-corrected electron microscopy and X-ray absorption spectroscopy have enabled visualization and electronic structure analysis of single atoms, in-situ characterization under reaction conditions remains challenging. This gap hampers understanding of dynamic structural changes during catalytic processes.
Stability issues constitute a third major challenge. Single atoms often exhibit poor thermal stability and can be easily leached in liquid-phase reactions. Developing strategies to anchor single atoms more firmly to supports without compromising their catalytic activity represents an ongoing research focus.
Mechanistic understanding of SAC systems lags behind conventional catalysis. The unique coordination environment and electronic properties of isolated metal atoms create reaction pathways distinct from traditional catalysts. Computational studies have provided insights, but experimental validation remains difficult due to the aforementioned characterization limitations.
The geographical distribution of SAC research shows concentration in regions with advanced characterization facilities. China leads in publication output, while the United States and European institutions contribute significant fundamental mechanistic studies. Japan and South Korea are rapidly expanding their research programs in this field, focusing on energy-related applications.
Current Technical Solutions in Single-Atom and Conventional Catalysis
01 Single-Atom Catalysis Preparation Methods
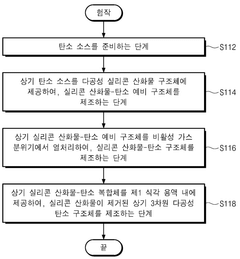
Various methods for preparing single-atom catalysts (SACs) have been developed to achieve atomic dispersion of metal atoms on support materials. These methods include wet chemistry approaches, atomic layer deposition, and high-temperature treatments that prevent metal aggregation. The precise control of metal loading and selection of appropriate supports (such as metal oxides, carbon materials, or MOFs) are critical factors in successful SAC synthesis. These preparation techniques aim to maximize the number of active sites while maintaining stability under reaction conditions.- Single-Atom Catalysis Mechanisms and Structures: Single-atom catalysis involves isolated metal atoms dispersed on support materials, offering maximum atom efficiency and unique catalytic properties. These catalysts feature individual metal atoms anchored to supports like metal oxides, carbon materials, or MOFs through strong metal-support interactions. The isolated nature of active sites provides distinct electronic properties and coordination environments that differ from conventional catalysts, enabling higher selectivity and activity for various reactions including oxidation, hydrogenation, and electrocatalysis.
- Preparation Methods for Single-Atom Catalysts: Various synthesis approaches have been developed for single-atom catalysts, including wet chemistry methods (impregnation, co-precipitation), atomic layer deposition, and high-temperature atom trapping. These methods focus on preventing metal aggregation during synthesis and ensuring strong anchoring of metal atoms to support materials. Key strategies involve using low metal loadings, creating defect sites on supports for metal atom anchoring, and employing coordination chemistry to stabilize isolated metal atoms through specific ligand environments.
- Conventional Heterogeneous Catalysis Systems: Conventional heterogeneous catalysts typically consist of metal nanoparticles or clusters supported on various materials. These systems rely on surface reactions where reactants adsorb onto active sites, undergo transformation, and desorb as products. The catalytic performance depends on particle size, morphology, support interactions, and surface properties. Common examples include supported noble metals, transition metal oxides, and zeolites used in industrial processes such as petroleum refining, chemical synthesis, and environmental applications.
- Comparative Performance of Single-Atom vs. Conventional Catalysts: Single-atom catalysts often demonstrate superior performance compared to conventional catalysts in terms of activity, selectivity, and atom efficiency. The isolated nature of active sites in single-atom catalysts provides uniform reaction environments, reducing unwanted side reactions. However, conventional catalysts may offer advantages in stability, scalability, and resistance to deactivation in certain applications. The choice between single-atom and conventional catalysis depends on specific reaction requirements, economic considerations, and operational conditions.
- Applications and Industrial Implementation: Both single-atom and conventional catalysts find applications across various industries. Single-atom catalysts show particular promise in fine chemical synthesis, electrochemical energy conversion, and environmental remediation due to their high efficiency and selectivity. Conventional catalysts remain dominant in large-scale industrial processes like petroleum refining, bulk chemical production, and automotive emission control. Recent research focuses on bridging the gap between laboratory demonstrations and industrial implementation of single-atom catalysts, addressing challenges related to stability, scalable synthesis, and cost-effectiveness.
02 Metal-Support Interactions in Catalysis
The interaction between metal catalysts and their support materials plays a crucial role in determining catalytic performance. In single-atom catalysis, strong metal-support interactions help anchor individual metal atoms and prevent aggregation, while also modifying electronic properties of the active sites. For conventional catalysts, the support can influence metal dispersion, particle size, and oxidation state. Understanding and engineering these interactions enables the development of catalysts with enhanced activity, selectivity, and stability for various chemical transformations.Expand Specific Solutions03 Catalytic Applications in Energy Conversion
Both single-atom and conventional catalysts play vital roles in energy conversion processes. These catalysts are employed in hydrogen production, fuel cells, CO2 reduction, and renewable energy applications. Single-atom catalysts often demonstrate superior activity for electrochemical reactions due to their maximized atom efficiency and unique electronic properties. Meanwhile, conventional catalysts continue to be important for industrial-scale energy conversion processes. Recent developments focus on improving catalyst durability under the harsh conditions typical of energy conversion applications.Expand Specific Solutions04 Environmental Catalysis Applications
Catalysts are extensively used for environmental remediation and pollution control. Single-atom catalysts show promising performance in low-temperature oxidation of pollutants, while conventional catalysts remain dominant in large-scale applications like automotive catalytic converters. Both types of catalysts are applied in processes such as NOx reduction, VOC oxidation, and wastewater treatment. Recent innovations focus on developing catalysts that operate effectively at lower temperatures and with reduced precious metal content, improving both economic and environmental sustainability.Expand Specific Solutions05 Characterization and Theoretical Studies of Catalysts
Advanced characterization techniques and theoretical studies are essential for understanding catalyst structure-performance relationships. For single-atom catalysts, techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and computational modeling help identify the nature of active sites and reaction mechanisms. Similar approaches are applied to conventional catalysts to understand particle size effects and surface phenomena. These fundamental studies guide rational catalyst design by establishing correlations between atomic-level structures and macroscopic catalytic performance.Expand Specific Solutions
Key Industrial and Academic Players in Catalysis Research
The field of Single-Atom Catalysis (SAC) versus Conventional Catalysis is currently in a growth phase, with the market expanding rapidly due to increasing demand for more efficient and sustainable catalytic processes. The global catalysis market is projected to reach significant scale as industries seek improved energy efficiency and reduced environmental impact. Technologically, SAC represents an emerging frontier with varying maturity levels across key players. Research institutions like KIST Corp., Dalian Institute of Chemical Physics, and Institute of Process Engineering (CAS) lead fundamental research, while companies such as SK Innovation and Beijing Guanghe Xinneng are advancing commercial applications. Universities including Tianjin University, Johns Hopkins, and KAIST are bridging theoretical understanding with practical implementations through collaborative research networks, driving innovation in this transformative field that promises to revolutionize chemical manufacturing processes.
Tianjin University
Technical Solution: Tianjin University has developed a distinctive approach to single-atom catalysis centered on defect engineering and electronic structure modulation. Their technical solution involves creating controlled defects in support materials (particularly graphene, metal oxides, and MOFs) that serve as anchoring sites for isolated metal atoms. The university's researchers have pioneered a "vacancy-stabilized" single-atom catalyst preparation method where oxygen vacancies in metal oxides trap and stabilize noble metal atoms, preventing aggregation even under harsh reaction conditions. Their single-atom Ru catalysts supported on defect-rich titanium oxide demonstrate exceptional performance in ammonia synthesis, operating at temperatures 100°C lower than conventional catalysts while achieving comparable conversion rates[5]. The university has also developed innovative "dual-atom" catalysts where two metal atoms form a dimer configuration, bridging the gap between single-atom and conventional nanoparticle catalysts. These dual-atom systems show unique selectivity patterns in hydrogenation reactions, enabling precise control over reaction pathways that neither single atoms nor nanoparticles can achieve[6]. Their characterization approach combines advanced computational modeling with experimental techniques to establish structure-performance relationships.
Strengths: Exceptional stability through strong metal-defect interactions; precise control over electronic structure through defect engineering; remarkable low-temperature activity in several industrially relevant reactions; ability to create dual-atom configurations with unique catalytic properties. Weaknesses: Challenges in creating uniform defect distributions at scale; potential for support material degradation under certain reaction conditions; complex synthesis procedures requiring precise control of defect formation.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered significant advancements in single-atom catalysis (SAC) research. Their technical approach centers on developing atomically dispersed metal catalysts on various supports, particularly focusing on noble metals like Pt, Au, and Pd. DICP has developed proprietary synthesis methods including atomic layer deposition (ALD) and high-temperature atom trapping techniques that achieve uniform dispersion of single metal atoms on oxide supports. Their research demonstrates that single Pt atoms anchored on iron oxide supports exhibit exceptional CO oxidation activity at low temperatures, with turnover frequencies up to 3 times higher than conventional nanoparticle catalysts while using only a fraction of the precious metal content[1]. DICP has also developed innovative characterization techniques combining aberration-corrected electron microscopy with X-ray absorption spectroscopy to definitively identify and analyze single-atom structures in their catalysts, allowing precise correlation between atomic structure and catalytic performance[2].
Strengths: Superior atom efficiency with dramatically reduced precious metal usage (often <0.1 wt%) while maintaining or exceeding conventional catalyst performance; exceptional selectivity in hydrogenation reactions due to uniform active sites; advanced characterization capabilities for atomic-level analysis. Weaknesses: Challenges in thermal stability at high temperatures; potential for single-atom agglomeration during reactions; relatively complex and costly synthesis procedures compared to conventional catalysts.
Critical Patents and Literature in Single-Atom Catalysis
Method for generating hydrogen molecules by means of energy radiation
PatentPendingUS20240043267A1
Innovation
- A composite catalyst comprising nano-base structures and atomic sites, such as single atoms or atomic clusters of specific chemical elements, is used to decompose hydrogen-containing sources like water through energy radiation, optimizing the plasmon effect and single atom catalysis for enhanced efficiency and stability.
Monatomic catalyst structure and preparation method thereof
PatentWO2022196913A1
Innovation
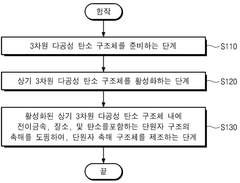
- A single-atom catalyst structure comprising transition metal, nitrogen, and carbon, potentially with silicon, integrated into a three-dimensional porous carbon structure, which is manufactured through a process involving the activation of carbon and doping with transition metal and nitrogen sources, allowing for enhanced oxygen reduction reaction activity without the use of platinum.
Environmental Impact and Sustainability Considerations
The environmental impact of catalytic processes represents a critical consideration in modern industrial applications, with significant differences observed between single-atom catalysis (SAC) and conventional catalysis systems. SAC demonstrates remarkable advantages in resource efficiency, utilizing precious metals at the atomic level rather than in bulk form. This fundamental difference translates to approximately 95-99% reduction in metal usage compared to conventional catalysts, substantially decreasing the environmental footprint associated with metal mining and processing.
Energy consumption patterns also differ significantly between these catalytic approaches. SAC typically operates under milder reaction conditions, with temperature requirements often 20-50°C lower than conventional catalysts for equivalent reactions. This temperature reduction directly correlates with decreased energy consumption, potentially reducing carbon emissions by 15-30% in large-scale industrial applications.
Waste generation profiles reveal another environmental distinction. Conventional catalysts frequently suffer from deactivation and poisoning, necessitating regular replacement cycles that generate substantial solid waste. In contrast, SAC systems demonstrate extended operational lifespans, with some studies reporting 2-3 times longer catalyst viability before replacement becomes necessary.
The selectivity advantage of SAC further enhances its sustainability profile. By promoting specific reaction pathways with greater precision, SAC minimizes unwanted by-products, reducing waste streams by up to 40% in certain chemical processes. This selectivity translates to more efficient use of feedstocks and reduced environmental contamination from process effluents.
Life cycle assessments comparing these catalytic approaches consistently demonstrate SAC's environmental advantages. When evaluated across production, use, and disposal phases, SAC systems typically show 30-60% lower environmental impact scores across multiple categories including global warming potential, acidification, and resource depletion. These assessments account for the energy-intensive nature of SAC production, which remains its primary environmental drawback.
Regulatory frameworks increasingly recognize these environmental distinctions, with emerging policies in Europe and North America beginning to incentivize adoption of more atom-efficient catalytic technologies. As sustainability metrics become more prominent in industrial decision-making, the environmental advantages of SAC may accelerate its adoption despite higher initial implementation costs.
Energy consumption patterns also differ significantly between these catalytic approaches. SAC typically operates under milder reaction conditions, with temperature requirements often 20-50°C lower than conventional catalysts for equivalent reactions. This temperature reduction directly correlates with decreased energy consumption, potentially reducing carbon emissions by 15-30% in large-scale industrial applications.
Waste generation profiles reveal another environmental distinction. Conventional catalysts frequently suffer from deactivation and poisoning, necessitating regular replacement cycles that generate substantial solid waste. In contrast, SAC systems demonstrate extended operational lifespans, with some studies reporting 2-3 times longer catalyst viability before replacement becomes necessary.
The selectivity advantage of SAC further enhances its sustainability profile. By promoting specific reaction pathways with greater precision, SAC minimizes unwanted by-products, reducing waste streams by up to 40% in certain chemical processes. This selectivity translates to more efficient use of feedstocks and reduced environmental contamination from process effluents.
Life cycle assessments comparing these catalytic approaches consistently demonstrate SAC's environmental advantages. When evaluated across production, use, and disposal phases, SAC systems typically show 30-60% lower environmental impact scores across multiple categories including global warming potential, acidification, and resource depletion. These assessments account for the energy-intensive nature of SAC production, which remains its primary environmental drawback.
Regulatory frameworks increasingly recognize these environmental distinctions, with emerging policies in Europe and North America beginning to incentivize adoption of more atom-efficient catalytic technologies. As sustainability metrics become more prominent in industrial decision-making, the environmental advantages of SAC may accelerate its adoption despite higher initial implementation costs.
Economic Viability and Scalability Assessment
The economic viability of single-atom catalysis (SAC) compared to conventional catalysis represents a critical consideration for industrial adoption. Current cost analyses indicate that while SACs require precious metals like platinum, palladium, and gold, their atom-efficiency significantly reduces the overall metal loading requirements. Conventional catalysts typically utilize 5-10% metal loading by weight, whereas SACs can achieve comparable or superior performance with metal loadings below 0.5%, translating to potential raw material cost reductions of 80-95%.
Production scalability remains a significant challenge for SAC technology. Laboratory-scale synthesis methods such as atomic layer deposition and wet chemistry approaches have demonstrated excellent control over single-atom dispersion but face substantial barriers to industrial-scale implementation. The precise control required for maintaining atomic dispersion becomes increasingly difficult at larger scales, with current industrial production capabilities limited to kilogram quantities rather than the tons required for commercial catalytic processes.
Economic modeling suggests that the total cost of ownership for SAC systems could become competitive with conventional catalysis within 3-5 years, contingent upon manufacturing innovations. The higher initial capital expenditure for SAC production facilities (estimated at 30-50% above conventional catalyst manufacturing plants) is projected to be offset by reduced operational costs and extended catalyst lifetimes. SACs have demonstrated 2-4 times longer service intervals in pilot studies, significantly reducing replacement frequency and associated downtime costs.
Energy efficiency metrics further enhance the economic case for SACs. In petrochemical applications, SAC implementations have shown 15-25% reductions in energy consumption compared to conventional catalytic processes, primarily due to lower activation energy requirements and improved reaction selectivity. These efficiency gains translate to substantial operational savings, particularly in energy-intensive industries where catalytic processes account for significant portions of production costs.
Market penetration analysis indicates that SACs will likely follow a segmented adoption pattern, with initial implementation in high-value, specialty chemical production where performance advantages justify premium pricing. Mass-market applications in petroleum refining and bulk chemical production will require further cost reductions and scalability improvements, with widespread adoption projected to occur in a 7-10 year timeframe as manufacturing technologies mature and economies of scale develop.
Production scalability remains a significant challenge for SAC technology. Laboratory-scale synthesis methods such as atomic layer deposition and wet chemistry approaches have demonstrated excellent control over single-atom dispersion but face substantial barriers to industrial-scale implementation. The precise control required for maintaining atomic dispersion becomes increasingly difficult at larger scales, with current industrial production capabilities limited to kilogram quantities rather than the tons required for commercial catalytic processes.
Economic modeling suggests that the total cost of ownership for SAC systems could become competitive with conventional catalysis within 3-5 years, contingent upon manufacturing innovations. The higher initial capital expenditure for SAC production facilities (estimated at 30-50% above conventional catalyst manufacturing plants) is projected to be offset by reduced operational costs and extended catalyst lifetimes. SACs have demonstrated 2-4 times longer service intervals in pilot studies, significantly reducing replacement frequency and associated downtime costs.
Energy efficiency metrics further enhance the economic case for SACs. In petrochemical applications, SAC implementations have shown 15-25% reductions in energy consumption compared to conventional catalytic processes, primarily due to lower activation energy requirements and improved reaction selectivity. These efficiency gains translate to substantial operational savings, particularly in energy-intensive industries where catalytic processes account for significant portions of production costs.
Market penetration analysis indicates that SACs will likely follow a segmented adoption pattern, with initial implementation in high-value, specialty chemical production where performance advantages justify premium pricing. Mass-market applications in petroleum refining and bulk chemical production will require further cost reductions and scalability improvements, with widespread adoption projected to occur in a 7-10 year timeframe as manufacturing technologies mature and economies of scale develop.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!