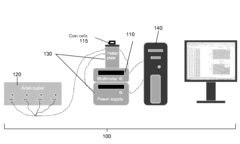
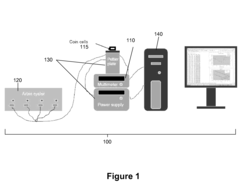
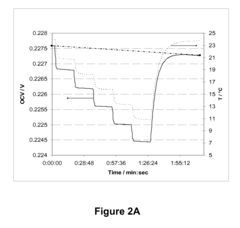
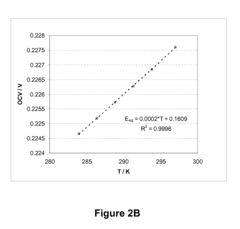
Benchmark Efficiency of Electrochemical Cell in Industrial Systems
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Benchmarking Background and Objectives
Electrochemical cells have evolved significantly since Alessandro Volta's pioneering work in the early 19th century, transforming from simple laboratory curiosities to sophisticated industrial systems powering various applications. The trajectory of development has accelerated dramatically in recent decades, driven by increasing demands for clean energy, efficient industrial processes, and sustainable manufacturing practices.
The benchmarking of electrochemical cell efficiency in industrial systems has become a critical focus area as industries seek to optimize performance, reduce operational costs, and minimize environmental impact. This technical exploration aims to establish standardized methodologies for evaluating electrochemical cell performance across diverse industrial applications, from energy storage and conversion to chemical manufacturing and waste treatment processes.
Current industrial implementations of electrochemical cells face significant efficiency challenges, with many systems operating well below their theoretical maximum efficiency. The gap between laboratory-scale performance and industrial-scale implementation remains substantial, highlighting the need for comprehensive benchmarking frameworks that can accurately assess real-world performance under varying operational conditions.
The primary objectives of this technical investigation include developing standardized protocols for measuring electrochemical cell efficiency in industrial environments, identifying key performance indicators that accurately reflect operational realities, and establishing industry-specific benchmarks that can guide future development efforts. Additionally, we aim to create comparative frameworks that enable meaningful assessment across different electrochemical technologies and applications.
Historical benchmarking approaches have often focused narrowly on specific performance metrics such as energy efficiency or reaction selectivity, without adequately addressing the complex interplay of factors that determine overall system performance in industrial settings. This investigation seeks to develop more holistic evaluation methodologies that consider factors including energy consumption, material utilization, operational stability, maintenance requirements, and lifecycle environmental impact.
Recent technological advancements in sensor technology, data analytics, and computational modeling have created new opportunities for more sophisticated benchmarking approaches. These developments enable real-time monitoring of electrochemical processes, predictive performance modeling, and data-driven optimization strategies that were previously unattainable.
The global transition toward renewable energy systems and sustainable industrial practices has further elevated the importance of efficient electrochemical technologies. As industries increasingly adopt these systems for applications ranging from grid-scale energy storage to carbon capture and utilization, the need for reliable benchmarking methodologies becomes increasingly critical for guiding investment decisions and technology development priorities.
The benchmarking of electrochemical cell efficiency in industrial systems has become a critical focus area as industries seek to optimize performance, reduce operational costs, and minimize environmental impact. This technical exploration aims to establish standardized methodologies for evaluating electrochemical cell performance across diverse industrial applications, from energy storage and conversion to chemical manufacturing and waste treatment processes.
Current industrial implementations of electrochemical cells face significant efficiency challenges, with many systems operating well below their theoretical maximum efficiency. The gap between laboratory-scale performance and industrial-scale implementation remains substantial, highlighting the need for comprehensive benchmarking frameworks that can accurately assess real-world performance under varying operational conditions.
The primary objectives of this technical investigation include developing standardized protocols for measuring electrochemical cell efficiency in industrial environments, identifying key performance indicators that accurately reflect operational realities, and establishing industry-specific benchmarks that can guide future development efforts. Additionally, we aim to create comparative frameworks that enable meaningful assessment across different electrochemical technologies and applications.
Historical benchmarking approaches have often focused narrowly on specific performance metrics such as energy efficiency or reaction selectivity, without adequately addressing the complex interplay of factors that determine overall system performance in industrial settings. This investigation seeks to develop more holistic evaluation methodologies that consider factors including energy consumption, material utilization, operational stability, maintenance requirements, and lifecycle environmental impact.
Recent technological advancements in sensor technology, data analytics, and computational modeling have created new opportunities for more sophisticated benchmarking approaches. These developments enable real-time monitoring of electrochemical processes, predictive performance modeling, and data-driven optimization strategies that were previously unattainable.
The global transition toward renewable energy systems and sustainable industrial practices has further elevated the importance of efficient electrochemical technologies. As industries increasingly adopt these systems for applications ranging from grid-scale energy storage to carbon capture and utilization, the need for reliable benchmarking methodologies becomes increasingly critical for guiding investment decisions and technology development priorities.
Industrial Market Demand for Efficient Electrochemical Systems
The electrochemical cell market is experiencing robust growth driven by increasing industrial demands for energy storage solutions, renewable energy integration, and sustainable manufacturing processes. Current market analysis indicates that the global electrochemical cell industry is valued at approximately $45 billion, with projections suggesting a compound annual growth rate of 8.7% through 2030, highlighting the significant commercial potential for efficiency improvements in this sector.
Industrial sectors including energy storage, automotive manufacturing, chemical processing, and water treatment represent the primary demand drivers for high-efficiency electrochemical systems. The automotive industry, particularly the electric vehicle segment, has emerged as a critical market, with battery efficiency directly impacting vehicle range, charging times, and overall consumer adoption rates.
Manufacturing industries are increasingly seeking electrochemical solutions that offer reduced energy consumption while maintaining or improving production output. This demand is particularly pronounced in regions with high energy costs or stringent environmental regulations, where efficiency improvements translate directly to operational cost savings and regulatory compliance.
The renewable energy sector presents another substantial market opportunity, as efficient electrochemical systems are essential for addressing intermittency challenges through energy storage. Grid-scale storage applications require electrochemical cells with high round-trip efficiency, extended cycle life, and improved energy density metrics to make renewable integration economically viable.
Market research indicates that industrial customers prioritize three key performance indicators when evaluating electrochemical systems: operational efficiency (energy input versus useful output), total cost of ownership (including maintenance and replacement costs), and system reliability under variable operating conditions. Benchmark efficiency improvements of even 2-3% can represent significant competitive advantages in this market landscape.
Regional analysis reveals differentiated market demands, with North American and European industries focusing on high-performance specifications and sustainability metrics, while emerging markets in Asia-Pacific regions demonstrate greater price sensitivity balanced against performance requirements. This geographical variation necessitates tailored benchmarking approaches for different market segments.
The industrial water treatment sector represents a rapidly expanding application area, with electrochemical technologies increasingly deployed for contaminant removal, disinfection, and resource recovery. Market demand in this segment is projected to grow at 12.3% annually, driven by water scarcity concerns and tightening discharge regulations across industrial operations.
Forward-looking market indicators suggest that industries are increasingly valuing standardized efficiency benchmarking protocols that enable direct comparison between competing electrochemical technologies, creating opportunities for organizations that can establish recognized performance certification methodologies.
Industrial sectors including energy storage, automotive manufacturing, chemical processing, and water treatment represent the primary demand drivers for high-efficiency electrochemical systems. The automotive industry, particularly the electric vehicle segment, has emerged as a critical market, with battery efficiency directly impacting vehicle range, charging times, and overall consumer adoption rates.
Manufacturing industries are increasingly seeking electrochemical solutions that offer reduced energy consumption while maintaining or improving production output. This demand is particularly pronounced in regions with high energy costs or stringent environmental regulations, where efficiency improvements translate directly to operational cost savings and regulatory compliance.
The renewable energy sector presents another substantial market opportunity, as efficient electrochemical systems are essential for addressing intermittency challenges through energy storage. Grid-scale storage applications require electrochemical cells with high round-trip efficiency, extended cycle life, and improved energy density metrics to make renewable integration economically viable.
Market research indicates that industrial customers prioritize three key performance indicators when evaluating electrochemical systems: operational efficiency (energy input versus useful output), total cost of ownership (including maintenance and replacement costs), and system reliability under variable operating conditions. Benchmark efficiency improvements of even 2-3% can represent significant competitive advantages in this market landscape.
Regional analysis reveals differentiated market demands, with North American and European industries focusing on high-performance specifications and sustainability metrics, while emerging markets in Asia-Pacific regions demonstrate greater price sensitivity balanced against performance requirements. This geographical variation necessitates tailored benchmarking approaches for different market segments.
The industrial water treatment sector represents a rapidly expanding application area, with electrochemical technologies increasingly deployed for contaminant removal, disinfection, and resource recovery. Market demand in this segment is projected to grow at 12.3% annually, driven by water scarcity concerns and tightening discharge regulations across industrial operations.
Forward-looking market indicators suggest that industries are increasingly valuing standardized efficiency benchmarking protocols that enable direct comparison between competing electrochemical technologies, creating opportunities for organizations that can establish recognized performance certification methodologies.
Current Status and Challenges in Electrochemical Cell Efficiency
The global landscape of electrochemical cell efficiency benchmarking reveals significant disparities across regions and industries. Currently, leading industrial applications achieve efficiency ratings between 60-85%, with advanced laboratory prototypes demonstrating potential for up to 92% efficiency under controlled conditions. However, these laboratory results rarely translate directly to industrial settings due to scaling challenges and variable operating environments.
A critical challenge facing the industry is the lack of standardized benchmarking protocols. Different manufacturers employ varied testing methodologies, making direct performance comparisons problematic. This inconsistency creates market confusion and hinders technological advancement as stakeholders struggle to identify truly superior solutions.
Material degradation remains a persistent obstacle to long-term efficiency. Current electrode materials experience performance decline of approximately 8-15% annually in industrial applications, necessitating costly replacements and maintenance. While novel materials show promise in laboratory settings, their durability under industrial conditions often falls short of theoretical projections.
Energy loss through heat generation represents another significant efficiency barrier. Industrial electrochemical cells typically operate at temperatures between 50-80°C, with cooling systems consuming 10-18% of the total energy input. This parasitic energy consumption substantially reduces overall system efficiency and increases operational costs.
Catalyst poisoning and electrode fouling accelerate in industrial environments where feedstock purity cannot match laboratory conditions. Current filtration and pretreatment technologies reduce but cannot eliminate these contaminants, resulting in gradual performance degradation that can reduce efficiency by 20-30% before scheduled maintenance intervals.
Scale-up challenges persist when transitioning from laboratory to industrial implementation. Phenomena such as uneven current distribution, mass transport limitations, and thermal management become increasingly problematic at larger scales. Engineering solutions that maintain laboratory-level efficiencies at industrial scales remain elusive despite significant research investment.
Geographical distribution of advanced electrochemical cell technology shows concentration in North America, Western Europe, and East Asia, with China, Germany, and the United States leading patent applications in this field. Emerging economies face additional challenges including limited access to advanced materials, technical expertise shortages, and inadequate infrastructure for high-efficiency operations.
Recent regulatory developments, particularly carbon pricing mechanisms and emissions standards, are creating new imperatives for efficiency improvements. Industries operating in regions with stringent environmental regulations report accelerated investment in electrochemical efficiency research, suggesting regulatory frameworks may be critical drivers for technological advancement in this field.
A critical challenge facing the industry is the lack of standardized benchmarking protocols. Different manufacturers employ varied testing methodologies, making direct performance comparisons problematic. This inconsistency creates market confusion and hinders technological advancement as stakeholders struggle to identify truly superior solutions.
Material degradation remains a persistent obstacle to long-term efficiency. Current electrode materials experience performance decline of approximately 8-15% annually in industrial applications, necessitating costly replacements and maintenance. While novel materials show promise in laboratory settings, their durability under industrial conditions often falls short of theoretical projections.
Energy loss through heat generation represents another significant efficiency barrier. Industrial electrochemical cells typically operate at temperatures between 50-80°C, with cooling systems consuming 10-18% of the total energy input. This parasitic energy consumption substantially reduces overall system efficiency and increases operational costs.
Catalyst poisoning and electrode fouling accelerate in industrial environments where feedstock purity cannot match laboratory conditions. Current filtration and pretreatment technologies reduce but cannot eliminate these contaminants, resulting in gradual performance degradation that can reduce efficiency by 20-30% before scheduled maintenance intervals.
Scale-up challenges persist when transitioning from laboratory to industrial implementation. Phenomena such as uneven current distribution, mass transport limitations, and thermal management become increasingly problematic at larger scales. Engineering solutions that maintain laboratory-level efficiencies at industrial scales remain elusive despite significant research investment.
Geographical distribution of advanced electrochemical cell technology shows concentration in North America, Western Europe, and East Asia, with China, Germany, and the United States leading patent applications in this field. Emerging economies face additional challenges including limited access to advanced materials, technical expertise shortages, and inadequate infrastructure for high-efficiency operations.
Recent regulatory developments, particularly carbon pricing mechanisms and emissions standards, are creating new imperatives for efficiency improvements. Industries operating in regions with stringent environmental regulations report accelerated investment in electrochemical efficiency research, suggesting regulatory frameworks may be critical drivers for technological advancement in this field.
Current Benchmarking Methodologies for Electrochemical Cells
01 Electrode materials and catalysts for improved efficiency
The choice of electrode materials and catalysts significantly impacts electrochemical cell efficiency. Advanced materials such as noble metals, metal oxides, and composite structures can enhance electron transfer rates and reduce activation energy barriers. Catalysts designed with high surface area and specific active sites can accelerate electrochemical reactions while minimizing energy losses. These materials can be engineered at the nanoscale to optimize their catalytic properties and stability under operating conditions.- Electrode materials and catalysts for improved efficiency: Advanced electrode materials and catalysts play a crucial role in enhancing electrochemical cell efficiency. These materials can reduce activation energy, increase reaction rates, and improve overall energy conversion. Innovations include novel catalyst compositions, nanostructured electrodes, and composite materials that facilitate electron transfer and ionic conductivity, resulting in higher power output and reduced internal resistance in electrochemical cells.
- Electrolyte composition and optimization: The composition and properties of electrolytes significantly impact electrochemical cell efficiency. Research focuses on developing electrolytes with enhanced ionic conductivity, stability, and compatibility with electrode materials. Innovations include novel electrolyte formulations, additives to prevent side reactions, and optimization of electrolyte concentration to minimize resistance and maximize ion transport, thereby improving overall cell performance and energy efficiency.
- Cell design and structural improvements: Structural design innovations in electrochemical cells contribute to efficiency improvements through better mass transport, reduced internal resistance, and optimized reaction kinetics. These include novel cell architectures, improved flow field designs, advanced membrane configurations, and optimized electrode spacing. Such design enhancements facilitate more efficient reactant distribution, product removal, and overall energy conversion within the electrochemical system.
- Temperature management and control systems: Effective temperature management is critical for maintaining optimal electrochemical cell efficiency. Systems that regulate operating temperature can prevent degradation of components, optimize reaction kinetics, and extend cell lifetime. Innovations include advanced cooling systems, thermal management materials, heat recovery mechanisms, and temperature monitoring technologies that maintain cells within ideal operating ranges, thereby maximizing energy conversion efficiency and performance stability.
- Monitoring and control algorithms for efficiency optimization: Advanced monitoring systems and control algorithms enable real-time optimization of electrochemical cell efficiency. These technologies utilize sensors, data analytics, and feedback mechanisms to adjust operating parameters based on performance metrics. Innovations include predictive maintenance algorithms, adaptive control systems, machine learning approaches for parameter optimization, and integrated monitoring platforms that identify inefficiencies and automatically implement corrective measures to maximize energy conversion and utilization.
02 Electrolyte composition and optimization
The composition and properties of electrolytes play a crucial role in electrochemical cell efficiency. Optimized electrolyte formulations can enhance ionic conductivity, reduce internal resistance, and improve mass transport within the cell. Additives and supporting electrolytes can be incorporated to stabilize electrochemical reactions and prevent side reactions that decrease efficiency. Temperature control and concentration gradients within the electrolyte also affect overall cell performance and energy conversion rates.Expand Specific Solutions03 Cell design and configuration for efficiency enhancement
The physical design and configuration of electrochemical cells significantly impact their efficiency. Optimized cell geometries can reduce internal resistance and improve mass transport. Flow field designs that ensure uniform distribution of reactants across electrode surfaces enhance reaction rates and prevent localized depletion. Advanced manufacturing techniques allow for precise control of electrode spacing, membrane integration, and current collector placement, all of which contribute to higher energy conversion efficiency and reduced losses.Expand Specific Solutions04 Temperature and pressure management systems
Effective temperature and pressure management systems are essential for maintaining optimal electrochemical cell efficiency. Controlled operating conditions prevent degradation of cell components and ensure consistent reaction kinetics. Thermal management systems that regulate heat generation and dissipation can prevent efficiency losses due to temperature gradients. Pressure control mechanisms optimize reactant solubility and mass transport properties, particularly in gas-evolving or gas-consuming electrochemical processes, leading to improved overall cell performance.Expand Specific Solutions05 Advanced monitoring and control strategies
Implementation of advanced monitoring and control strategies enables real-time optimization of electrochemical cell efficiency. Sensors and analytical techniques that provide feedback on cell performance parameters allow for adaptive control of operating conditions. Machine learning algorithms can predict performance degradation and suggest preventive maintenance. Integrated control systems that balance multiple parameters simultaneously can maintain cells at their efficiency peak despite changing external conditions or internal degradation processes.Expand Specific Solutions
Key Industry Players in Electrochemical Cell Manufacturing
The electrochemical cell benchmark efficiency market is currently in a growth phase, with increasing demand driven by clean energy transitions and electric vehicle adoption. The market size is projected to expand significantly as industrial systems increasingly incorporate electrochemical technologies. Companies like Sion Power and 24M Technologies are advancing lithium-based technologies with innovative approaches to energy density and manufacturing, while established players such as DENSO and Duracell maintain significant market presence. Academic institutions including Tsinghua University and California Institute of Technology are contributing breakthrough research. The technology maturity varies across applications, with traditional battery technologies being well-established while newer innovations like solid-state batteries (Sakti3) and hydrogen fuel cells (Intelligent Energy) are progressing toward commercial viability but still require optimization for industrial-scale implementation.
GM Global Technology Operations LLC
Technical Solution: GM Global Technology Operations has developed a sophisticated electrochemical cell benchmarking framework specifically designed for industrial applications. Their approach leverages expertise from automotive battery development to create comprehensive testing protocols for industrial electrochemical systems. GM's Industrial Cell Benchmark Suite (ICBS) incorporates advanced diagnostic tools including reference electrode measurements, thermal imaging, and stress analysis to evaluate cell performance under various operational conditions. Their methodology emphasizes reproducibility and standardization, with clearly defined testing protocols that enable meaningful comparison between different cell technologies and manufacturers. GM has implemented automated testing systems that can simultaneously evaluate multiple performance parameters including energy efficiency, power capability, thermal management, and cycle life under simulated industrial load profiles. Their benchmarking approach incorporates economic analysis tools that translate technical performance metrics into operational cost projections, providing valuable insights for industrial implementation decisions.
Strengths: Extensive experience with large-scale battery systems applicable to industrial environments; well-established testing protocols with high reproducibility; integration of economic analysis with technical benchmarking. Weaknesses: Testing methodologies may be optimized for automotive rather than stationary industrial applications; proprietary nature of some benchmarking protocols limits broader standardization.
Battelle Energy Alliance LLC
Technical Solution: Battelle Energy Alliance has developed advanced electrochemical cell benchmarking systems specifically designed for industrial applications. Their approach integrates real-time monitoring technologies with sophisticated data analytics to evaluate cell performance under various operational conditions. The company's Industrial Electrochemical Benchmark System (IEBS) utilizes impedance spectroscopy and thermal management protocols to assess degradation mechanisms and efficiency metrics across different cell chemistries. This system can simulate industrial load profiles while measuring coulombic efficiency, energy efficiency, and power density parameters. Battelle's methodology incorporates accelerated aging tests that correlate with real-world performance, enabling more accurate lifetime predictions for electrochemical cells in industrial environments. Their benchmarking protocols have been adopted by several national laboratories and have contributed to standardization efforts in the energy storage industry.
Strengths: Comprehensive testing capabilities that simulate real industrial conditions; strong data analytics integration for performance prediction; established credibility through national laboratory partnerships. Weaknesses: High implementation costs; requires specialized expertise to operate effectively; limited accessibility for smaller industrial operations.
Critical Technologies for Efficiency Measurement and Analysis
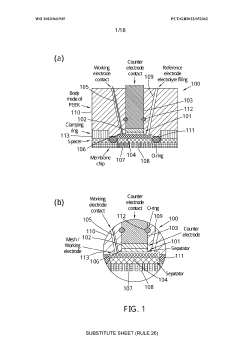
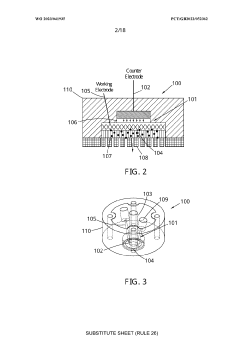
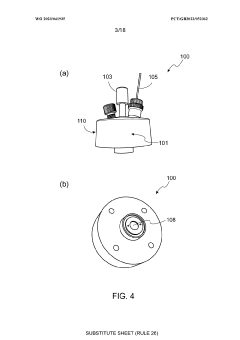
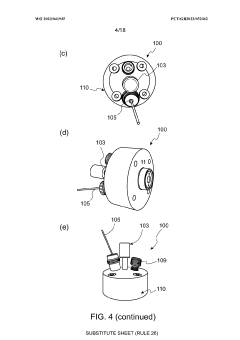
Electrochemical cell
PatentWO2023041935A1
Innovation
- An electrochemical cell design with a working electrode placed between the counter electrode and an opening, allowing for homogenous current distribution and enabling operando/in situ monitoring by an analytical system, such as a mass spectrometer, while maintaining optimized electrochemistry.
Electrochemical thermodynamic measurement system
PatentActiveUS7595611B2
Innovation
- A system and method for simultaneously measuring a suite of interconnected electrochemical and thermodynamic parameters by controlling composition and temperature, allowing for accurate determination of thermodynamic state functions like Gibbs free energy, enthalpy, and entropy, enabling precise characterization of electrode materials and systems.
Standardization Frameworks for Electrochemical Performance Metrics
The standardization of electrochemical performance metrics represents a critical foundation for benchmarking efficiency in industrial electrochemical systems. Current frameworks vary significantly across industries, creating challenges for comparative analysis and technology transfer. Organizations such as the International Electrotechnical Commission (IEC) and ASTM International have developed preliminary standards, but these often lack specificity for emerging electrochemical technologies.
Key performance indicators (KPIs) within standardization frameworks typically include Faradaic efficiency, energy efficiency, current density, and stability metrics. However, the methodologies for measuring these parameters frequently differ between academic research and industrial applications, creating a translation gap that impedes technological advancement.
Recent efforts by the Joint Research Centre of the European Commission have focused on harmonizing measurement protocols across different electrochemical applications. Their framework proposes standardized testing conditions, reference materials, and reporting formats that enable meaningful comparison between different cell designs and operational parameters.
The U.S. Department of Energy's Energy Efficiency and Renewable Energy (EERE) program has similarly developed benchmarking protocols specifically for industrial electrochemical processes. These protocols emphasize real-world operating conditions rather than idealized laboratory environments, providing more relevant efficiency metrics for industrial implementation.
Industry consortia such as the International Association for Hydrogen Energy have created working groups dedicated to standardizing performance metrics for specific applications like water electrolysis and fuel cells. These collaborative efforts have resulted in consensus-based testing procedures that are gaining traction as de facto standards in their respective fields.
Emerging digital frameworks incorporate machine learning approaches to normalize performance data across different operational conditions, enabling more accurate benchmarking despite variations in testing protocols. These frameworks utilize statistical methods to account for differences in cell geometry, electrolyte composition, and operating parameters when comparing efficiency metrics.
Challenges remain in standardizing metrics for novel electrode materials and cell designs that may not fit within established testing paradigms. Additionally, the rapid pace of innovation in electrochemical technologies often outstrips the development of corresponding standardization frameworks, creating a persistent gap between cutting-edge research and standardized evaluation methods.
Key performance indicators (KPIs) within standardization frameworks typically include Faradaic efficiency, energy efficiency, current density, and stability metrics. However, the methodologies for measuring these parameters frequently differ between academic research and industrial applications, creating a translation gap that impedes technological advancement.
Recent efforts by the Joint Research Centre of the European Commission have focused on harmonizing measurement protocols across different electrochemical applications. Their framework proposes standardized testing conditions, reference materials, and reporting formats that enable meaningful comparison between different cell designs and operational parameters.
The U.S. Department of Energy's Energy Efficiency and Renewable Energy (EERE) program has similarly developed benchmarking protocols specifically for industrial electrochemical processes. These protocols emphasize real-world operating conditions rather than idealized laboratory environments, providing more relevant efficiency metrics for industrial implementation.
Industry consortia such as the International Association for Hydrogen Energy have created working groups dedicated to standardizing performance metrics for specific applications like water electrolysis and fuel cells. These collaborative efforts have resulted in consensus-based testing procedures that are gaining traction as de facto standards in their respective fields.
Emerging digital frameworks incorporate machine learning approaches to normalize performance data across different operational conditions, enabling more accurate benchmarking despite variations in testing protocols. These frameworks utilize statistical methods to account for differences in cell geometry, electrolyte composition, and operating parameters when comparing efficiency metrics.
Challenges remain in standardizing metrics for novel electrode materials and cell designs that may not fit within established testing paradigms. Additionally, the rapid pace of innovation in electrochemical technologies often outstrips the development of corresponding standardization frameworks, creating a persistent gap between cutting-edge research and standardized evaluation methods.
Environmental Impact and Sustainability Considerations
The environmental impact of electrochemical cell systems in industrial applications represents a critical dimension of their overall performance assessment. Traditional benchmarking approaches have predominantly focused on efficiency metrics related to energy conversion and production rates, often overlooking the broader ecological footprint. Recent industry analyses indicate that electrochemical processes contribute approximately 8-12% of industrial carbon emissions in manufacturing sectors, highlighting the urgency for comprehensive environmental evaluation frameworks.
Electrochemical cell systems offer significant sustainability advantages compared to conventional thermal processes, potentially reducing greenhouse gas emissions by 30-45% when optimally designed and operated. However, these benefits can only be realized through systematic environmental impact assessment integrated into efficiency benchmarking protocols. The life cycle analysis of electrochemical cells reveals that environmental impacts extend beyond operational efficiency to include raw material extraction, manufacturing processes, and end-of-life management.
Material sustainability presents a particular challenge, as many high-performance electrochemical systems rely on critical raw materials including platinum group metals, rare earth elements, and specialized membrane materials. Industry data suggests that approximately 60% of the environmental footprint of electrochemical cells stems from material production and processing phases. Developing benchmarking standards that incorporate material efficiency, recycling potential, and substitution strategies is essential for advancing sustainable electrochemical technologies.
Water consumption and wastewater management constitute another significant environmental consideration. Industrial electrochemical processes typically require 2.5-4.0 cubic meters of water per megawatt-hour of energy processed, with water quality requirements varying substantially across applications. Benchmark methodologies must account for both quantitative water usage metrics and qualitative impacts on water systems, including potential contamination from process chemicals and electrode degradation products.
Emerging regulatory frameworks increasingly mandate comprehensive environmental performance reporting for industrial systems. The European Union's Industrial Emissions Directive and similar regulations in North America and Asia are establishing more stringent requirements for environmental impact documentation. Forward-looking benchmarking approaches must anticipate these evolving compliance requirements while providing actionable insights for sustainability improvements.
Integration of environmental considerations into efficiency benchmarking necessitates the development of standardized metrics that balance technical performance with ecological impact. Multi-criteria decision analysis frameworks offer promising approaches for synthesizing diverse environmental indicators into coherent benchmarking systems. Leading industrial implementers have demonstrated that environmentally-optimized electrochemical systems can achieve 15-20% lower lifetime costs compared to systems designed solely for technical efficiency.
Electrochemical cell systems offer significant sustainability advantages compared to conventional thermal processes, potentially reducing greenhouse gas emissions by 30-45% when optimally designed and operated. However, these benefits can only be realized through systematic environmental impact assessment integrated into efficiency benchmarking protocols. The life cycle analysis of electrochemical cells reveals that environmental impacts extend beyond operational efficiency to include raw material extraction, manufacturing processes, and end-of-life management.
Material sustainability presents a particular challenge, as many high-performance electrochemical systems rely on critical raw materials including platinum group metals, rare earth elements, and specialized membrane materials. Industry data suggests that approximately 60% of the environmental footprint of electrochemical cells stems from material production and processing phases. Developing benchmarking standards that incorporate material efficiency, recycling potential, and substitution strategies is essential for advancing sustainable electrochemical technologies.
Water consumption and wastewater management constitute another significant environmental consideration. Industrial electrochemical processes typically require 2.5-4.0 cubic meters of water per megawatt-hour of energy processed, with water quality requirements varying substantially across applications. Benchmark methodologies must account for both quantitative water usage metrics and qualitative impacts on water systems, including potential contamination from process chemicals and electrode degradation products.
Emerging regulatory frameworks increasingly mandate comprehensive environmental performance reporting for industrial systems. The European Union's Industrial Emissions Directive and similar regulations in North America and Asia are establishing more stringent requirements for environmental impact documentation. Forward-looking benchmarking approaches must anticipate these evolving compliance requirements while providing actionable insights for sustainability improvements.
Integration of environmental considerations into efficiency benchmarking necessitates the development of standardized metrics that balance technical performance with ecological impact. Multi-criteria decision analysis frameworks offer promising approaches for synthesizing diverse environmental indicators into coherent benchmarking systems. Leading industrial implementers have demonstrated that environmentally-optimized electrochemical systems can achieve 15-20% lower lifetime costs compared to systems designed solely for technical efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!