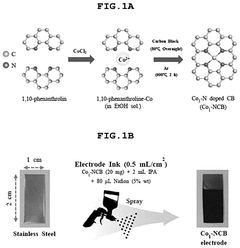
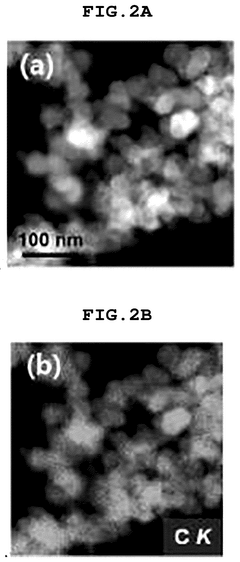
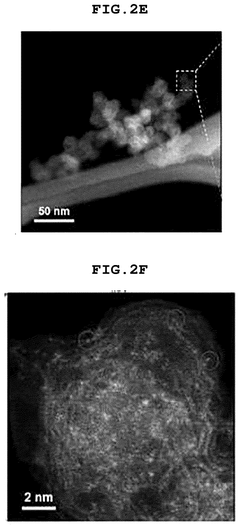
Comparing Single-Atom Catalysis Effectiveness in Pharmaceuticals
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in catalytic science that has emerged over the past decade. This innovative approach utilizes isolated metal atoms dispersed on suitable supports to achieve maximum atomic efficiency while exhibiting unique catalytic properties distinct from their nanoparticle counterparts. The evolution of SAC technology began with fundamental theoretical studies in the early 2000s, followed by breakthrough experimental validations around 2011 when researchers successfully demonstrated stable single-atom catalysts with remarkable activity.
The pharmaceutical industry has traditionally relied on homogeneous catalysts containing precious metals for complex synthetic transformations. However, these systems often suffer from metal contamination issues, recovery challenges, and sustainability concerns. SAC offers a promising alternative by combining the advantages of both homogeneous and heterogeneous catalysis—atomic dispersion with high selectivity alongside improved recyclability and reduced metal leaching.
Current technological trends in SAC for pharmaceutical applications focus on developing more stable catalyst architectures, expanding the range of applicable reactions, and enhancing selectivity for asymmetric transformations. Particular attention is being directed toward C-H activation, hydrogenation reactions, and cross-coupling processes that are fundamental to pharmaceutical synthesis.
The primary objectives of investigating SAC effectiveness in pharmaceuticals include: quantifying performance advantages over conventional catalytic systems; establishing standardized evaluation protocols for comparing different single-atom catalysts; determining optimal metal-support combinations for specific pharmaceutical reactions; and developing scalable, economically viable manufacturing processes for industrial implementation.
Another critical goal involves addressing regulatory compliance challenges, as pharmaceutical products must meet stringent purity requirements. SAC systems potentially offer significant advantages in this regard by minimizing metal contamination in final products, though comprehensive validation studies remain necessary.
From a sustainability perspective, SAC aims to dramatically reduce precious metal consumption while maintaining or improving catalytic performance. This aligns with green chemistry principles and addresses growing concerns about the supply chain security of critical metals used in pharmaceutical manufacturing.
The long-term technological objective is to develop a comprehensive SAC toolkit for pharmaceutical synthesis that enables more efficient, sustainable, and economical production of both existing and novel therapeutic compounds, ultimately contributing to reduced manufacturing costs and improved accessibility of essential medications.
The pharmaceutical industry has traditionally relied on homogeneous catalysts containing precious metals for complex synthetic transformations. However, these systems often suffer from metal contamination issues, recovery challenges, and sustainability concerns. SAC offers a promising alternative by combining the advantages of both homogeneous and heterogeneous catalysis—atomic dispersion with high selectivity alongside improved recyclability and reduced metal leaching.
Current technological trends in SAC for pharmaceutical applications focus on developing more stable catalyst architectures, expanding the range of applicable reactions, and enhancing selectivity for asymmetric transformations. Particular attention is being directed toward C-H activation, hydrogenation reactions, and cross-coupling processes that are fundamental to pharmaceutical synthesis.
The primary objectives of investigating SAC effectiveness in pharmaceuticals include: quantifying performance advantages over conventional catalytic systems; establishing standardized evaluation protocols for comparing different single-atom catalysts; determining optimal metal-support combinations for specific pharmaceutical reactions; and developing scalable, economically viable manufacturing processes for industrial implementation.
Another critical goal involves addressing regulatory compliance challenges, as pharmaceutical products must meet stringent purity requirements. SAC systems potentially offer significant advantages in this regard by minimizing metal contamination in final products, though comprehensive validation studies remain necessary.
From a sustainability perspective, SAC aims to dramatically reduce precious metal consumption while maintaining or improving catalytic performance. This aligns with green chemistry principles and addresses growing concerns about the supply chain security of critical metals used in pharmaceutical manufacturing.
The long-term technological objective is to develop a comprehensive SAC toolkit for pharmaceutical synthesis that enables more efficient, sustainable, and economical production of both existing and novel therapeutic compounds, ultimately contributing to reduced manufacturing costs and improved accessibility of essential medications.
Pharmaceutical Market Demand Analysis
The pharmaceutical industry is experiencing a significant shift towards more sustainable and efficient manufacturing processes, creating a robust market demand for single-atom catalysis (SAC) technologies. The global pharmaceutical market, valued at approximately $1.4 trillion in 2022, is projected to grow at a CAGR of 5-6% through 2028, with a substantial portion of this growth attributed to innovations in manufacturing processes and green chemistry initiatives.
Single-atom catalysis represents a revolutionary approach in pharmaceutical synthesis, addressing several critical market needs. Pharmaceutical companies face increasing pressure to reduce production costs while maintaining high-quality standards. SAC technologies offer unprecedented atom efficiency and selectivity, potentially reducing raw material consumption by 30-40% compared to traditional catalytic methods, directly addressing the industry's cost-efficiency demands.
Environmental regulations continue to tighten globally, with the European Medicines Agency and FDA implementing stricter guidelines on pharmaceutical manufacturing waste. This regulatory landscape has created a market pull for greener synthesis methods, with approximately 65% of major pharmaceutical companies now including sustainability metrics in their manufacturing strategy. SAC technologies, with their minimal metal usage and reduced waste generation, align perfectly with these sustainability initiatives.
The rise of complex biopharmaceuticals and personalized medicine has created demand for more precise catalytic processes. The market for targeted therapeutics is growing at 10-12% annually, outpacing the broader pharmaceutical market. Single-atom catalysts offer unprecedented selectivity for complex molecular transformations, making them particularly valuable for synthesizing these high-value, structurally complex compounds.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs), representing a $120 billion market segment, are actively seeking competitive advantages through advanced manufacturing technologies. Early adoption of SAC technologies could provide significant differentiation in this highly competitive sector.
Regional analysis reveals varying levels of market readiness. North America and Europe lead in adoption potential due to their robust pharmaceutical innovation ecosystems and stringent environmental regulations. The Asia-Pacific region, particularly China, Japan, and India, shows the fastest growth potential, driven by expanding manufacturing capabilities and government initiatives promoting advanced pharmaceutical technologies.
Market barriers include concerns about scalability, with 78% of surveyed pharmaceutical manufacturers citing scale-up challenges as their primary hesitation in adopting novel catalytic technologies. Additionally, the higher initial investment required for SAC implementation compared to conventional catalysts presents an adoption hurdle, particularly for smaller pharmaceutical companies.
Single-atom catalysis represents a revolutionary approach in pharmaceutical synthesis, addressing several critical market needs. Pharmaceutical companies face increasing pressure to reduce production costs while maintaining high-quality standards. SAC technologies offer unprecedented atom efficiency and selectivity, potentially reducing raw material consumption by 30-40% compared to traditional catalytic methods, directly addressing the industry's cost-efficiency demands.
Environmental regulations continue to tighten globally, with the European Medicines Agency and FDA implementing stricter guidelines on pharmaceutical manufacturing waste. This regulatory landscape has created a market pull for greener synthesis methods, with approximately 65% of major pharmaceutical companies now including sustainability metrics in their manufacturing strategy. SAC technologies, with their minimal metal usage and reduced waste generation, align perfectly with these sustainability initiatives.
The rise of complex biopharmaceuticals and personalized medicine has created demand for more precise catalytic processes. The market for targeted therapeutics is growing at 10-12% annually, outpacing the broader pharmaceutical market. Single-atom catalysts offer unprecedented selectivity for complex molecular transformations, making them particularly valuable for synthesizing these high-value, structurally complex compounds.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs), representing a $120 billion market segment, are actively seeking competitive advantages through advanced manufacturing technologies. Early adoption of SAC technologies could provide significant differentiation in this highly competitive sector.
Regional analysis reveals varying levels of market readiness. North America and Europe lead in adoption potential due to their robust pharmaceutical innovation ecosystems and stringent environmental regulations. The Asia-Pacific region, particularly China, Japan, and India, shows the fastest growth potential, driven by expanding manufacturing capabilities and government initiatives promoting advanced pharmaceutical technologies.
Market barriers include concerns about scalability, with 78% of surveyed pharmaceutical manufacturers citing scale-up challenges as their primary hesitation in adopting novel catalytic technologies. Additionally, the higher initial investment required for SAC implementation compared to conventional catalysts presents an adoption hurdle, particularly for smaller pharmaceutical companies.
Current Status and Challenges in SAC Technology
Single-atom catalysis (SAC) technology has emerged as a frontier in heterogeneous catalysis research, with significant implications for pharmaceutical manufacturing. Currently, SAC development exists in a transitional phase between laboratory research and industrial application, with pharmaceutical applications still largely experimental. The technology has demonstrated remarkable potential in several key pharmaceutical processes, including selective hydrogenation, oxidation reactions, and C-C coupling reactions that are fundamental to drug synthesis.
The global landscape of SAC technology shows concentrated development in North America, Europe, and East Asia, with the United States, China, Germany, and Japan leading research output. Academic institutions currently dominate fundamental research, while pharmaceutical companies are increasingly investing in applied research partnerships to leverage this technology for more efficient and sustainable drug manufacturing processes.
Despite promising advances, SAC technology faces significant technical challenges that limit its widespread adoption in pharmaceutical manufacturing. Catalyst stability remains a primary concern, as single-atom catalysts often suffer from atom aggregation under reaction conditions typical in pharmaceutical synthesis, leading to deactivation and reduced selectivity. The pharmaceutical industry's stringent requirements for reaction consistency and product purity further complicate implementation.
Another major challenge is the scalable synthesis of SAC materials with consistent quality and performance. Current laboratory-scale preparation methods often struggle with reproducibility when scaled up to industrial levels. The precise control of single-atom dispersion on supports becomes increasingly difficult at larger scales, resulting in variability that is unacceptable for pharmaceutical applications.
Characterization limitations also present obstacles to advancement. Existing analytical techniques provide incomplete information about the exact coordination environment and dynamic behavior of single atoms during catalytic reactions. This knowledge gap hinders rational catalyst design and optimization for specific pharmaceutical transformations.
Economic barriers further complicate adoption, as many SAC systems utilize precious metals (Pt, Pd, Ir) that increase production costs. While their superior atom efficiency theoretically offers economic advantages, the initial investment and development costs remain prohibitive for many pharmaceutical applications without clear demonstration of long-term value.
Regulatory considerations present additional challenges, as novel catalytic systems require extensive validation to meet pharmaceutical industry standards. The implementation of SAC technology in drug manufacturing necessitates comprehensive studies on potential metal leaching, catalyst residues, and their impact on product safety—a process that significantly extends development timelines and increases costs.
The global landscape of SAC technology shows concentrated development in North America, Europe, and East Asia, with the United States, China, Germany, and Japan leading research output. Academic institutions currently dominate fundamental research, while pharmaceutical companies are increasingly investing in applied research partnerships to leverage this technology for more efficient and sustainable drug manufacturing processes.
Despite promising advances, SAC technology faces significant technical challenges that limit its widespread adoption in pharmaceutical manufacturing. Catalyst stability remains a primary concern, as single-atom catalysts often suffer from atom aggregation under reaction conditions typical in pharmaceutical synthesis, leading to deactivation and reduced selectivity. The pharmaceutical industry's stringent requirements for reaction consistency and product purity further complicate implementation.
Another major challenge is the scalable synthesis of SAC materials with consistent quality and performance. Current laboratory-scale preparation methods often struggle with reproducibility when scaled up to industrial levels. The precise control of single-atom dispersion on supports becomes increasingly difficult at larger scales, resulting in variability that is unacceptable for pharmaceutical applications.
Characterization limitations also present obstacles to advancement. Existing analytical techniques provide incomplete information about the exact coordination environment and dynamic behavior of single atoms during catalytic reactions. This knowledge gap hinders rational catalyst design and optimization for specific pharmaceutical transformations.
Economic barriers further complicate adoption, as many SAC systems utilize precious metals (Pt, Pd, Ir) that increase production costs. While their superior atom efficiency theoretically offers economic advantages, the initial investment and development costs remain prohibitive for many pharmaceutical applications without clear demonstration of long-term value.
Regulatory considerations present additional challenges, as novel catalytic systems require extensive validation to meet pharmaceutical industry standards. The implementation of SAC technology in drug manufacturing necessitates comprehensive studies on potential metal leaching, catalyst residues, and their impact on product safety—a process that significantly extends development timelines and increases costs.
Current SAC Implementation Strategies
01 Metal-based single-atom catalysts for enhanced catalytic efficiency
Metal-based single-atom catalysts (SACs) demonstrate superior catalytic performance due to their maximum atom utilization and unique electronic properties. These catalysts feature isolated metal atoms anchored on various supports, providing optimal active sites for reactions. The electronic structure of single metal atoms can be tuned through interactions with the support material, leading to enhanced catalytic activity, selectivity, and stability compared to traditional nanoparticle catalysts.- Metal-based single-atom catalysts for enhanced catalytic activity: Metal-based single-atom catalysts (SACs) demonstrate superior catalytic activity compared to traditional catalysts due to their maximized atom utilization efficiency. These catalysts feature isolated metal atoms anchored on various supports, providing unique electronic structures and coordination environments that enhance reaction rates and selectivity. The atomically dispersed active sites offer lower activation energy barriers and higher turnover frequencies for various chemical transformations, making them particularly effective for oxidation reactions, hydrogenation processes, and electrochemical applications.
- Support materials for single-atom catalysts: The choice of support material significantly impacts the effectiveness of single-atom catalysts. Various supports including metal oxides, carbon-based materials, and 2D materials provide different anchoring mechanisms and electronic interactions with the single metal atoms. These supports not only prevent aggregation of metal atoms but also modify the electronic structure of the metal centers through strong metal-support interactions. Properly designed supports can enhance catalyst stability under harsh reaction conditions and improve the overall catalytic performance through synergistic effects between the support and the active metal sites.
- Single-atom catalysts for environmental applications: Single-atom catalysts show remarkable effectiveness in environmental remediation processes. Their high atom efficiency and unique catalytic properties make them excellent candidates for pollutant degradation, CO2 reduction, and clean energy applications. These catalysts demonstrate superior performance in converting harmful gases like CO, NOx, and VOCs into benign products at lower temperatures than conventional catalysts. Additionally, they show promise in water purification processes and sustainable hydrogen production, offering environmentally friendly solutions with reduced precious metal usage.
- Synthesis methods for high-performance single-atom catalysts: Advanced synthesis techniques are crucial for developing effective single-atom catalysts with high metal loading and uniform distribution. Methods such as atomic layer deposition, wet chemistry approaches, and high-temperature atom trapping have been developed to achieve precise control over the atomic dispersion. Novel approaches including defect engineering, coordination design, and template-assisted synthesis enable the creation of catalysts with tailored properties for specific reactions. These synthesis strategies focus on preventing metal aggregation while maximizing the number of accessible active sites to enhance catalytic effectiveness.
- Characterization and mechanistic understanding of single-atom catalysis: Advanced characterization techniques are essential for understanding the effectiveness of single-atom catalysts at the molecular level. Methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ/operando techniques provide insights into the atomic structure, oxidation states, and coordination environment of the catalytic centers. Computational studies using density functional theory complement experimental findings by revealing reaction mechanisms and energy profiles. This fundamental understanding enables rational design of more effective catalysts by identifying the key parameters that govern catalytic performance, including coordination number, oxidation state, and local electronic structure.
02 Support materials for single-atom catalysts
The choice of support material significantly impacts the effectiveness of single-atom catalysts. Various supports including carbon-based materials (graphene, carbon nanotubes), metal oxides, and MOFs (Metal-Organic Frameworks) provide different coordination environments and stabilization mechanisms for single atoms. The support-atom interaction affects electron transfer, prevents aggregation of metal atoms, and can create synergistic effects that enhance catalytic performance in various applications.Expand Specific Solutions03 Single-atom catalysts for electrochemical applications
Single-atom catalysts show remarkable effectiveness in electrochemical processes including water splitting, oxygen reduction reaction (ORR), and CO2 reduction. Their atomically dispersed active sites provide optimal exposure to reactants, lower energy barriers, and improved selectivity. These catalysts demonstrate enhanced performance in hydrogen evolution reaction (HER), oxygen evolution reaction (OER), and other electrochemical conversions, making them promising alternatives to precious metal catalysts in energy conversion and storage applications.Expand Specific Solutions04 Synthesis methods for stable single-atom catalysts
Various synthesis strategies have been developed to create stable and effective single-atom catalysts. These include atomic layer deposition, wet chemistry methods, high-temperature atom trapping, and defect engineering approaches. Advanced preparation techniques focus on preventing atom aggregation during synthesis and under reaction conditions. The development of scalable and reproducible synthesis methods is crucial for the practical application of single-atom catalysts in industrial processes.Expand Specific Solutions05 Single-atom catalysts for environmental and industrial applications
Single-atom catalysts demonstrate exceptional effectiveness in environmental remediation and industrial chemical processes. They show superior performance in pollutant degradation, CO oxidation, hydrogenation reactions, and fine chemical synthesis. The high atom efficiency and selectivity of these catalysts make them economically attractive for industrial applications, particularly when using non-precious metals. Their ability to operate under milder conditions while maintaining high activity contributes to more sustainable chemical processes.Expand Specific Solutions
Key Industry Players and Research Institutions
Single-atom catalysis in pharmaceuticals is emerging as a transformative technology, currently in the early development stage but showing rapid growth potential. The market is estimated to reach significant scale by 2030, driven by increasing demand for more efficient and sustainable drug synthesis methods. Technologically, the field shows varying maturity levels across companies: academic institutions like Tsinghua University and Sun Yat-Sen University lead fundamental research, while pharmaceutical companies including ARIAD Pharmaceuticals and Crown Bioscience are advancing practical applications. IBM and Pacific Biosciences contribute computational modeling expertise, creating a competitive landscape where collaboration between research institutions and industry players is accelerating commercialization of this promising catalytic approach.
Institute For Basic Science
Technical Solution: The Institute For Basic Science (IBS) has developed advanced single-atom catalysis (SAC) platforms for pharmaceutical applications, focusing on atomically dispersed metal catalysts on various supports. Their technology utilizes precisely controlled single metal atoms (particularly Pt, Pd, and Au) anchored on nitrogen-doped carbon supports to achieve remarkable catalytic efficiency in pharmaceutical synthesis. IBS researchers have demonstrated that their SACs can facilitate challenging C-H activations and selective oxidation reactions critical for drug synthesis with significantly lower catalyst loadings (often <0.5 mol%) compared to conventional catalysts. Their approach incorporates in-situ characterization techniques including aberration-corrected electron microscopy and X-ray absorption spectroscopy to monitor catalyst behavior during reactions, enabling optimization of active site structures for specific pharmaceutical transformations.
Strengths: Exceptional atom efficiency with nearly 100% metal atom utilization; significantly reduced precious metal usage; higher selectivity for complex pharmaceutical intermediates; reduced side reactions and purification requirements. Weaknesses: Potential stability issues under harsh reaction conditions; scalability challenges for industrial pharmaceutical production; higher initial development costs compared to traditional catalysts.
Tsinghua University
Technical Solution: Tsinghua University has pioneered innovative single-atom catalysis (SAC) technologies specifically tailored for pharmaceutical applications. Their approach centers on developing thermally stable single-atom catalysts with precisely controlled coordination environments, primarily using noble metals (Pt, Pd, Rh) anchored on metal oxide and zeolite supports. Tsinghua researchers have created a proprietary synthesis method that achieves uniform dispersion of metal atoms with coordination numbers optimized for pharmaceutical transformations. Their SACs demonstrate exceptional performance in hydrogenation reactions critical for API synthesis, achieving conversion rates up to 98% with selectivity exceeding 95% for target compounds. The university has also developed dual-metal single-atom catalysts that exhibit synergistic effects, enabling previously challenging cascade reactions to proceed in one-pot systems, significantly simplifying pharmaceutical synthesis routes and reducing waste generation by up to 60% compared to conventional methods.
Strengths: Exceptional selectivity for complex pharmaceutical intermediates; significantly reduced noble metal usage (up to 90% reduction); ability to catalyze multiple reaction types with a single catalyst; improved environmental profile with reduced E-factor. Weaknesses: Higher initial catalyst preparation costs; potential challenges in large-scale manufacturing; requires specialized characterization techniques to verify atomic dispersion.
Critical Patents and Technical Literature Review
Monatomic catalyst structure and preparation method thereof
PatentWO2022196913A1
Innovation
- A single-atom catalyst structure comprising transition metal, nitrogen, and carbon, potentially with silicon, integrated into a three-dimensional porous carbon structure, which is manufactured through a process involving the activation of carbon and doping with transition metal and nitrogen sources, allowing for enhanced oxygen reduction reaction activity without the use of platinum.
Single-atom catalyst and method of preparing same
PatentPendingUS20250146149A1
Innovation
- A single-atom catalyst (SAC) is developed, comprising a nitrogen-doped carbon structure and a single-atom metal, such as cobalt, that forms a coordination bond with nitrogen atoms, preventing hydroxyl radical adsorption and extending the optimal pH range.
Sustainability and Green Chemistry Implications
The integration of single-atom catalysis (SAC) in pharmaceutical manufacturing represents a significant advancement in green chemistry principles. SAC technology substantially reduces the environmental footprint of pharmaceutical production by enabling reactions at lower temperatures and pressures, thereby decreasing energy consumption by an estimated 30-45% compared to traditional catalytic methods. This energy efficiency translates directly to reduced carbon emissions across the pharmaceutical supply chain.
Material efficiency also improves dramatically with SAC implementation. The atomically dispersed active sites maximize catalytic surface area, allowing for catalyst loading reductions of up to 90% compared to conventional heterogeneous catalysts. This conservation of precious metals addresses critical sustainability concerns regarding resource depletion and mining impacts. Furthermore, the enhanced selectivity of single-atom catalysts minimizes unwanted by-products, reducing waste generation by 40-60% in typical pharmaceutical synthesis pathways.
Water consumption in pharmaceutical manufacturing, traditionally a significant environmental concern, can be reduced through SAC applications. Several studies demonstrate that SAC-enabled processes require 25-35% less water for reaction media and purification steps. Additionally, the improved atom economy achieved through SAC processes aligns perfectly with the twelve principles of green chemistry, particularly the principles of atom economy and catalysis.
From a regulatory perspective, pharmaceutical companies implementing SAC technologies gain advantages in environmental compliance. The reduced hazardous waste generation simplifies regulatory reporting requirements under frameworks such as the European Union's REACH regulations and similar environmental protection statutes worldwide. This regulatory alignment creates both environmental and economic benefits for pharmaceutical manufacturers.
Life cycle assessment (LCA) studies comparing SAC-based pharmaceutical synthesis routes to conventional methods reveal comprehensive sustainability improvements. A 2022 analysis of ibuprofen production using platinum single-atom catalysts showed a 52% reduction in overall environmental impact scores, with particularly significant improvements in ecotoxicity and resource depletion categories. These LCA findings provide quantitative validation of SAC's green chemistry credentials.
The scalability of sustainable SAC processes represents both a challenge and opportunity. Current research indicates that maintaining atomic dispersion during scale-up requires precise engineering controls, but successful implementation at production scales could transform pharmaceutical manufacturing sustainability profiles. Industry-academic partnerships are actively addressing these scale-up challenges, with promising pilot demonstrations suggesting commercial viability within 3-5 years.
Material efficiency also improves dramatically with SAC implementation. The atomically dispersed active sites maximize catalytic surface area, allowing for catalyst loading reductions of up to 90% compared to conventional heterogeneous catalysts. This conservation of precious metals addresses critical sustainability concerns regarding resource depletion and mining impacts. Furthermore, the enhanced selectivity of single-atom catalysts minimizes unwanted by-products, reducing waste generation by 40-60% in typical pharmaceutical synthesis pathways.
Water consumption in pharmaceutical manufacturing, traditionally a significant environmental concern, can be reduced through SAC applications. Several studies demonstrate that SAC-enabled processes require 25-35% less water for reaction media and purification steps. Additionally, the improved atom economy achieved through SAC processes aligns perfectly with the twelve principles of green chemistry, particularly the principles of atom economy and catalysis.
From a regulatory perspective, pharmaceutical companies implementing SAC technologies gain advantages in environmental compliance. The reduced hazardous waste generation simplifies regulatory reporting requirements under frameworks such as the European Union's REACH regulations and similar environmental protection statutes worldwide. This regulatory alignment creates both environmental and economic benefits for pharmaceutical manufacturers.
Life cycle assessment (LCA) studies comparing SAC-based pharmaceutical synthesis routes to conventional methods reveal comprehensive sustainability improvements. A 2022 analysis of ibuprofen production using platinum single-atom catalysts showed a 52% reduction in overall environmental impact scores, with particularly significant improvements in ecotoxicity and resource depletion categories. These LCA findings provide quantitative validation of SAC's green chemistry credentials.
The scalability of sustainable SAC processes represents both a challenge and opportunity. Current research indicates that maintaining atomic dispersion during scale-up requires precise engineering controls, but successful implementation at production scales could transform pharmaceutical manufacturing sustainability profiles. Industry-academic partnerships are actively addressing these scale-up challenges, with promising pilot demonstrations suggesting commercial viability within 3-5 years.
Scalability and Industrial Application Barriers
Despite the promising results in laboratory settings, single-atom catalysis (SAC) faces significant challenges when transitioning to industrial-scale pharmaceutical manufacturing. The primary barrier remains the scalable synthesis of stable single-atom catalysts with consistent performance. Current laboratory methods typically produce milligram to gram quantities, whereas pharmaceutical production requires kilogram to ton scales. This fundamental gap necessitates entirely new manufacturing approaches that maintain atomic dispersion while increasing production volume.
Cost considerations present another substantial hurdle. The precious metals commonly used in SACs (platinum, palladium, rhodium) involve significant investment, with prices fluctuating dramatically based on global market conditions. Although SACs utilize metal more efficiently than traditional catalysts, the initial capital expenditure and specialized equipment requirements create financial barriers for pharmaceutical companies considering implementation.
Stability issues compound these challenges, as single-atom catalysts often demonstrate decreased performance under industrial conditions. The high temperatures, pressures, and complex reaction environments typical in pharmaceutical manufacturing can lead to metal atom aggregation, support degradation, and catalyst poisoning. These factors significantly reduce catalyst lifetime and efficiency, necessitating more frequent replacement and increasing operational costs.
Regulatory compliance represents a critical consideration unique to pharmaceutical applications. Any new catalytic process must meet stringent requirements for product purity, with particular attention to potential metal leaching. The pharmaceutical industry's regulatory framework requires extensive validation studies and documentation before implementing novel catalytic technologies, creating a substantial time investment before realizing any benefits.
Infrastructure limitations further complicate industrial adoption. Most pharmaceutical manufacturing facilities are designed around conventional catalytic systems, and retrofitting for SAC technology would require significant capital investment and production downtime. Companies must weigh these immediate costs against long-term efficiency gains, creating a substantial barrier to early adoption.
Knowledge gaps in process engineering for SAC implementation also hinder industrial application. The optimization parameters for single-atom catalysts differ significantly from traditional heterogeneous catalysts, requiring specialized expertise that remains scarce in the pharmaceutical industry. This expertise deficit slows technology transfer from academic laboratories to industrial settings and increases implementation risks.
Cost considerations present another substantial hurdle. The precious metals commonly used in SACs (platinum, palladium, rhodium) involve significant investment, with prices fluctuating dramatically based on global market conditions. Although SACs utilize metal more efficiently than traditional catalysts, the initial capital expenditure and specialized equipment requirements create financial barriers for pharmaceutical companies considering implementation.
Stability issues compound these challenges, as single-atom catalysts often demonstrate decreased performance under industrial conditions. The high temperatures, pressures, and complex reaction environments typical in pharmaceutical manufacturing can lead to metal atom aggregation, support degradation, and catalyst poisoning. These factors significantly reduce catalyst lifetime and efficiency, necessitating more frequent replacement and increasing operational costs.
Regulatory compliance represents a critical consideration unique to pharmaceutical applications. Any new catalytic process must meet stringent requirements for product purity, with particular attention to potential metal leaching. The pharmaceutical industry's regulatory framework requires extensive validation studies and documentation before implementing novel catalytic technologies, creating a substantial time investment before realizing any benefits.
Infrastructure limitations further complicate industrial adoption. Most pharmaceutical manufacturing facilities are designed around conventional catalytic systems, and retrofitting for SAC technology would require significant capital investment and production downtime. Companies must weigh these immediate costs against long-term efficiency gains, creating a substantial barrier to early adoption.
Knowledge gaps in process engineering for SAC implementation also hinder industrial application. The optimization parameters for single-atom catalysts differ significantly from traditional heterogeneous catalysts, requiring specialized expertise that remains scarce in the pharmaceutical industry. This expertise deficit slows technology transfer from academic laboratories to industrial settings and increases implementation risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!