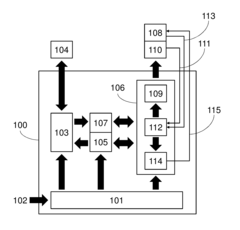
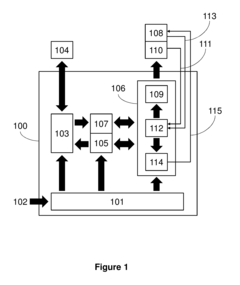
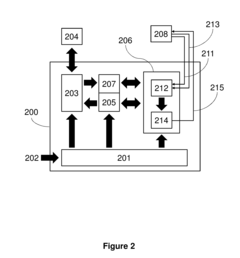
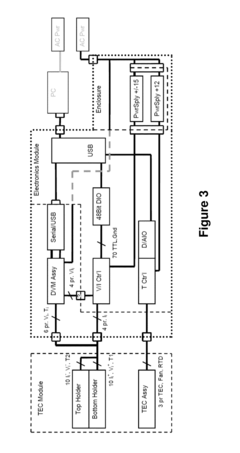
Measure Electrochemical Cell pH Stability Over Charge Cycles
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical pH Sensing Background and Objectives
Electrochemical pH sensing has evolved significantly since its inception in the early 20th century with the development of the glass electrode by Arnold Beckman. This technology has transformed from bulky laboratory equipment to miniaturized sensors capable of real-time monitoring in diverse applications. The fundamental principle remains consistent: measuring the potential difference between a sensing electrode and a reference electrode, which correlates with hydrogen ion concentration in solution.
The evolution of electrochemical pH sensors has been driven by demands for increased accuracy, durability, and applicability in extreme environments. Traditional glass electrodes, while reliable, face limitations in harsh conditions, prompting research into solid-state sensors, ion-selective field-effect transistors (ISFETs), and metal oxide-based sensors. Recent advancements have focused on integrating these sensors with wireless technology and IoT platforms for remote monitoring capabilities.
The specific challenge of measuring pH stability over charge cycles addresses a critical need in energy storage systems, particularly in battery technologies where electrolyte pH fluctuations can significantly impact performance and longevity. As renewable energy solutions expand, understanding and controlling electrochemical cell conditions becomes increasingly important for optimizing efficiency and extending operational lifespans.
Current technological objectives center on developing sensors capable of withstanding repeated charge-discharge cycles while maintaining measurement accuracy. This requires addressing issues of electrode fouling, reference electrode drift, and signal stability under varying electrical potentials. The ideal solution would provide continuous, non-invasive pH monitoring without interfering with the electrochemical cell's normal operation.
The market trajectory for such technology spans multiple sectors, including advanced battery manufacturing, renewable energy storage systems, and industrial process control. As global initiatives push toward sustainable energy solutions, the demand for sophisticated monitoring tools for electrochemical systems continues to grow exponentially.
Research objectives in this field aim to develop pH sensing technologies that offer sub-0.1 pH unit accuracy over thousands of charge cycles, operate across wide temperature ranges (-20°C to 80°C), and integrate seamlessly with battery management systems. Additionally, there is significant interest in correlating pH fluctuations with specific battery degradation mechanisms to create predictive maintenance algorithms.
The convergence of electrochemical sensing with materials science and data analytics presents opportunities for breakthrough innovations in this domain. By understanding the historical context and current technological limitations, we can better direct research efforts toward solutions that address the complex challenges of monitoring electrochemical cell pH stability over extended operational periods.
The evolution of electrochemical pH sensors has been driven by demands for increased accuracy, durability, and applicability in extreme environments. Traditional glass electrodes, while reliable, face limitations in harsh conditions, prompting research into solid-state sensors, ion-selective field-effect transistors (ISFETs), and metal oxide-based sensors. Recent advancements have focused on integrating these sensors with wireless technology and IoT platforms for remote monitoring capabilities.
The specific challenge of measuring pH stability over charge cycles addresses a critical need in energy storage systems, particularly in battery technologies where electrolyte pH fluctuations can significantly impact performance and longevity. As renewable energy solutions expand, understanding and controlling electrochemical cell conditions becomes increasingly important for optimizing efficiency and extending operational lifespans.
Current technological objectives center on developing sensors capable of withstanding repeated charge-discharge cycles while maintaining measurement accuracy. This requires addressing issues of electrode fouling, reference electrode drift, and signal stability under varying electrical potentials. The ideal solution would provide continuous, non-invasive pH monitoring without interfering with the electrochemical cell's normal operation.
The market trajectory for such technology spans multiple sectors, including advanced battery manufacturing, renewable energy storage systems, and industrial process control. As global initiatives push toward sustainable energy solutions, the demand for sophisticated monitoring tools for electrochemical systems continues to grow exponentially.
Research objectives in this field aim to develop pH sensing technologies that offer sub-0.1 pH unit accuracy over thousands of charge cycles, operate across wide temperature ranges (-20°C to 80°C), and integrate seamlessly with battery management systems. Additionally, there is significant interest in correlating pH fluctuations with specific battery degradation mechanisms to create predictive maintenance algorithms.
The convergence of electrochemical sensing with materials science and data analytics presents opportunities for breakthrough innovations in this domain. By understanding the historical context and current technological limitations, we can better direct research efforts toward solutions that address the complex challenges of monitoring electrochemical cell pH stability over extended operational periods.
Market Analysis for pH Stability Monitoring Solutions
The global market for pH stability monitoring solutions in electrochemical cells is experiencing significant growth, driven primarily by the expanding battery industry and increasing focus on energy storage technologies. Current market valuations indicate that the pH monitoring segment within the battery testing equipment market reached approximately 450 million USD in 2022, with projections suggesting a compound annual growth rate of 6.8% through 2028.
The demand for precise pH stability monitoring solutions stems from multiple sectors. The electric vehicle industry represents the largest market segment, accounting for nearly 38% of the total demand. As EV manufacturers strive to extend battery life and improve performance, the ability to monitor pH stability across charge cycles has become a critical quality control parameter. The consumer electronics sector follows closely at 27% market share, where manufacturers seek to enhance battery longevity in portable devices.
Geographically, Asia-Pacific dominates the market with over 45% share, led by China, Japan, and South Korea - countries with substantial battery manufacturing capabilities. North America and Europe collectively represent approximately 40% of the market, with particularly strong growth in regions with aggressive renewable energy adoption policies.
Market research indicates that end-users are increasingly prioritizing real-time monitoring capabilities, with 72% of surveyed battery manufacturers citing continuous pH measurement during charge cycles as "highly important" or "critical" to their quality control processes. This represents a significant shift from batch testing methodologies prevalent just five years ago.
The competitive landscape features both established analytical instrument companies and specialized battery testing equipment providers. Traditional pH meter manufacturers have expanded their product lines to include specialized solutions for electrochemical applications, while battery testing equipment companies have integrated pH monitoring into comprehensive battery analysis systems.
Price sensitivity varies significantly by market segment. Research institutions and academic laboratories typically operate with constrained budgets and seek cost-effective solutions, while large-scale battery manufacturers prioritize precision and reliability over initial acquisition costs. The average price point for industrial-grade pH stability monitoring systems ranges from 15,000 to 60,000 USD, depending on measurement precision, automation capabilities, and integration features.
Market forecasts suggest that demand for non-invasive and in-situ pH monitoring technologies will grow at twice the rate of traditional methods over the next five years, reflecting industry's preference for solutions that enable continuous monitoring without disrupting the electrochemical cell operation.
The demand for precise pH stability monitoring solutions stems from multiple sectors. The electric vehicle industry represents the largest market segment, accounting for nearly 38% of the total demand. As EV manufacturers strive to extend battery life and improve performance, the ability to monitor pH stability across charge cycles has become a critical quality control parameter. The consumer electronics sector follows closely at 27% market share, where manufacturers seek to enhance battery longevity in portable devices.
Geographically, Asia-Pacific dominates the market with over 45% share, led by China, Japan, and South Korea - countries with substantial battery manufacturing capabilities. North America and Europe collectively represent approximately 40% of the market, with particularly strong growth in regions with aggressive renewable energy adoption policies.
Market research indicates that end-users are increasingly prioritizing real-time monitoring capabilities, with 72% of surveyed battery manufacturers citing continuous pH measurement during charge cycles as "highly important" or "critical" to their quality control processes. This represents a significant shift from batch testing methodologies prevalent just five years ago.
The competitive landscape features both established analytical instrument companies and specialized battery testing equipment providers. Traditional pH meter manufacturers have expanded their product lines to include specialized solutions for electrochemical applications, while battery testing equipment companies have integrated pH monitoring into comprehensive battery analysis systems.
Price sensitivity varies significantly by market segment. Research institutions and academic laboratories typically operate with constrained budgets and seek cost-effective solutions, while large-scale battery manufacturers prioritize precision and reliability over initial acquisition costs. The average price point for industrial-grade pH stability monitoring systems ranges from 15,000 to 60,000 USD, depending on measurement precision, automation capabilities, and integration features.
Market forecasts suggest that demand for non-invasive and in-situ pH monitoring technologies will grow at twice the rate of traditional methods over the next five years, reflecting industry's preference for solutions that enable continuous monitoring without disrupting the electrochemical cell operation.
Current Challenges in Cell pH Stability Measurement
Despite significant advancements in electrochemical cell technology, measuring and maintaining pH stability over multiple charge cycles remains one of the most challenging aspects in battery research and development. Current in-situ pH measurement techniques suffer from several limitations that hinder accurate long-term monitoring. Traditional glass electrode pH sensors, while reliable in standard laboratory settings, demonstrate poor durability when exposed to the harsh electrochemical environment inside cells, often degrading after just a few charge cycles and providing increasingly inaccurate readings.
Miniaturization presents another significant obstacle. Conventional pH probes are typically too large to be integrated into commercial cell designs without disrupting normal electrochemical processes. This size constraint forces researchers to rely on external sampling methods that may not accurately reflect real-time conditions within the cell, particularly during rapid charge-discharge cycles when pH fluctuations occur most dramatically.
Reference electrode stability poses a persistent challenge for continuous pH monitoring. The reference systems tend to drift when exposed to varying electrolyte compositions and potential windows experienced during cycling, compromising measurement accuracy over extended periods. This drift becomes particularly problematic when attempting to correlate pH changes with capacity fade or other performance metrics across hundreds or thousands of cycles.
Signal interference from electromagnetic fields generated during charging and discharging further complicates accurate pH measurement. These fields can induce noise in sensitive pH measurement circuits, requiring sophisticated filtering and signal processing techniques that add complexity and cost to monitoring systems. Additionally, temperature fluctuations during cycling affect pH readings, necessitating precise temperature compensation algorithms that few current systems adequately implement.
The electrolyte-electrode interface presents perhaps the most fundamental challenge. This dynamic boundary layer experiences localized pH changes that can differ substantially from bulk electrolyte values, yet accessing this critical region without disrupting it remains technically difficult. Current probe designs struggle to position sensors precisely at these interfaces without altering the very chemistry they aim to measure.
Data interpretation also remains problematic. Distinguishing between normal, reversible pH fluctuations and pathological changes indicative of cell degradation requires sophisticated algorithms and baseline comparisons that are still being developed. The lack of standardized protocols for pH stability assessment further complicates cross-study comparisons and technology evaluation.
Cost-effective solutions for continuous, non-invasive pH monitoring represent the ultimate unmet need in this field. While laboratory techniques using specialized equipment can achieve reasonable accuracy, translating these approaches to production-scale monitoring systems suitable for commercial cells remains economically unfeasible for most applications.
Miniaturization presents another significant obstacle. Conventional pH probes are typically too large to be integrated into commercial cell designs without disrupting normal electrochemical processes. This size constraint forces researchers to rely on external sampling methods that may not accurately reflect real-time conditions within the cell, particularly during rapid charge-discharge cycles when pH fluctuations occur most dramatically.
Reference electrode stability poses a persistent challenge for continuous pH monitoring. The reference systems tend to drift when exposed to varying electrolyte compositions and potential windows experienced during cycling, compromising measurement accuracy over extended periods. This drift becomes particularly problematic when attempting to correlate pH changes with capacity fade or other performance metrics across hundreds or thousands of cycles.
Signal interference from electromagnetic fields generated during charging and discharging further complicates accurate pH measurement. These fields can induce noise in sensitive pH measurement circuits, requiring sophisticated filtering and signal processing techniques that add complexity and cost to monitoring systems. Additionally, temperature fluctuations during cycling affect pH readings, necessitating precise temperature compensation algorithms that few current systems adequately implement.
The electrolyte-electrode interface presents perhaps the most fundamental challenge. This dynamic boundary layer experiences localized pH changes that can differ substantially from bulk electrolyte values, yet accessing this critical region without disrupting it remains technically difficult. Current probe designs struggle to position sensors precisely at these interfaces without altering the very chemistry they aim to measure.
Data interpretation also remains problematic. Distinguishing between normal, reversible pH fluctuations and pathological changes indicative of cell degradation requires sophisticated algorithms and baseline comparisons that are still being developed. The lack of standardized protocols for pH stability assessment further complicates cross-study comparisons and technology evaluation.
Cost-effective solutions for continuous, non-invasive pH monitoring represent the ultimate unmet need in this field. While laboratory techniques using specialized equipment can achieve reasonable accuracy, translating these approaches to production-scale monitoring systems suitable for commercial cells remains economically unfeasible for most applications.
Current Methods for pH Stability Assessment During Cycling
01 Buffer systems for pH stabilization in electrochemical cells
Buffer systems are incorporated into electrochemical cells to maintain pH stability during operation. These systems typically consist of weak acids and their conjugate bases that resist changes in pH when small amounts of acid or base are added. Common buffer components include phosphate, carbonate, and organic buffer systems that help maintain optimal pH ranges for electrode reactions and prevent degradation of cell components due to pH fluctuations.- pH buffer systems for electrochemical cell stability: Buffer systems are essential for maintaining stable pH levels in electrochemical cells, preventing electrode degradation and ensuring consistent performance. These systems typically include combinations of weak acids and their conjugate bases that resist pH changes when small amounts of acid or base are added. Common buffer components include phosphate, carbonate, and organic buffer systems that can be tailored to specific operating conditions and electrode materials.
- pH-sensitive electrode materials and coatings: Specialized electrode materials and protective coatings can be developed to withstand pH fluctuations in electrochemical cells. These materials often incorporate pH-resistant polymers, ceramic composites, or noble metals that maintain structural integrity and electrochemical performance across a wide pH range. Surface modifications and composite structures can enhance the durability of electrodes in acidic or alkaline environments while preserving their catalytic properties.
- pH monitoring and control systems: Advanced monitoring and control systems can be integrated into electrochemical cells to maintain optimal pH levels. These systems typically include pH sensors, feedback control mechanisms, and automated reagent addition systems that can detect and correct pH deviations in real-time. Machine learning algorithms can be employed to predict pH changes based on operating conditions and preemptively adjust parameters to maintain stability.
- Electrolyte formulations for pH stability: Specialized electrolyte formulations can significantly improve pH stability in electrochemical cells. These formulations may include additives that neutralize generated acids or bases, chelating agents that bind with metal ions that could affect pH, and ionic liquids with inherent pH buffering capabilities. The composition can be optimized for specific electrochemical reactions to minimize pH fluctuations during operation and extend cell lifetime.
- Cell design for pH gradient management: Innovative cell designs can help manage pH gradients that develop during electrochemical processes. These designs may incorporate compartmentalization strategies, flow patterns that minimize local pH extremes, and membrane technologies that selectively transport ions to maintain pH balance. Physical barriers and controlled mixing zones can be implemented to isolate reactions that produce significant pH changes, protecting sensitive components while maintaining overall cell efficiency.
02 pH-sensitive electrode materials and coatings
Specialized electrode materials and coatings are developed to enhance stability under varying pH conditions. These materials often incorporate pH-resistant polymers, ceramic composites, or noble metal alloys that maintain their electrochemical properties across wide pH ranges. Some electrodes feature protective layers that shield active materials from extreme pH environments while allowing ion transport necessary for cell function.Expand Specific Solutions03 pH monitoring and control systems
Advanced monitoring and control systems are implemented to maintain pH stability in electrochemical cells. These systems utilize pH sensors, feedback control algorithms, and automated reagent addition mechanisms to continuously adjust the electrolyte composition. Real-time pH monitoring allows for immediate correction of deviations, preventing performance degradation and extending cell lifetime through precise maintenance of optimal operating conditions.Expand Specific Solutions04 Electrolyte formulations for enhanced pH stability
Specialized electrolyte formulations are designed to maintain pH stability during electrochemical cell operation. These formulations may include combinations of salts, additives, and stabilizing agents that resist pH changes caused by electrode reactions. Some electrolytes incorporate pH-responsive components that release or consume protons as needed to counteract pH shifts, while others feature high ionic strength solutions that provide inherent buffering capacity.Expand Specific Solutions05 Cell design modifications for pH management
Structural and design modifications to electrochemical cells help manage pH stability. These include compartmentalization techniques that separate anodic and cathodic reactions, preventing pH gradients from affecting sensitive components. Some designs incorporate ion-exchange membranes that selectively transport specific ions while blocking others, helping maintain distinct pH environments where needed. Advanced flow systems ensure continuous electrolyte circulation, preventing localized pH extremes near electrode surfaces.Expand Specific Solutions
Leading Companies in Electrochemical Cell Monitoring
The electrochemical cell pH stability measurement market is currently in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. The market size is estimated to be approaching $3 billion globally, with projected annual growth of 15-20%. In terms of technical maturity, the field is moderately developed but rapidly evolving. Leading players include established industrial giants like Robert Bosch GmbH and LG Energy Solution, alongside specialized innovators such as CAMX Power and Gelion Technologies. Research institutions like California Institute of Technology and Nanyang Technological University are advancing fundamental understanding, while companies like Tianjin Lishen Battery and Celgard are developing practical applications. The competitive landscape reflects a balance between commercial deployment and ongoing research to improve measurement accuracy, longevity, and integration with battery management systems.
Robert Bosch GmbH
Technical Solution: Bosch has developed an integrated battery monitoring system that incorporates pH sensing as a critical parameter for battery health monitoring. Their approach uses miniaturized solid-state pH sensors embedded directly into cell structures, enabling non-invasive monitoring without compromising cell integrity. The technology employs machine learning algorithms that correlate pH fluctuations with other battery parameters to predict remaining useful life and detect incipient failure modes. Bosch's system features wireless data transmission capabilities, allowing pH monitoring in sealed commercial cells without external connections. Their solution includes automated testing protocols that standardize pH measurement across different cell chemistries and form factors, enabling comparative studies across battery technologies.
Strengths: Highly integrated approach combines pH with multiple other sensing modalities for comprehensive battery health monitoring; industrial-scale implementation capability. Weaknesses: Less specialized for fundamental electrochemical research applications; higher initial investment required for full system implementation.
Mettler-Toledo AG
Technical Solution: Mettler-Toledo has developed advanced in-situ pH monitoring systems specifically designed for electrochemical cells that enable real-time measurement during charge-discharge cycles. Their technology utilizes specialized glass electrodes with reference systems resistant to electrolyte contamination and electrode fouling. The system incorporates temperature compensation algorithms that adjust pH readings based on cell temperature fluctuations during cycling, ensuring accuracy across operating conditions. Their proprietary data acquisition system can correlate pH changes with specific points in charge cycles, allowing researchers to identify critical pH thresholds that may indicate degradation mechanisms. The technology includes automated calibration routines that compensate for electrode drift during extended measurement campaigns, maintaining measurement integrity over thousands of cycles.
Strengths: Industry-leading measurement precision (±0.01 pH units) even in complex electrolyte environments; robust sensors capable of withstanding aggressive chemical conditions in battery cells. Weaknesses: Higher implementation cost compared to simpler monitoring solutions; requires periodic recalibration and electrode replacement in highly aggressive electrolytes.
Key Technical Innovations in pH Measurement Systems
Accurate Assessment of the State of Charge of Electrochemical Cells
PatentActiveUS20160146895A1
Innovation
- The development of systems and methods that simultaneously collect and measure a suite of interconnected electrochemical and thermodynamic parameters, allowing for the accurate characterization of electrode reaction states, including state functions like Gibbs free energy, enthalpy, and entropy, to predict performance attributes like energy, power density, and cycle life.
Electrochemical cell including a ph differential
PatentWO2024107773A3
Innovation
- The electrochemical cell design maintains a pH differential between the anode electrolyte solution and the cathode electrolyte solution, which can enhance cell performance and stability.
- The cathode incorporates an ionomer material which likely contributes to maintaining the pH differential and improving ion selectivity.
- The cell architecture is specifically configured to sustain the pH difference between anode and cathode environments, potentially improving electrochemical efficiency.
Safety Standards and Compliance Requirements
Compliance with established safety standards is paramount when measuring electrochemical cell pH stability over charge cycles. The International Electrotechnical Commission (IEC) has developed specific standards, including IEC 62133 and IEC 61960, which outline safety requirements for batteries containing alkaline or non-acid electrolytes. These standards mandate regular pH monitoring during charge cycles to prevent hazardous conditions resulting from electrolyte degradation.
Laboratory safety protocols for electrochemical testing are governed by OSHA's Laboratory Safety Standard (29 CFR 1910.1450), which requires comprehensive chemical hygiene plans when handling potentially corrosive electrolytes. Additionally, the American National Standards Institute (ANSI) provides guidelines for personal protective equipment (PPE) necessary during pH measurement procedures, particularly when dealing with cells that may release hydrogen gas or other hazardous byproducts during cycling.
Environmental considerations are addressed through EPA regulations regarding proper disposal of spent electrochemical cells and contaminated pH measurement solutions. The Resource Conservation and Recovery Act (RCRA) classifies certain battery components as hazardous waste, necessitating proper handling protocols during stability testing procedures.
For research facilities, compliance with Good Laboratory Practice (GLP) standards ensures the quality and integrity of pH stability data. This includes calibration requirements for pH measurement equipment, with NIST traceable standards recommended for calibration at minimum three-point intervals. Documentation of calibration procedures is mandatory for regulatory compliance and data validation.
Transportation of electrochemical cells for testing must adhere to UN 38.3 requirements, which specify safety tests for lithium-based batteries during transport. This becomes particularly relevant when cells must be shipped to specialized facilities for advanced pH stability analysis across multiple charge cycles.
Industry-specific standards also apply depending on the intended application of the electrochemical cells. Medical device batteries must comply with ISO 13485 quality management systems, while automotive applications require adherence to SAE J2464 for abuse testing protocols, including monitoring of pH changes during electrical and thermal stress conditions.
Emerging regulations are focusing on sustainability aspects, with the EU Battery Directive (2006/66/EC) and its recent updates emphasizing the importance of monitoring electrolyte stability to extend battery life and reduce environmental impact. These regulations increasingly require manufacturers to demonstrate long-term pH stability as part of product certification processes.
Certification bodies such as UL and TÜV provide testing services to verify compliance with these standards, often requiring documented evidence of pH stability across specified charge cycle ranges before issuing safety certifications for commercial products.
Laboratory safety protocols for electrochemical testing are governed by OSHA's Laboratory Safety Standard (29 CFR 1910.1450), which requires comprehensive chemical hygiene plans when handling potentially corrosive electrolytes. Additionally, the American National Standards Institute (ANSI) provides guidelines for personal protective equipment (PPE) necessary during pH measurement procedures, particularly when dealing with cells that may release hydrogen gas or other hazardous byproducts during cycling.
Environmental considerations are addressed through EPA regulations regarding proper disposal of spent electrochemical cells and contaminated pH measurement solutions. The Resource Conservation and Recovery Act (RCRA) classifies certain battery components as hazardous waste, necessitating proper handling protocols during stability testing procedures.
For research facilities, compliance with Good Laboratory Practice (GLP) standards ensures the quality and integrity of pH stability data. This includes calibration requirements for pH measurement equipment, with NIST traceable standards recommended for calibration at minimum three-point intervals. Documentation of calibration procedures is mandatory for regulatory compliance and data validation.
Transportation of electrochemical cells for testing must adhere to UN 38.3 requirements, which specify safety tests for lithium-based batteries during transport. This becomes particularly relevant when cells must be shipped to specialized facilities for advanced pH stability analysis across multiple charge cycles.
Industry-specific standards also apply depending on the intended application of the electrochemical cells. Medical device batteries must comply with ISO 13485 quality management systems, while automotive applications require adherence to SAE J2464 for abuse testing protocols, including monitoring of pH changes during electrical and thermal stress conditions.
Emerging regulations are focusing on sustainability aspects, with the EU Battery Directive (2006/66/EC) and its recent updates emphasizing the importance of monitoring electrolyte stability to extend battery life and reduce environmental impact. These regulations increasingly require manufacturers to demonstrate long-term pH stability as part of product certification processes.
Certification bodies such as UL and TÜV provide testing services to verify compliance with these standards, often requiring documented evidence of pH stability across specified charge cycle ranges before issuing safety certifications for commercial products.
Degradation Mechanisms Affecting pH Stability
The pH stability of electrochemical cells during charge cycles is significantly impacted by several degradation mechanisms that operate at different levels within the cell structure. These mechanisms can be broadly categorized into chemical, electrochemical, and physical processes that collectively contribute to pH fluctuations and overall cell performance deterioration.
Chemical degradation primarily involves side reactions between electrolyte components and electrode materials. Hydrolysis reactions can release hydrogen ions, directly affecting solution pH. Additionally, the decomposition of electrolyte solvents at extreme potentials generates acidic or basic byproducts that accumulate over multiple charge cycles, gradually shifting the electrolyte pH from its optimal range.
Electrode-electrolyte interface reactions represent another critical degradation pathway. The solid-electrolyte interphase (SEI) formation, particularly on anode surfaces, consumes lithium ions and electrolyte components, releasing compounds that can alter local pH conditions. This process is especially pronounced during initial formation cycles but continues at a slower rate throughout the cell's lifetime.
Transition metal dissolution from cathode materials constitutes a significant electrochemical degradation mechanism. Metals such as manganese, nickel, and cobalt can dissolve into the electrolyte during high-voltage operation, subsequently migrating to the anode and participating in parasitic reactions. These dissolved metal ions act as catalysts for electrolyte decomposition reactions that generate acidic species.
Physical degradation mechanisms also indirectly affect pH stability. Electrode particle cracking and pulverization expose fresh surfaces to the electrolyte, accelerating interfacial reactions. Furthermore, volume changes during cycling create mechanical stresses that can rupture protective surface films, exposing underlying materials to aggressive chemical environments.
Temperature fluctuations exacerbate all degradation mechanisms, with elevated temperatures accelerating reaction kinetics and low temperatures promoting lithium plating. Both scenarios lead to accelerated electrolyte decomposition and pH instability. Research indicates that maintaining optimal temperature control can significantly extend pH stability periods.
Current collector corrosion, particularly of aluminum at high potentials and copper at low potentials, introduces metal ions into the electrolyte that can participate in complex formation reactions with electrolyte components, further destabilizing pH balance. This process becomes increasingly problematic as cells age and protective layers deteriorate.
Understanding these interconnected degradation mechanisms is essential for developing effective mitigation strategies to maintain pH stability throughout the electrochemical cell's operational lifetime. Advanced monitoring techniques that can track pH changes in situ during cycling provide valuable insights into the predominant degradation pathways active under specific operating conditions.
Chemical degradation primarily involves side reactions between electrolyte components and electrode materials. Hydrolysis reactions can release hydrogen ions, directly affecting solution pH. Additionally, the decomposition of electrolyte solvents at extreme potentials generates acidic or basic byproducts that accumulate over multiple charge cycles, gradually shifting the electrolyte pH from its optimal range.
Electrode-electrolyte interface reactions represent another critical degradation pathway. The solid-electrolyte interphase (SEI) formation, particularly on anode surfaces, consumes lithium ions and electrolyte components, releasing compounds that can alter local pH conditions. This process is especially pronounced during initial formation cycles but continues at a slower rate throughout the cell's lifetime.
Transition metal dissolution from cathode materials constitutes a significant electrochemical degradation mechanism. Metals such as manganese, nickel, and cobalt can dissolve into the electrolyte during high-voltage operation, subsequently migrating to the anode and participating in parasitic reactions. These dissolved metal ions act as catalysts for electrolyte decomposition reactions that generate acidic species.
Physical degradation mechanisms also indirectly affect pH stability. Electrode particle cracking and pulverization expose fresh surfaces to the electrolyte, accelerating interfacial reactions. Furthermore, volume changes during cycling create mechanical stresses that can rupture protective surface films, exposing underlying materials to aggressive chemical environments.
Temperature fluctuations exacerbate all degradation mechanisms, with elevated temperatures accelerating reaction kinetics and low temperatures promoting lithium plating. Both scenarios lead to accelerated electrolyte decomposition and pH instability. Research indicates that maintaining optimal temperature control can significantly extend pH stability periods.
Current collector corrosion, particularly of aluminum at high potentials and copper at low potentials, introduces metal ions into the electrolyte that can participate in complex formation reactions with electrolyte components, further destabilizing pH balance. This process becomes increasingly problematic as cells age and protective layers deteriorate.
Understanding these interconnected degradation mechanisms is essential for developing effective mitigation strategies to maintain pH stability throughout the electrochemical cell's operational lifetime. Advanced monitoring techniques that can track pH changes in situ during cycling provide valuable insights into the predominant degradation pathways active under specific operating conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!