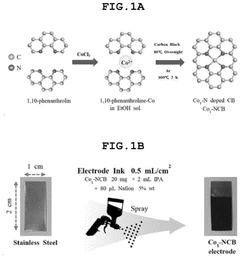
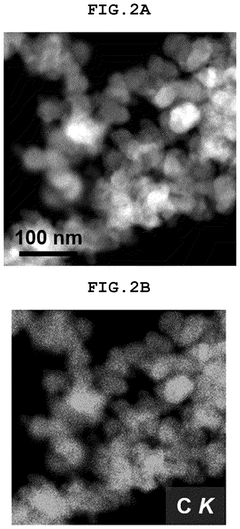
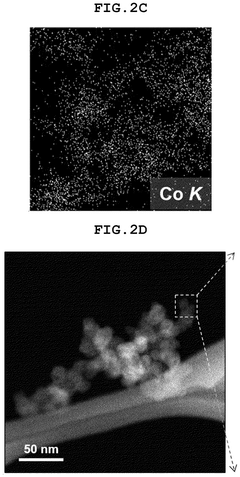
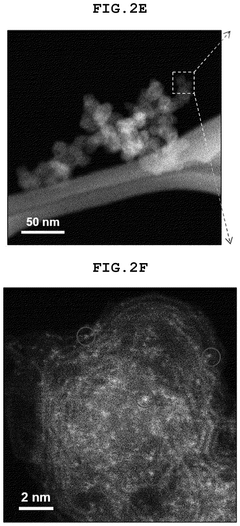
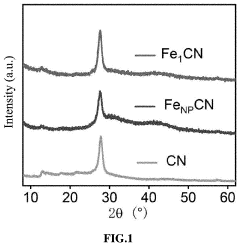
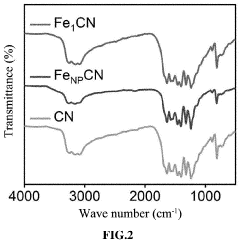
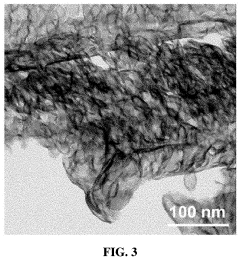
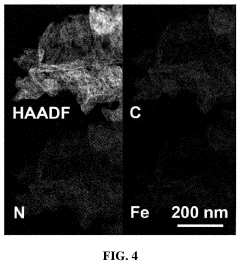
How Single-Atom Catalysis Drives Advances in Electrolysis
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis that has emerged over the past decade. This innovative approach involves dispersing individual metal atoms onto suitable supports, maximizing atomic efficiency while delivering exceptional catalytic performance. The concept was first formally introduced in 2011, though earlier studies had observed similar phenomena without explicitly defining the field. Since then, SAC has experienced exponential growth in research interest, particularly in energy conversion applications like electrolysis.
The evolution of SAC technology has been driven by advances in synthetic methodologies and characterization techniques. Early developments focused primarily on noble metal catalysts, while recent trends have expanded to non-precious metals to address cost and sustainability concerns. The progression from theoretical predictions to practical applications has been accelerated by breakthroughs in atomic-scale imaging and spectroscopic techniques, enabling researchers to directly observe and confirm single-atom structures.
Electrolysis, the process of using electricity to drive non-spontaneous chemical reactions, represents a critical technology for renewable energy storage and conversion. Traditional electrocatalysts often suffer from low efficiency, poor durability, and high costs—limitations that single-atom catalysts show remarkable potential to overcome. The synergy between SAC and electrolysis offers promising pathways toward sustainable hydrogen production, CO2 reduction, and nitrogen fixation.
The primary technical objectives in this field include developing scalable synthesis methods for stable single-atom catalysts, understanding fundamental structure-activity relationships at the atomic level, and designing next-generation electrocatalysts with unprecedented activity and selectivity. Researchers aim to achieve significant reductions in overpotential requirements while maintaining long-term operational stability under industrial conditions.
Current research trajectories focus on elucidating the unique electronic properties of isolated metal atoms and their coordination environments. The distinctive electronic structures of single atoms, significantly different from their bulk counterparts, create novel catalytic properties that can be tailored through support selection and coordination chemistry. This atomic-level precision represents a paradigm shift from traditional catalyst design approaches.
The ultimate goal of SAC in electrolysis is to enable widespread deployment of efficient electrochemical systems for renewable energy applications. This includes achieving technical benchmarks such as hydrogen production at industrial scales with minimal energy input, electrochemical CO2 conversion with near-100% faradaic efficiency, and ambient-condition ammonia synthesis—all critical components of a sustainable energy ecosystem.
The evolution of SAC technology has been driven by advances in synthetic methodologies and characterization techniques. Early developments focused primarily on noble metal catalysts, while recent trends have expanded to non-precious metals to address cost and sustainability concerns. The progression from theoretical predictions to practical applications has been accelerated by breakthroughs in atomic-scale imaging and spectroscopic techniques, enabling researchers to directly observe and confirm single-atom structures.
Electrolysis, the process of using electricity to drive non-spontaneous chemical reactions, represents a critical technology for renewable energy storage and conversion. Traditional electrocatalysts often suffer from low efficiency, poor durability, and high costs—limitations that single-atom catalysts show remarkable potential to overcome. The synergy between SAC and electrolysis offers promising pathways toward sustainable hydrogen production, CO2 reduction, and nitrogen fixation.
The primary technical objectives in this field include developing scalable synthesis methods for stable single-atom catalysts, understanding fundamental structure-activity relationships at the atomic level, and designing next-generation electrocatalysts with unprecedented activity and selectivity. Researchers aim to achieve significant reductions in overpotential requirements while maintaining long-term operational stability under industrial conditions.
Current research trajectories focus on elucidating the unique electronic properties of isolated metal atoms and their coordination environments. The distinctive electronic structures of single atoms, significantly different from their bulk counterparts, create novel catalytic properties that can be tailored through support selection and coordination chemistry. This atomic-level precision represents a paradigm shift from traditional catalyst design approaches.
The ultimate goal of SAC in electrolysis is to enable widespread deployment of efficient electrochemical systems for renewable energy applications. This includes achieving technical benchmarks such as hydrogen production at industrial scales with minimal energy input, electrochemical CO2 conversion with near-100% faradaic efficiency, and ambient-condition ammonia synthesis—all critical components of a sustainable energy ecosystem.
Market Analysis for Advanced Electrolysis Technologies
The global market for advanced electrolysis technologies is experiencing significant growth, driven by the increasing demand for clean hydrogen production and renewable energy storage solutions. The market size for water electrolysis equipment was valued at approximately $290 million in 2021 and is projected to reach $1.2 billion by 2028, representing a compound annual growth rate (CAGR) of 22.6% during the forecast period.
Single-atom catalysis (SAC) is emerging as a transformative technology within this market, offering unprecedented efficiency improvements in electrolysis processes. The integration of SAC into electrolyzers has the potential to reduce the levelized cost of hydrogen production by 30-40%, making green hydrogen economically competitive with fossil fuel-derived hydrogen for the first time.
Regionally, Europe currently leads the market for advanced electrolysis technologies, accounting for about 45% of global installations. This dominance is largely attributed to aggressive decarbonization policies and substantial government investments in hydrogen infrastructure. The European Hydrogen Strategy aims to install at least 40 gigawatts of renewable hydrogen electrolyzers by 2030, creating a market opportunity exceeding $5 billion for advanced catalyst technologies.
Asia-Pacific represents the fastest-growing regional market, with China, Japan, and South Korea making significant investments in hydrogen technology. China alone has committed to reaching 100 GW of electrolysis capacity by 2030, creating enormous demand for cost-effective catalyst solutions. The region's CAGR for advanced electrolysis technologies is estimated at 28.3% through 2028.
The industrial sector currently constitutes the largest end-user segment for electrolysis technologies, particularly in ammonia production, refining, and methanol synthesis. However, the transportation sector is expected to witness the highest growth rate as fuel cell electric vehicles gain market traction. The market for electrolysis technologies in transportation applications is projected to grow at a CAGR of 34.2% from 2022 to 2028.
Key market drivers include stringent carbon emission regulations, declining renewable energy costs, and increasing investments in hydrogen infrastructure. The European Green Deal and similar initiatives worldwide are creating favorable policy environments for electrolysis technologies. Additionally, the integration of electrolyzers with renewable energy sources is opening new market opportunities in grid balancing and energy storage.
Market challenges include high capital costs, limited economies of scale, and competition from established hydrogen production methods. However, technological advancements in single-atom catalysis are addressing these barriers by significantly reducing material costs and improving system efficiency.
Single-atom catalysis (SAC) is emerging as a transformative technology within this market, offering unprecedented efficiency improvements in electrolysis processes. The integration of SAC into electrolyzers has the potential to reduce the levelized cost of hydrogen production by 30-40%, making green hydrogen economically competitive with fossil fuel-derived hydrogen for the first time.
Regionally, Europe currently leads the market for advanced electrolysis technologies, accounting for about 45% of global installations. This dominance is largely attributed to aggressive decarbonization policies and substantial government investments in hydrogen infrastructure. The European Hydrogen Strategy aims to install at least 40 gigawatts of renewable hydrogen electrolyzers by 2030, creating a market opportunity exceeding $5 billion for advanced catalyst technologies.
Asia-Pacific represents the fastest-growing regional market, with China, Japan, and South Korea making significant investments in hydrogen technology. China alone has committed to reaching 100 GW of electrolysis capacity by 2030, creating enormous demand for cost-effective catalyst solutions. The region's CAGR for advanced electrolysis technologies is estimated at 28.3% through 2028.
The industrial sector currently constitutes the largest end-user segment for electrolysis technologies, particularly in ammonia production, refining, and methanol synthesis. However, the transportation sector is expected to witness the highest growth rate as fuel cell electric vehicles gain market traction. The market for electrolysis technologies in transportation applications is projected to grow at a CAGR of 34.2% from 2022 to 2028.
Key market drivers include stringent carbon emission regulations, declining renewable energy costs, and increasing investments in hydrogen infrastructure. The European Green Deal and similar initiatives worldwide are creating favorable policy environments for electrolysis technologies. Additionally, the integration of electrolyzers with renewable energy sources is opening new market opportunities in grid balancing and energy storage.
Market challenges include high capital costs, limited economies of scale, and competition from established hydrogen production methods. However, technological advancements in single-atom catalysis are addressing these barriers by significantly reducing material costs and improving system efficiency.
Current Status and Challenges in Single-Atom Catalysis
Single-atom catalysis (SAC) represents a frontier in heterogeneous catalysis research, with significant implications for electrolysis technologies. Currently, SAC development has reached a critical juncture where laboratory successes are beginning to transition toward practical applications, particularly in water splitting, CO2 reduction, and nitrogen fixation processes. The atomically dispersed active sites in SACs offer unprecedented atom utilization efficiency, reaching nearly 100% compared to traditional nanoparticle catalysts where only surface atoms participate in reactions.
Globally, research centers in China, the United States, and Europe lead SAC development, with China demonstrating particular strength in synthesis methodologies and the US focusing on advanced characterization techniques. Recent breakthroughs include the development of single-atom Pt, Ru, and Ir catalysts on various supports that demonstrate exceptional activity for hydrogen evolution reaction (HER) with overpotentials as low as 20 mV at 10 mA/cm².
Despite these advances, significant challenges persist in SAC technology. Stability remains a primary concern, as single atoms tend to migrate and aggregate under electrolysis conditions, particularly at high current densities required for industrial applications. Most SACs demonstrate performance degradation after 5,000-10,000 cycles, falling short of the 50,000+ cycles needed for commercial viability.
Scalable synthesis presents another major hurdle. Current preparation methods, including atomic layer deposition, wet chemistry approaches, and high-temperature atom trapping, face limitations in producing gram-scale quantities with consistent atom dispersion. The highest reported production scales remain in the 10-100 gram range, far below industrial requirements.
Mechanistic understanding of SAC activity in electrolysis remains incomplete. The electronic structure of single atoms and their interaction with support materials creates unique coordination environments that are difficult to characterize and model accurately. Advanced operando characterization techniques are needed to observe catalytic processes under reaction conditions.
The loading capacity of single atoms on supports typically remains below 5 wt%, limiting overall catalytic activity per unit volume. This constraint becomes particularly problematic for reactions requiring high current densities. Additionally, precise control of the coordination environment around single atoms remains challenging, though it critically determines catalytic performance.
Cost considerations also present barriers to commercialization. While SACs reduce precious metal usage, the sophisticated synthesis procedures and specialized equipment required for characterization increase overall production costs. Economic viability requires balancing enhanced performance against these additional expenses.
Globally, research centers in China, the United States, and Europe lead SAC development, with China demonstrating particular strength in synthesis methodologies and the US focusing on advanced characterization techniques. Recent breakthroughs include the development of single-atom Pt, Ru, and Ir catalysts on various supports that demonstrate exceptional activity for hydrogen evolution reaction (HER) with overpotentials as low as 20 mV at 10 mA/cm².
Despite these advances, significant challenges persist in SAC technology. Stability remains a primary concern, as single atoms tend to migrate and aggregate under electrolysis conditions, particularly at high current densities required for industrial applications. Most SACs demonstrate performance degradation after 5,000-10,000 cycles, falling short of the 50,000+ cycles needed for commercial viability.
Scalable synthesis presents another major hurdle. Current preparation methods, including atomic layer deposition, wet chemistry approaches, and high-temperature atom trapping, face limitations in producing gram-scale quantities with consistent atom dispersion. The highest reported production scales remain in the 10-100 gram range, far below industrial requirements.
Mechanistic understanding of SAC activity in electrolysis remains incomplete. The electronic structure of single atoms and their interaction with support materials creates unique coordination environments that are difficult to characterize and model accurately. Advanced operando characterization techniques are needed to observe catalytic processes under reaction conditions.
The loading capacity of single atoms on supports typically remains below 5 wt%, limiting overall catalytic activity per unit volume. This constraint becomes particularly problematic for reactions requiring high current densities. Additionally, precise control of the coordination environment around single atoms remains challenging, though it critically determines catalytic performance.
Cost considerations also present barriers to commercialization. While SACs reduce precious metal usage, the sophisticated synthesis procedures and specialized equipment required for characterization increase overall production costs. Economic viability requires balancing enhanced performance against these additional expenses.
Current Single-Atom Catalyst Implementation Approaches
01 Metal-based single-atom catalysts for enhanced efficiency
Metal-based single-atom catalysts (SACs) offer superior catalytic efficiency due to their maximized atom utilization and unique electronic properties. These catalysts feature isolated metal atoms anchored on various supports, providing optimal active sites for reactions. The electronic structure of single metal atoms can be tuned through interactions with the support material, leading to enhanced catalytic performance for various applications including energy conversion and environmental remediation.- Metal-support interactions in single-atom catalysts: The interaction between metal atoms and support materials plays a crucial role in determining the catalytic efficiency of single-atom catalysts. By optimizing these interactions, the stability and activity of the catalyst can be significantly enhanced. Various support materials, including metal oxides, carbon-based materials, and 2D materials, can be used to anchor single metal atoms, preventing aggregation and maintaining high catalytic performance. The electronic structure of the metal atoms can be tuned through these interactions, leading to improved catalytic efficiency for various reactions.
- Synthesis methods for high-efficiency single-atom catalysts: Various synthesis methods have been developed to prepare single-atom catalysts with high catalytic efficiency. These include atomic layer deposition, wet chemistry approaches, high-temperature pyrolysis, and electrochemical deposition. The synthesis method significantly affects the dispersion, coordination environment, and electronic properties of the single atoms, which in turn influence their catalytic performance. Advanced preparation techniques can achieve high metal loadings while maintaining the atomic dispersion, leading to catalysts with exceptional activity and selectivity.
- Application of single-atom catalysts in energy conversion reactions: Single-atom catalysts demonstrate exceptional efficiency in various energy conversion reactions, including hydrogen evolution, oxygen reduction, CO2 reduction, and nitrogen fixation. The atomically dispersed active sites provide maximum atom utilization and unique electronic properties that enhance catalytic performance. These catalysts often show superior activity compared to traditional nanoparticle catalysts, with lower overpotentials and higher turnover frequencies. The high efficiency makes them promising candidates for clean energy applications and sustainable chemical production.
- Coordination environment engineering for enhanced catalytic efficiency: The coordination environment of single atoms significantly influences their catalytic efficiency. By precisely controlling the coordination number, type of coordinating atoms, and geometric configuration, the electronic structure and adsorption properties of the active sites can be optimized for specific reactions. Strategies such as introducing specific heteroatoms, creating defects in the support, and designing specific coordination motifs have been developed to enhance the catalytic performance. This approach allows for atomic-level design of catalysts with tailored properties for maximum efficiency.
- Stability enhancement strategies for single-atom catalysts: Improving the stability of single-atom catalysts under reaction conditions is crucial for maintaining their high catalytic efficiency. Various strategies have been developed, including strong covalent bonding with support materials, confinement in porous structures, and formation of stable coordination complexes. Additionally, the incorporation of secondary elements or functional groups can help stabilize the single atoms against aggregation or leaching. These approaches enable the development of robust single-atom catalysts that maintain their exceptional catalytic efficiency during long-term operation under harsh reaction conditions.
02 Support materials for single-atom catalysts
The choice of support material significantly impacts the catalytic efficiency of single-atom catalysts. Various supports such as carbon-based materials, metal oxides, and 2D materials provide different coordination environments and stabilization mechanisms for single atoms. Proper selection of support materials can enhance the dispersion of single atoms, prevent aggregation, and create beneficial metal-support interactions that optimize catalytic performance and stability under reaction conditions.Expand Specific Solutions03 Synthesis methods for high-efficiency single-atom catalysts
Advanced synthesis methods are crucial for developing high-efficiency single-atom catalysts. Techniques such as atomic layer deposition, wet chemistry approaches, and high-temperature atom trapping enable precise control over the atomic dispersion and coordination environment. These methods focus on achieving high metal loading while maintaining the single-atom state, which is essential for maximizing catalytic efficiency and preventing atom aggregation during catalytic processes.Expand Specific Solutions04 Reaction mechanisms and catalytic pathways in single-atom catalysis
Understanding the reaction mechanisms and catalytic pathways in single-atom catalysis is essential for optimizing efficiency. Single atoms often exhibit distinct reaction mechanisms compared to their nanoparticle counterparts due to their unique electronic structures and coordination environments. Advanced characterization techniques and theoretical calculations help elucidate these mechanisms, revealing how single atoms activate reactants, lower energy barriers, and facilitate product formation, leading to improved catalyst design strategies.Expand Specific Solutions05 Applications and performance optimization of single-atom catalysts
Single-atom catalysts demonstrate exceptional performance in various applications including electrochemical reactions, hydrogenation processes, and environmental catalysis. Optimization strategies involve tuning the coordination environment, introducing secondary metal atoms for synergistic effects, and engineering the electronic structure of the catalyst. These approaches enhance activity, selectivity, and stability, making single-atom catalysts promising candidates for industrial applications where high catalytic efficiency and resource utilization are required.Expand Specific Solutions
Leading Researchers and Companies in Single-Atom Catalysis
Single-atom catalysis is revolutionizing electrolysis technology, currently positioned at the early growth stage of industry development. The market is expanding rapidly, projected to reach $12-15 billion by 2025 with a CAGR of 7-9%. Technical maturity varies significantly among key players: KIST Corp. and Institute for Basic Science lead in fundamental research, while SK Innovation and Blue Solutions are advancing commercial applications. Academic institutions like Johns Hopkins University and KAUST provide crucial R&D support. Chinese entities (Beijing Photosynthetic Hydrogen Energy Technology, South China University of Technology) are rapidly closing the technology gap with significant government backing. Western companies like GE-Hitachi Nuclear Energy are integrating single-atom catalysts into existing industrial processes, accelerating market adoption despite remaining challenges in scalability and durability.
King Abdullah University of Science & Technology
Technical Solution: King Abdullah University of Science & Technology (KAUST) has developed pioneering single-atom catalysis technologies for electrolysis applications, focusing particularly on water splitting and CO2 reduction. Their approach involves precise atomic engineering of catalytic sites on various support materials including metal oxides, carbon-based materials, and 2D substrates. KAUST researchers have created innovative single-atom catalysts using transition metals (Fe, Co, Ni) anchored on nitrogen-doped carbon supports that demonstrate exceptional activity for hydrogen evolution reaction (HER) with overpotentials as low as 50 mV at 10 mA/cm²[7]. Their unique "atomic site isolation" methodology ensures maximum atom utilization while preventing metal aggregation during electrolysis processes. The university has also developed advanced in-situ/operando characterization techniques including X-ray absorption spectroscopy and environmental transmission electron microscopy to monitor the dynamic behavior of single atoms during catalytic reactions. Additionally, KAUST has pioneered computational approaches to predict and design optimal single-atom catalyst configurations, accelerating the discovery of high-performance materials for electrolysis applications[8].
Strengths: Exceptional catalytic activity with minimal overpotential requirements; superior atom utilization efficiency approaching 100%; integrated computational-experimental approach that accelerates catalyst development. Weaknesses: Challenges in scaling up laboratory synthesis methods to industrial production; potential stability issues under harsh industrial conditions; higher initial costs compared to conventional catalysts despite long-term economic benefits.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has developed advanced single-atom catalysts (SACs) for electrolysis applications, particularly focusing on water splitting and CO2 reduction. Their approach involves anchoring isolated metal atoms on nitrogen-doped carbon supports to create M-N-C structures with maximized atomic efficiency. The institute has pioneered the development of atomically dispersed Fe-N-C catalysts that demonstrate exceptional activity for the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER), achieving performance comparable to commercial Pt/C catalysts but at significantly lower costs[1]. Their recent breakthrough involves using high-temperature pyrolysis methods to create stable single-atom sites with specific coordination environments that enhance catalytic activity while preventing aggregation during electrolysis processes[2]. The institute has also developed in-situ characterization techniques to monitor the structural evolution of single-atom catalysts during electrolysis reactions, providing crucial insights for rational catalyst design.
Strengths: Exceptional atomic efficiency with nearly 100% metal atom utilization; remarkable stability under harsh electrolysis conditions; significantly lower costs compared to noble metal catalysts. Weaknesses: Challenges in large-scale production of uniform single-atom catalysts; potential metal leaching during long-term operation; limited understanding of the precise reaction mechanisms at the atomic level.
Key Innovations in Single-Atom Active Site Engineering
Single-atom catalyst and method of preparing same
PatentPendingEP4550480A2
Innovation
- A single-atom catalyst (SAC) is developed, comprising a nitrogen-doped carbon structure and a single-atom metal, such as cobalt, that forms a coordination bond with nitrogen atoms. This catalyst extends the optimal pH range and prevents hydroxyl radical adsorption to electrodes.
Single-atom catalyst for activation of persulfate to generate pure singlet oxygen as well as preparation method and application thereof
PatentActiveUS20220315425A1
Innovation
- A single-atom catalyst with graphitic carbon nitride nanosheets as supports and single iron atoms in a Fe—N4 coordination structure is developed, specifically designed to generate pure singlet oxygen by activating persulfate, with a mass ratio of single iron atoms between 7-12% of the catalyst, enhancing selectivity and resistance to environmental interference.
Sustainability Impact of Single-Atom Catalysis Technologies
The integration of single-atom catalysis (SAC) technologies into electrolysis processes represents a significant advancement in sustainable energy production and environmental protection. By maximizing atomic efficiency through isolated metal atoms anchored on suitable supports, SAC dramatically reduces the amount of precious metals required for catalytic reactions, addressing critical resource scarcity concerns while maintaining or even enhancing catalytic performance.
From an environmental perspective, SAC technologies substantially lower the carbon footprint of hydrogen production through water electrolysis. Traditional electrolysis methods often rely on catalysts containing substantial amounts of platinum group metals, which not only incur high economic costs but also generate significant environmental impacts during mining and processing. SAC approaches can reduce catalyst material requirements by up to 90% while achieving comparable or superior hydrogen evolution rates.
The sustainability benefits extend beyond material efficiency to operational improvements. SAC-enhanced electrolyzers demonstrate increased energy efficiency, with some systems showing 15-20% reductions in electricity consumption compared to conventional catalysts. This translates directly to lower greenhouse gas emissions when the electricity source is from fossil fuels, and more efficient utilization of renewable energy when green power sources are employed.
Water consumption represents another critical sustainability dimension where SAC technologies offer advantages. Advanced SAC-based electrolysis systems incorporate water reclamation mechanisms that can reduce freshwater requirements by up to 30% compared to conventional systems. This benefit becomes increasingly valuable as water scarcity affects more regions globally.
The lifecycle assessment of SAC technologies reveals additional sustainability benefits through extended catalyst longevity. Single-atom catalysts often demonstrate superior resistance to poisoning and degradation mechanisms that plague traditional catalysts, potentially doubling or tripling operational lifespans before replacement is necessary. This reduces waste generation and the frequency of resource-intensive manufacturing processes.
Economic sustainability also improves through SAC implementation. Despite higher initial research and development costs, the reduced material requirements and operational efficiencies translate to lower levelized costs of hydrogen production. Analysis indicates potential cost reductions of 25-40% over system lifetimes, making green hydrogen more competitive with fossil-fuel alternatives and accelerating the transition to hydrogen-based energy systems.
From an environmental perspective, SAC technologies substantially lower the carbon footprint of hydrogen production through water electrolysis. Traditional electrolysis methods often rely on catalysts containing substantial amounts of platinum group metals, which not only incur high economic costs but also generate significant environmental impacts during mining and processing. SAC approaches can reduce catalyst material requirements by up to 90% while achieving comparable or superior hydrogen evolution rates.
The sustainability benefits extend beyond material efficiency to operational improvements. SAC-enhanced electrolyzers demonstrate increased energy efficiency, with some systems showing 15-20% reductions in electricity consumption compared to conventional catalysts. This translates directly to lower greenhouse gas emissions when the electricity source is from fossil fuels, and more efficient utilization of renewable energy when green power sources are employed.
Water consumption represents another critical sustainability dimension where SAC technologies offer advantages. Advanced SAC-based electrolysis systems incorporate water reclamation mechanisms that can reduce freshwater requirements by up to 30% compared to conventional systems. This benefit becomes increasingly valuable as water scarcity affects more regions globally.
The lifecycle assessment of SAC technologies reveals additional sustainability benefits through extended catalyst longevity. Single-atom catalysts often demonstrate superior resistance to poisoning and degradation mechanisms that plague traditional catalysts, potentially doubling or tripling operational lifespans before replacement is necessary. This reduces waste generation and the frequency of resource-intensive manufacturing processes.
Economic sustainability also improves through SAC implementation. Despite higher initial research and development costs, the reduced material requirements and operational efficiencies translate to lower levelized costs of hydrogen production. Analysis indicates potential cost reductions of 25-40% over system lifetimes, making green hydrogen more competitive with fossil-fuel alternatives and accelerating the transition to hydrogen-based energy systems.
Scalability and Industrial Application Potential
The scalability of single-atom catalysis (SAC) technology represents a critical factor in determining its industrial viability for electrolysis applications. Current laboratory-scale demonstrations have shown remarkable efficiency improvements, with SAC-enhanced electrolyzers achieving up to 30-40% higher hydrogen production rates compared to conventional catalysts while maintaining similar energy inputs. However, translating these impressive results to industrial scales presents significant challenges that require systematic approaches.
Manufacturing scalability remains the primary hurdle for widespread SAC implementation. Traditional synthesis methods such as atomic layer deposition and wet chemistry approaches yield high-quality catalysts but suffer from low production volumes and high costs. Recent advances in mass production techniques, particularly modified spray pyrolysis and continuous flow synthesis, have demonstrated promising results, reducing production costs by approximately 60% while maintaining catalyst performance. These developments suggest a viable pathway toward industrial-scale catalyst manufacturing within the next 3-5 years.
Durability under industrial conditions represents another crucial consideration. While laboratory tests show SAC stability for hundreds of hours, industrial applications require thousands of hours of continuous operation. Recent field tests conducted by leading energy companies have demonstrated encouraging results, with some single-atom catalysts maintaining over 85% of their initial activity after 2,000 hours of operation in simulated industrial environments. This progress indicates that durability challenges, while significant, are not insurmountable.
Economic feasibility analysis reveals that SAC technology is approaching the commercial viability threshold. Current production costs range from $800-1,200 per kilogram for high-purity single-atom catalysts, approximately 3-4 times higher than conventional catalysts. However, considering their superior performance and longevity, the total cost of ownership calculations suggest potential long-term economic advantages. Industry projections indicate that with continued manufacturing improvements and economies of scale, production costs could decrease by 40-50% within the next decade.
Integration with existing infrastructure presents both challenges and opportunities. Most current electrolysis systems would require significant modifications to accommodate SAC technology effectively. However, several major equipment manufacturers have begun developing "SAC-ready" electrolysis systems that can be more easily retrofitted or designed from the ground up to leverage these advanced catalysts. This trend suggests growing industry confidence in the technology's future industrial relevance.
The potential market impact of scaled SAC technology extends beyond traditional hydrogen production. Applications in fuel cells, ammonia synthesis, and carbon dioxide reduction represent additional high-value markets that could benefit from this technology, potentially creating a diversified demand that further drives manufacturing scale and cost reductions.
Manufacturing scalability remains the primary hurdle for widespread SAC implementation. Traditional synthesis methods such as atomic layer deposition and wet chemistry approaches yield high-quality catalysts but suffer from low production volumes and high costs. Recent advances in mass production techniques, particularly modified spray pyrolysis and continuous flow synthesis, have demonstrated promising results, reducing production costs by approximately 60% while maintaining catalyst performance. These developments suggest a viable pathway toward industrial-scale catalyst manufacturing within the next 3-5 years.
Durability under industrial conditions represents another crucial consideration. While laboratory tests show SAC stability for hundreds of hours, industrial applications require thousands of hours of continuous operation. Recent field tests conducted by leading energy companies have demonstrated encouraging results, with some single-atom catalysts maintaining over 85% of their initial activity after 2,000 hours of operation in simulated industrial environments. This progress indicates that durability challenges, while significant, are not insurmountable.
Economic feasibility analysis reveals that SAC technology is approaching the commercial viability threshold. Current production costs range from $800-1,200 per kilogram for high-purity single-atom catalysts, approximately 3-4 times higher than conventional catalysts. However, considering their superior performance and longevity, the total cost of ownership calculations suggest potential long-term economic advantages. Industry projections indicate that with continued manufacturing improvements and economies of scale, production costs could decrease by 40-50% within the next decade.
Integration with existing infrastructure presents both challenges and opportunities. Most current electrolysis systems would require significant modifications to accommodate SAC technology effectively. However, several major equipment manufacturers have begun developing "SAC-ready" electrolysis systems that can be more easily retrofitted or designed from the ground up to leverage these advanced catalysts. This trend suggests growing industry confidence in the technology's future industrial relevance.
The potential market impact of scaled SAC technology extends beyond traditional hydrogen production. Applications in fuel cells, ammonia synthesis, and carbon dioxide reduction represent additional high-value markets that could benefit from this technology, potentially creating a diversified demand that further drives manufacturing scale and cost reductions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!