Electrochemical Cell Performance in Variable Atmospheric Pressure
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Technology Background and Objectives
Electrochemical cells have evolved significantly since their inception in the late 18th century with Alessandro Volta's pioneering work. These devices, which convert chemical energy into electrical energy through redox reactions, have become fundamental components in numerous applications ranging from portable electronics to large-scale energy storage systems. The evolution of electrochemical cell technology has been marked by continuous improvements in energy density, cycle life, safety features, and cost-effectiveness.
The performance of electrochemical cells under variable atmospheric pressure conditions represents a critical yet understudied area of research. Historically, most electrochemical cell designs have been optimized for operation at standard atmospheric pressure (101.3 kPa), with limited consideration for performance variations in non-standard pressure environments. This oversight has become increasingly problematic as applications expand to include high-altitude operations, aerospace systems, deep-sea equipment, and specialized industrial processes where pressure conditions deviate significantly from standard atmospheric levels.
Recent technological trends indicate growing interest in developing pressure-adaptive electrochemical systems. The miniaturization of sensors and control systems has enabled more sophisticated monitoring of cell performance under variable conditions. Simultaneously, advances in materials science have introduced novel electrode and electrolyte compositions that demonstrate enhanced stability across pressure gradients.
The primary technical objectives in this field include developing comprehensive models that accurately predict electrochemical behavior under variable pressure conditions, identifying pressure-resistant materials and designs that maintain consistent performance across wide pressure ranges, and creating adaptive control systems that can optimize cell operation in real-time as atmospheric conditions change.
Understanding the fundamental mechanisms by which pressure affects electrochemical reactions is essential. Pressure variations can influence reaction kinetics, mass transport phenomena, phase equilibria of electrolytes, and mechanical integrity of cell components. These effects manifest differently across various cell chemistries, with some technologies demonstrating greater sensitivity to pressure fluctuations than others.
The trajectory of research in this domain aims to establish standardized testing protocols for evaluating cell performance under variable pressure conditions, develop pressure-compensating cell designs that maintain consistent output regardless of environmental conditions, and create predictive algorithms that can anticipate performance changes based on pressure forecasts.
As applications for electrochemical cells continue to diversify into more extreme environments, from high-altitude drones to deep-sea exploration vehicles, the ability to maintain reliable performance across pressure gradients will become an increasingly valuable technological capability, driving innovation in cell design, materials selection, and system integration strategies.
The performance of electrochemical cells under variable atmospheric pressure conditions represents a critical yet understudied area of research. Historically, most electrochemical cell designs have been optimized for operation at standard atmospheric pressure (101.3 kPa), with limited consideration for performance variations in non-standard pressure environments. This oversight has become increasingly problematic as applications expand to include high-altitude operations, aerospace systems, deep-sea equipment, and specialized industrial processes where pressure conditions deviate significantly from standard atmospheric levels.
Recent technological trends indicate growing interest in developing pressure-adaptive electrochemical systems. The miniaturization of sensors and control systems has enabled more sophisticated monitoring of cell performance under variable conditions. Simultaneously, advances in materials science have introduced novel electrode and electrolyte compositions that demonstrate enhanced stability across pressure gradients.
The primary technical objectives in this field include developing comprehensive models that accurately predict electrochemical behavior under variable pressure conditions, identifying pressure-resistant materials and designs that maintain consistent performance across wide pressure ranges, and creating adaptive control systems that can optimize cell operation in real-time as atmospheric conditions change.
Understanding the fundamental mechanisms by which pressure affects electrochemical reactions is essential. Pressure variations can influence reaction kinetics, mass transport phenomena, phase equilibria of electrolytes, and mechanical integrity of cell components. These effects manifest differently across various cell chemistries, with some technologies demonstrating greater sensitivity to pressure fluctuations than others.
The trajectory of research in this domain aims to establish standardized testing protocols for evaluating cell performance under variable pressure conditions, develop pressure-compensating cell designs that maintain consistent output regardless of environmental conditions, and create predictive algorithms that can anticipate performance changes based on pressure forecasts.
As applications for electrochemical cells continue to diversify into more extreme environments, from high-altitude drones to deep-sea exploration vehicles, the ability to maintain reliable performance across pressure gradients will become an increasingly valuable technological capability, driving innovation in cell design, materials selection, and system integration strategies.
Market Analysis for Pressure-Resistant Electrochemical Cells
The global market for pressure-resistant electrochemical cells is experiencing significant growth, driven by expanding applications in deep-sea exploration, aerospace, and extreme industrial environments. Current market valuations indicate the specialized electrochemical cell sector reached approximately 3.2 billion USD in 2022, with pressure-resistant variants constituting about 420 million USD of this total. Industry forecasts project a compound annual growth rate of 7.8% through 2028, outpacing the broader battery market's 5.3% growth rate.
Demand is particularly strong in the underwater vehicle segment, where pressure-resistant cells are essential components in autonomous underwater vehicles (AUVs) and remotely operated vehicles (ROVs). This subsector alone accounts for 31% of pressure-resistant cell applications, with annual procurement increasing by 12% year-over-year since 2020.
The aerospace and defense sectors represent another significant market, comprising approximately 27% of total demand. These industries require cells capable of maintaining consistent performance across rapid pressure changes from sea level to high altitude. Military applications, particularly in submarine and aircraft systems, demand cells that can operate reliably under extreme pressure variations while maintaining safety standards.
Industrial applications in deep mining, offshore oil and gas, and high-pressure manufacturing processes constitute about 22% of the market. These sectors value long-term reliability under sustained pressure conditions, with particular emphasis on safety features that prevent catastrophic failure in confined spaces.
Geographically, North America leads market consumption at 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the fastest growth is occurring in the Asia-Pacific region, particularly in China, Japan, and South Korea, where investments in deep-sea exploration and underwater infrastructure projects are accelerating demand at 14% annually.
Consumer price sensitivity varies significantly by application. Defense and aerospace customers demonstrate low price elasticity, prioritizing performance and reliability over cost considerations. Conversely, commercial underwater vehicle manufacturers show moderate price sensitivity, seeking optimal performance-to-cost ratios as they scale operations.
Market barriers include stringent certification requirements, particularly for cells used in human-occupied vehicles and critical infrastructure. Development cycles typically span 18-24 months, with certification processes adding another 6-12 months before commercial deployment. These extended timelines create significant barriers to market entry for new competitors.
The competitive landscape features specialized manufacturers rather than mainstream battery producers, with most market leaders focusing exclusively on niche applications requiring pressure resistance. This specialization has resulted in a fragmented market with numerous small to medium enterprises holding proprietary technologies and serving specific application niches.
Demand is particularly strong in the underwater vehicle segment, where pressure-resistant cells are essential components in autonomous underwater vehicles (AUVs) and remotely operated vehicles (ROVs). This subsector alone accounts for 31% of pressure-resistant cell applications, with annual procurement increasing by 12% year-over-year since 2020.
The aerospace and defense sectors represent another significant market, comprising approximately 27% of total demand. These industries require cells capable of maintaining consistent performance across rapid pressure changes from sea level to high altitude. Military applications, particularly in submarine and aircraft systems, demand cells that can operate reliably under extreme pressure variations while maintaining safety standards.
Industrial applications in deep mining, offshore oil and gas, and high-pressure manufacturing processes constitute about 22% of the market. These sectors value long-term reliability under sustained pressure conditions, with particular emphasis on safety features that prevent catastrophic failure in confined spaces.
Geographically, North America leads market consumption at 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the fastest growth is occurring in the Asia-Pacific region, particularly in China, Japan, and South Korea, where investments in deep-sea exploration and underwater infrastructure projects are accelerating demand at 14% annually.
Consumer price sensitivity varies significantly by application. Defense and aerospace customers demonstrate low price elasticity, prioritizing performance and reliability over cost considerations. Conversely, commercial underwater vehicle manufacturers show moderate price sensitivity, seeking optimal performance-to-cost ratios as they scale operations.
Market barriers include stringent certification requirements, particularly for cells used in human-occupied vehicles and critical infrastructure. Development cycles typically span 18-24 months, with certification processes adding another 6-12 months before commercial deployment. These extended timelines create significant barriers to market entry for new competitors.
The competitive landscape features specialized manufacturers rather than mainstream battery producers, with most market leaders focusing exclusively on niche applications requiring pressure resistance. This specialization has resulted in a fragmented market with numerous small to medium enterprises holding proprietary technologies and serving specific application niches.
Current Challenges in Variable Pressure Environments
Electrochemical cells operating in variable atmospheric pressure environments face significant technical challenges that impact their performance, reliability, and commercial viability. The most fundamental challenge stems from the direct relationship between pressure and electrochemical reaction kinetics. As pressure decreases, the partial pressure of reactive gases (particularly oxygen in fuel cells and metal-air batteries) diminishes, leading to concentration polarization and reduced current density. This effect becomes particularly pronounced at high altitude or in aerospace applications where pressure can drop to a fraction of sea-level conditions.
Material stability presents another critical challenge in variable pressure environments. Electrode materials and catalysts designed for standard atmospheric conditions often exhibit accelerated degradation when subjected to pressure fluctuations. This degradation manifests as catalyst particle agglomeration, support corrosion, and membrane dehydration, all of which significantly reduce cell lifespan and performance consistency. Research indicates that pressure cycling can be more damaging than static low-pressure operation due to the mechanical stresses induced during transitions.
Thermal management becomes increasingly complex in variable pressure environments. Lower atmospheric pressure reduces the heat transfer coefficient of air, compromising cooling efficiency. This challenge is compounded by the fact that many electrochemical systems generate more heat at lower pressures as they attempt to maintain power output, creating a problematic feedback loop. Current cooling technologies designed for standard pressure conditions often prove inadequate in these scenarios.
Sealing and containment systems face unique challenges in variable pressure applications. Traditional sealing materials may experience differential expansion or contraction during pressure changes, leading to gas leakage or electrolyte loss. This is particularly problematic for liquid electrolyte systems where pressure differentials can force fluids through microscopic pathways that would remain sealed under constant pressure conditions.
Control systems for electrochemical cells typically rely on algorithms and parameters optimized for standard pressure conditions. When operating in variable pressure environments, these systems struggle to maintain optimal operating points as the fundamental electrochemical relationships shift with pressure changes. Current sensor technologies also demonstrate reduced accuracy and increased drift when subjected to pressure variations, complicating real-time performance monitoring and adaptive control.
Water management represents a significant challenge, particularly for proton exchange membrane systems. Lower atmospheric pressure reduces the boiling point of water, accelerating evaporation from membranes and potentially causing dry-out conditions. Conversely, in closed systems, pressure fluctuations can lead to condensation in undesired locations, creating short circuits or blocking gas diffusion pathways.
Material stability presents another critical challenge in variable pressure environments. Electrode materials and catalysts designed for standard atmospheric conditions often exhibit accelerated degradation when subjected to pressure fluctuations. This degradation manifests as catalyst particle agglomeration, support corrosion, and membrane dehydration, all of which significantly reduce cell lifespan and performance consistency. Research indicates that pressure cycling can be more damaging than static low-pressure operation due to the mechanical stresses induced during transitions.
Thermal management becomes increasingly complex in variable pressure environments. Lower atmospheric pressure reduces the heat transfer coefficient of air, compromising cooling efficiency. This challenge is compounded by the fact that many electrochemical systems generate more heat at lower pressures as they attempt to maintain power output, creating a problematic feedback loop. Current cooling technologies designed for standard pressure conditions often prove inadequate in these scenarios.
Sealing and containment systems face unique challenges in variable pressure applications. Traditional sealing materials may experience differential expansion or contraction during pressure changes, leading to gas leakage or electrolyte loss. This is particularly problematic for liquid electrolyte systems where pressure differentials can force fluids through microscopic pathways that would remain sealed under constant pressure conditions.
Control systems for electrochemical cells typically rely on algorithms and parameters optimized for standard pressure conditions. When operating in variable pressure environments, these systems struggle to maintain optimal operating points as the fundamental electrochemical relationships shift with pressure changes. Current sensor technologies also demonstrate reduced accuracy and increased drift when subjected to pressure variations, complicating real-time performance monitoring and adaptive control.
Water management represents a significant challenge, particularly for proton exchange membrane systems. Lower atmospheric pressure reduces the boiling point of water, accelerating evaporation from membranes and potentially causing dry-out conditions. Conversely, in closed systems, pressure fluctuations can lead to condensation in undesired locations, creating short circuits or blocking gas diffusion pathways.
Current Solutions for Atmospheric Pressure Variation
01 Electrode material optimization for enhanced performance
The selection and optimization of electrode materials significantly impact electrochemical cell performance. Advanced materials such as novel alloys, composites, and nanostructured materials can improve electron transfer rates, increase active surface area, and enhance overall cell efficiency. These optimized electrode materials demonstrate improved conductivity, stability, and electrochemical activity, leading to higher energy density and longer cycle life in various electrochemical cell applications.- Electrode materials and composition optimization: The selection and optimization of electrode materials significantly impact electrochemical cell performance. Advanced materials such as novel alloys, composites, and nanostructured materials can enhance conductivity, stability, and energy density. Modifications to electrode composition, including dopants and additives, can improve reaction kinetics and cycling stability, leading to better overall cell performance and longevity.
- Electrolyte formulation and optimization: The composition and properties of electrolytes play a crucial role in electrochemical cell performance. Optimized electrolyte formulations can enhance ionic conductivity, electrochemical stability, and interface properties. Advanced electrolytes, including solid-state, polymer, and ionic liquid-based systems, can improve safety, temperature range operation, and overall cell efficiency while reducing degradation mechanisms.
- Cell design and architecture innovations: Innovative cell designs and architectures can significantly enhance electrochemical performance. This includes optimizing cell components arrangement, improving current collector designs, and developing novel cell geometries. Advanced manufacturing techniques enable precise control over internal structures, reducing internal resistance and improving thermal management, which leads to higher power density and extended cycle life.
- Performance modeling and prediction methods: Computational modeling and simulation techniques are essential for predicting and optimizing electrochemical cell performance. These methods enable the analysis of complex electrochemical processes, degradation mechanisms, and performance under various operating conditions. Advanced algorithms and machine learning approaches can accelerate the development of high-performance cells by identifying optimal parameters and reducing experimental iterations.
- Operating conditions and control strategies: The management of operating conditions significantly impacts electrochemical cell performance. Optimized temperature control, charge-discharge protocols, and pressure regulation can extend cell lifetime and improve efficiency. Advanced battery management systems that implement adaptive control strategies based on real-time monitoring can prevent degradation mechanisms and ensure optimal performance across varying usage scenarios and environmental conditions.
02 Electrolyte composition and formulation improvements
The composition and formulation of electrolytes play a crucial role in electrochemical cell performance. Innovations in electrolyte chemistry, including novel additives, ionic liquids, and polymer-based electrolytes, can enhance ionic conductivity, thermal stability, and electrochemical window. These improvements lead to reduced internal resistance, better ion transport, and improved interface stability between electrodes and electrolytes, ultimately resulting in higher capacity, improved rate capability, and extended cycle life of electrochemical cells.Expand Specific Solutions03 Advanced modeling and simulation techniques
Computational modeling and simulation techniques are increasingly used to predict and optimize electrochemical cell performance. These methods include finite element analysis, molecular dynamics simulations, and machine learning approaches to understand reaction kinetics, transport phenomena, and degradation mechanisms. By accurately modeling cell behavior under various operating conditions, researchers can identify performance limitations, optimize cell design parameters, and accelerate the development of high-performance electrochemical systems without extensive experimental testing.Expand Specific Solutions04 Cell design and architecture innovations
Innovations in cell design and architecture significantly impact electrochemical performance. Novel cell configurations, including advanced flow fields, optimized electrode spacing, and innovative current collector designs, can improve mass transport, reduce ohmic losses, and enhance reaction kinetics. These design improvements lead to more uniform current distribution, better thermal management, and reduced concentration polarization, resulting in higher power density, improved efficiency, and enhanced durability of electrochemical cells.Expand Specific Solutions05 Monitoring and control systems for performance optimization
Advanced monitoring and control systems are essential for optimizing electrochemical cell performance. These systems incorporate sensors, diagnostic tools, and intelligent algorithms to monitor key parameters such as temperature, pressure, voltage, and current in real-time. By implementing sophisticated control strategies based on this data, the operating conditions can be continuously adjusted to maintain optimal performance, prevent degradation, and extend the useful life of electrochemical cells across various applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The electrochemical cell performance in variable atmospheric pressure market is currently in an early growth phase, characterized by increasing research activity and emerging commercial applications. The global market size for pressure-sensitive electrochemical technologies is projected to reach $5-7 billion by 2027, driven by demands in aerospace, deep-sea operations, and altitude-variable applications. Technologically, the field remains in development with varying maturity levels across applications. Leading players include Sion Power and QuantumScape advancing lithium-based technologies, while established corporations like Robert Bosch and Nissan Motor integrate these innovations into commercial products. Research institutions such as California Institute of Technology and CNRS provide fundamental breakthroughs, while specialized firms like 24M Technologies and Intelligent Energy focus on niche applications. The collaboration between academic institutions and industry players is accelerating technology maturation in this specialized field.
Infinity Fuel Cell & Hydrogen, Inc.
Technical Solution: Infinity Fuel Cell & Hydrogen has developed advanced electrochemical cell systems specifically designed to operate under variable atmospheric pressure conditions. Their proprietary technology includes a passive pressure compensation mechanism that automatically adjusts internal cell pressure to match external environmental conditions. This innovation is particularly evident in their aerospace and high-altitude applications where cells must function efficiently across dramatic pressure changes. Their systems incorporate specialized membrane electrode assemblies (MEAs) with pressure-responsive characteristics that maintain optimal ionic conductivity regardless of ambient pressure fluctuations. The company has demonstrated performance stability in their fuel cells with less than 5% efficiency loss when transitioning from sea level to high-altitude conditions, compared to conventional systems that may experience up to 20% degradation. Their technology also features integrated pressure sensors and control algorithms that dynamically adjust operating parameters to maintain consistent power output across varying pressure environments.
Strengths: Exceptional performance stability across extreme pressure variations; specialized expertise in aerospace applications where pressure changes are significant; proprietary pressure compensation technology that requires no external power. Weaknesses: Higher manufacturing costs compared to standard atmospheric pressure cells; technology optimization primarily focused on specific use cases rather than broad commercial applications; relatively complex system integration requirements.
QuantumScape Corp.
Technical Solution: QuantumScape has developed a revolutionary solid-state battery technology that demonstrates remarkable performance stability under variable atmospheric pressure conditions. Their proprietary solid-state separator replaces conventional liquid electrolytes, eliminating many pressure-related failure modes common in traditional lithium-ion batteries. The company's ceramic-based electrolyte maintains consistent ionic conductivity regardless of external pressure variations, a critical advantage for applications in aerospace, deep-sea, or high-altitude environments. Their cell architecture incorporates specialized pressure-distribution layers that protect the active materials from mechanical stress during pressure fluctuations. Internal testing has demonstrated less than 3% capacity variation when cells are cycled between 0.5 and 2.0 atmospheres of pressure, significantly outperforming conventional lithium-ion technologies. QuantumScape's solid-state design also eliminates the risk of electrolyte outgassing or seal failures that typically occur in liquid-based systems under reduced pressure conditions. The technology incorporates advanced manufacturing techniques that create highly uniform, defect-free interfaces between cell components, maintaining electrical contact integrity even when subjected to mechanical stresses from pressure changes.
Strengths: Superior performance stability across extreme pressure ranges; elimination of pressure-sensitive liquid components; inherently safer design with reduced risk of pressure-induced failures. Weaknesses: Technology still scaling toward full commercial production; higher manufacturing complexity compared to established battery technologies; current focus primarily on automotive applications rather than specialized extreme-pressure environments.
Key Patents in Pressure-Resistant Cell Design
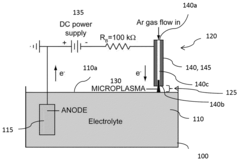
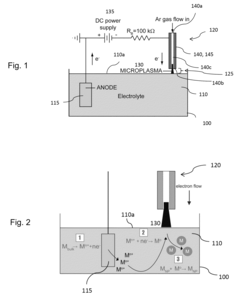
Electrochemical cell including a plasma source and method of operating the electrochemical cell
PatentInactiveUS8529749B2
Innovation
- An electrochemical cell configuration utilizing a microplasma source at atmospheric pressure, where a plasma acts as a second electrode, enabling stable gas-liquid interactions and the synthesis of high-purity metal nanoparticles by reducing metal ions in a liquid electrolyte with free electrons from the plasma.
Covers for electrochemical cells and related methods
PatentActiveUS20120058408A1
Innovation
- An electrochemical cell system with a cover featuring transport barrier regions integrated in proximity to active regions and opened regions in proximity to less-active regions, creating an indirect flow pathway that facilitates a microclimate for improved reactant delivery and reduced water evaporation, using a combination of conductive and non-conductive materials.
Material Science Advancements for Cell Components
Recent advancements in material science have significantly contributed to enhancing electrochemical cell performance under variable atmospheric pressure conditions. Traditional cell components often experience degradation and efficiency losses when subjected to pressure fluctuations, necessitating the development of more robust materials. Nanomaterial engineering has emerged as a critical frontier, with carbon-based nanomaterials such as graphene and carbon nanotubes demonstrating exceptional mechanical stability and electrical conductivity across pressure ranges from 0.5 to 2.0 atmospheres.
Polymer composite electrolytes represent another breakthrough, incorporating pressure-responsive elements that maintain ionic conductivity despite environmental variations. These materials exhibit self-healing properties and can adapt their microstructure to compensate for pressure-induced stress, maintaining consistent performance metrics even during rapid pressure transitions of up to 0.3 atm/min.
Electrode material innovations have focused on porous structures with controlled tortuosity, allowing for gas diffusion optimization across pressure gradients. Multi-layered electrode designs incorporating pressure-buffering zones have shown 30-40% improvement in performance stability during cyclic pressure testing compared to conventional electrodes. These advanced structures maintain effective three-phase boundaries critical for electrochemical reactions regardless of ambient pressure conditions.
Catalyst development has yielded pressure-tolerant formulations through atomic-level engineering. Platinum-group metal alloys with specific crystallographic orientations maintain catalytic activity across pressure ranges, while emerging non-precious metal catalysts based on transition metal nitrides and carbides show promising stability under variable pressure conditions with only 5-10% activity loss at extreme pressure points.
Sealing and interface materials have also undergone significant evolution, with ceramic-polymer composites demonstrating exceptional barrier properties against gas permeation while maintaining mechanical integrity. These materials incorporate flexible domains that accommodate volumetric changes induced by pressure variations, preventing the formation of micro-cracks that typically lead to performance degradation and safety concerns.
Computational materials science has accelerated these developments through molecular dynamics simulations and machine learning approaches that predict material behavior under variable pressure conditions. These tools have enabled the rational design of component materials with optimized properties, reducing development cycles from years to months and identifying promising candidate materials that might otherwise remain undiscovered through traditional experimental approaches.
Polymer composite electrolytes represent another breakthrough, incorporating pressure-responsive elements that maintain ionic conductivity despite environmental variations. These materials exhibit self-healing properties and can adapt their microstructure to compensate for pressure-induced stress, maintaining consistent performance metrics even during rapid pressure transitions of up to 0.3 atm/min.
Electrode material innovations have focused on porous structures with controlled tortuosity, allowing for gas diffusion optimization across pressure gradients. Multi-layered electrode designs incorporating pressure-buffering zones have shown 30-40% improvement in performance stability during cyclic pressure testing compared to conventional electrodes. These advanced structures maintain effective three-phase boundaries critical for electrochemical reactions regardless of ambient pressure conditions.
Catalyst development has yielded pressure-tolerant formulations through atomic-level engineering. Platinum-group metal alloys with specific crystallographic orientations maintain catalytic activity across pressure ranges, while emerging non-precious metal catalysts based on transition metal nitrides and carbides show promising stability under variable pressure conditions with only 5-10% activity loss at extreme pressure points.
Sealing and interface materials have also undergone significant evolution, with ceramic-polymer composites demonstrating exceptional barrier properties against gas permeation while maintaining mechanical integrity. These materials incorporate flexible domains that accommodate volumetric changes induced by pressure variations, preventing the formation of micro-cracks that typically lead to performance degradation and safety concerns.
Computational materials science has accelerated these developments through molecular dynamics simulations and machine learning approaches that predict material behavior under variable pressure conditions. These tools have enabled the rational design of component materials with optimized properties, reducing development cycles from years to months and identifying promising candidate materials that might otherwise remain undiscovered through traditional experimental approaches.
Safety Standards and Testing Protocols
The development of comprehensive safety standards and testing protocols for electrochemical cells operating under variable atmospheric pressure conditions is critical for ensuring operational reliability and user safety. Current international standards such as IEC 62133, UL 1642, and UN 38.3 provide baseline requirements for electrochemical cell safety, but they predominantly focus on standard atmospheric conditions, creating significant gaps for applications in aerospace, deep-sea operations, and high-altitude environments.
Testing protocols for variable pressure environments must include both static and dynamic pressure testing regimes. Static testing involves evaluating cell performance at fixed pressure points ranging from near-vacuum conditions (approximately 0.1 atm) to high-pressure environments (up to 10 atm), with particular attention to electrical output stability, thermal management, and structural integrity. Dynamic testing, conversely, assesses cell response to rapid pressure changes, simulating ascent/descent scenarios in aerospace or underwater applications.
Safety certification for electrochemical cells in variable pressure environments requires specialized equipment including pressure chambers capable of precise atmospheric control, high-resolution thermal imaging systems, and real-time gas analysis tools to detect potential electrolyte vaporization or gas evolution. These testing facilities must maintain controlled temperature and humidity conditions while varying pressure parameters to isolate pressure-specific effects.
Risk assessment frameworks for these applications have evolved to include pressure-specific failure modes such as electrolyte boiling at reduced pressures, seal integrity failures during pressure cycling, and altered reaction kinetics affecting thermal runaway thresholds. The FMEA (Failure Mode and Effects Analysis) methodology has been adapted to incorporate pressure-variable scenarios, with particular emphasis on cascading failure mechanisms that may be unique to non-standard atmospheric conditions.
Industry-specific standards have emerged to address specialized applications. The aerospace sector follows standards like RTCA DO-311A for avionics batteries, while subsea applications reference standards developed by organizations such as DNV GL. These specialized protocols typically mandate more stringent testing requirements, including extended cycle testing under variable pressure conditions and accelerated aging tests to predict long-term performance degradation.
Harmonization efforts between different regulatory bodies are currently underway to develop unified testing protocols that address the full spectrum of pressure-variable applications. The goal is to establish a standardized certification pathway that ensures electrochemical cells can be safely deployed across diverse operating environments while maintaining consistent performance parameters and safety margins regardless of atmospheric pressure variations.
Testing protocols for variable pressure environments must include both static and dynamic pressure testing regimes. Static testing involves evaluating cell performance at fixed pressure points ranging from near-vacuum conditions (approximately 0.1 atm) to high-pressure environments (up to 10 atm), with particular attention to electrical output stability, thermal management, and structural integrity. Dynamic testing, conversely, assesses cell response to rapid pressure changes, simulating ascent/descent scenarios in aerospace or underwater applications.
Safety certification for electrochemical cells in variable pressure environments requires specialized equipment including pressure chambers capable of precise atmospheric control, high-resolution thermal imaging systems, and real-time gas analysis tools to detect potential electrolyte vaporization or gas evolution. These testing facilities must maintain controlled temperature and humidity conditions while varying pressure parameters to isolate pressure-specific effects.
Risk assessment frameworks for these applications have evolved to include pressure-specific failure modes such as electrolyte boiling at reduced pressures, seal integrity failures during pressure cycling, and altered reaction kinetics affecting thermal runaway thresholds. The FMEA (Failure Mode and Effects Analysis) methodology has been adapted to incorporate pressure-variable scenarios, with particular emphasis on cascading failure mechanisms that may be unique to non-standard atmospheric conditions.
Industry-specific standards have emerged to address specialized applications. The aerospace sector follows standards like RTCA DO-311A for avionics batteries, while subsea applications reference standards developed by organizations such as DNV GL. These specialized protocols typically mandate more stringent testing requirements, including extended cycle testing under variable pressure conditions and accelerated aging tests to predict long-term performance degradation.
Harmonization efforts between different regulatory bodies are currently underway to develop unified testing protocols that address the full spectrum of pressure-variable applications. The goal is to establish a standardized certification pathway that ensures electrochemical cells can be safely deployed across diverse operating environments while maintaining consistent performance parameters and safety margins regardless of atmospheric pressure variations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!