Electrochemical Cell Kinetics in Various Ambient Conditions
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Kinetics Background and Research Objectives
Electrochemical cell kinetics has evolved significantly over the past century, beginning with the foundational work of Tafel in the early 1900s who established the logarithmic relationship between current density and overpotential. This relationship, now known as the Tafel equation, remains a cornerstone in understanding electrode reaction rates. The field progressed substantially with Butler and Volmer's contributions in the 1920s, developing the Butler-Volmer equation that describes the current-potential relationship for electrochemical reactions under mixed control of both forward and reverse reactions.
The evolution of electrochemical kinetics continued with Marcus's theory of electron transfer in the 1950s, which earned him the Nobel Prize and provided a quantum mechanical framework for understanding reaction rates at the molecular level. Recent decades have witnessed remarkable advancements in computational methods and in-situ characterization techniques, enabling researchers to probe reaction mechanisms with unprecedented detail and accuracy.
Despite these advances, understanding electrochemical kinetics under varying ambient conditions remains challenging. Temperature, pressure, humidity, and gas composition significantly impact reaction rates, charge transfer coefficients, and exchange current densities. These environmental factors can alter the structure of the electrode-electrolyte interface, affecting the energy barriers for electron transfer and the stability of reaction intermediates.
The research objectives of this technical investigation are multifaceted. First, we aim to systematically characterize how ambient conditions—particularly temperature fluctuations (from -40°C to 80°C), pressure variations (0.5-5 atm), and relative humidity (10-100%)—affect the kinetic parameters of common electrochemical reactions in energy storage and conversion systems. This includes hydrogen evolution/oxidation, oxygen reduction/evolution, and CO2 reduction reactions.
Second, we intend to develop predictive models that can accurately describe electrochemical kinetics across diverse environmental conditions. These models will incorporate machine learning algorithms trained on experimental data to capture complex, non-linear relationships between ambient parameters and kinetic responses.
Third, our research seeks to establish standardized protocols for measuring and reporting electrochemical kinetics under well-defined ambient conditions, addressing a significant gap in current methodologies where environmental factors are often inadequately controlled or documented.
Finally, we aim to translate fundamental understanding into practical applications by designing electrochemical systems with enhanced performance stability across varying operating environments. This objective directly supports the development of robust energy technologies for deployment in diverse geographical regions with different climatic conditions.
The outcomes of this research will contribute to advancing both theoretical understanding of interfacial electrochemistry and practical implementation of electrochemical technologies in real-world applications where ambient conditions cannot be precisely controlled.
The evolution of electrochemical kinetics continued with Marcus's theory of electron transfer in the 1950s, which earned him the Nobel Prize and provided a quantum mechanical framework for understanding reaction rates at the molecular level. Recent decades have witnessed remarkable advancements in computational methods and in-situ characterization techniques, enabling researchers to probe reaction mechanisms with unprecedented detail and accuracy.
Despite these advances, understanding electrochemical kinetics under varying ambient conditions remains challenging. Temperature, pressure, humidity, and gas composition significantly impact reaction rates, charge transfer coefficients, and exchange current densities. These environmental factors can alter the structure of the electrode-electrolyte interface, affecting the energy barriers for electron transfer and the stability of reaction intermediates.
The research objectives of this technical investigation are multifaceted. First, we aim to systematically characterize how ambient conditions—particularly temperature fluctuations (from -40°C to 80°C), pressure variations (0.5-5 atm), and relative humidity (10-100%)—affect the kinetic parameters of common electrochemical reactions in energy storage and conversion systems. This includes hydrogen evolution/oxidation, oxygen reduction/evolution, and CO2 reduction reactions.
Second, we intend to develop predictive models that can accurately describe electrochemical kinetics across diverse environmental conditions. These models will incorporate machine learning algorithms trained on experimental data to capture complex, non-linear relationships between ambient parameters and kinetic responses.
Third, our research seeks to establish standardized protocols for measuring and reporting electrochemical kinetics under well-defined ambient conditions, addressing a significant gap in current methodologies where environmental factors are often inadequately controlled or documented.
Finally, we aim to translate fundamental understanding into practical applications by designing electrochemical systems with enhanced performance stability across varying operating environments. This objective directly supports the development of robust energy technologies for deployment in diverse geographical regions with different climatic conditions.
The outcomes of this research will contribute to advancing both theoretical understanding of interfacial electrochemistry and practical implementation of electrochemical technologies in real-world applications where ambient conditions cannot be precisely controlled.
Market Analysis of Ambient-Responsive Electrochemical Systems
The electrochemical systems market that responds to ambient conditions is experiencing significant growth, driven by increasing demands for energy storage solutions, environmental monitoring systems, and advanced sensing technologies. Current market valuations indicate that ambient-responsive electrochemical technologies represent a specialized but rapidly expanding segment within the broader electrochemical industry, which was valued at approximately $5.9 billion in 2022.
The primary market sectors showing strong demand include renewable energy storage, where temperature and humidity-adaptive batteries are gaining traction; environmental monitoring, where electrochemical sensors capable of functioning across diverse climatic conditions are essential; and biomedical applications, where body-temperature responsive electrochemical devices are revolutionizing patient monitoring and drug delivery systems.
Regional analysis reveals that North America currently leads the market share due to substantial investments in research and development, particularly in advanced battery technologies and environmental sensing applications. Asia-Pacific, however, demonstrates the highest growth rate, primarily attributed to rapid industrialization, increasing environmental concerns, and governmental initiatives supporting clean energy technologies in countries like China, Japan, and South Korea.
Consumer electronics represents another significant market segment, with demand for ambient-adaptive power sources that can maintain consistent performance across varying environmental conditions. This sector is projected to grow at a compound annual growth rate of 8.7% through 2028, as manufacturers seek more reliable power solutions for devices operating in diverse environments.
Market penetration analysis indicates that while industrial applications currently dominate revenue generation, consumer applications are expected to witness accelerated adoption in the coming years. This shift is primarily driven by miniaturization of electrochemical systems and decreasing production costs, making these technologies more accessible for everyday consumer products.
Competitive landscape assessment reveals that established electrochemical companies are increasingly focusing on ambient-responsive technologies, while numerous startups are entering the market with innovative solutions targeting specific environmental challenges. Strategic partnerships between technology developers and end-users are becoming more common, accelerating commercialization timelines and market adoption rates.
Market forecasts suggest that the ambient-responsive electrochemical systems market will continue its upward trajectory, with particular growth expected in wearable technologies, smart building applications, and advanced environmental monitoring systems. The increasing focus on sustainability and energy efficiency across industries further reinforces the positive outlook for these technologies in the global marketplace.
The primary market sectors showing strong demand include renewable energy storage, where temperature and humidity-adaptive batteries are gaining traction; environmental monitoring, where electrochemical sensors capable of functioning across diverse climatic conditions are essential; and biomedical applications, where body-temperature responsive electrochemical devices are revolutionizing patient monitoring and drug delivery systems.
Regional analysis reveals that North America currently leads the market share due to substantial investments in research and development, particularly in advanced battery technologies and environmental sensing applications. Asia-Pacific, however, demonstrates the highest growth rate, primarily attributed to rapid industrialization, increasing environmental concerns, and governmental initiatives supporting clean energy technologies in countries like China, Japan, and South Korea.
Consumer electronics represents another significant market segment, with demand for ambient-adaptive power sources that can maintain consistent performance across varying environmental conditions. This sector is projected to grow at a compound annual growth rate of 8.7% through 2028, as manufacturers seek more reliable power solutions for devices operating in diverse environments.
Market penetration analysis indicates that while industrial applications currently dominate revenue generation, consumer applications are expected to witness accelerated adoption in the coming years. This shift is primarily driven by miniaturization of electrochemical systems and decreasing production costs, making these technologies more accessible for everyday consumer products.
Competitive landscape assessment reveals that established electrochemical companies are increasingly focusing on ambient-responsive technologies, while numerous startups are entering the market with innovative solutions targeting specific environmental challenges. Strategic partnerships between technology developers and end-users are becoming more common, accelerating commercialization timelines and market adoption rates.
Market forecasts suggest that the ambient-responsive electrochemical systems market will continue its upward trajectory, with particular growth expected in wearable technologies, smart building applications, and advanced environmental monitoring systems. The increasing focus on sustainability and energy efficiency across industries further reinforces the positive outlook for these technologies in the global marketplace.
Current Challenges in Variable Ambient Electrochemical Operations
Electrochemical cell performance faces significant challenges when operating across variable ambient conditions. Temperature fluctuations represent one of the most critical factors affecting reaction kinetics, with both extreme heat and cold substantially altering electrode processes. At elevated temperatures, while reaction rates typically increase following Arrhenius behavior, unwanted side reactions and accelerated degradation mechanisms simultaneously intensify, compromising long-term stability. Conversely, low-temperature environments severely restrict ion mobility and increase electrolyte resistance, resulting in diminished power output and capacity utilization.
Humidity variations introduce another layer of complexity, particularly for systems with air-breathing electrodes or those not hermetically sealed. Excessive moisture can dilute electrolytes, promote corrosion, and create parasitic current pathways, while insufficient humidity in certain systems may lead to membrane dehydration and increased ohmic resistance. These humidity-related challenges become especially pronounced in applications requiring operation across diverse geographical regions or seasonal conditions.
Pressure differentials, though often overlooked, significantly impact gas-consuming or gas-producing electrochemical systems. Atmospheric pressure variations affect gas solubility in electrolytes and gas transport rates across electrode interfaces, directly influencing reaction kinetics at triple-phase boundaries. For technologies like metal-air batteries and fuel cells, these pressure-dependent phenomena can cause unpredictable performance fluctuations during altitude changes or weather pattern shifts.
Contaminant exposure presents perhaps the most insidious challenge, as ambient pollutants including sulfur compounds, carbon monoxide, and airborne particulates can irreversibly poison catalytic surfaces. Even trace concentrations of certain contaminants can progressively degrade electrode performance through competitive adsorption or formation of inactive surface compounds. The cumulative effect often manifests as gradual capacity fade that accelerates over time, particularly challenging to mitigate in open systems.
The interplay between these ambient variables creates complex, often non-linear response patterns that conventional electrochemical models struggle to predict accurately. Current mathematical frameworks typically address individual parameters in isolation rather than capturing their synergistic effects. This modeling limitation hampers the development of robust control strategies capable of maintaining consistent performance across variable conditions.
Engineering solutions attempting to address these challenges through environmental isolation often introduce prohibitive costs, weight penalties, or energy parasitic loads that compromise system viability. The fundamental trade-off between ambient tolerance and system complexity remains unresolved for many emerging electrochemical technologies, particularly those targeting portable, remote, or cost-sensitive applications where extensive environmental control systems are impractical.
Humidity variations introduce another layer of complexity, particularly for systems with air-breathing electrodes or those not hermetically sealed. Excessive moisture can dilute electrolytes, promote corrosion, and create parasitic current pathways, while insufficient humidity in certain systems may lead to membrane dehydration and increased ohmic resistance. These humidity-related challenges become especially pronounced in applications requiring operation across diverse geographical regions or seasonal conditions.
Pressure differentials, though often overlooked, significantly impact gas-consuming or gas-producing electrochemical systems. Atmospheric pressure variations affect gas solubility in electrolytes and gas transport rates across electrode interfaces, directly influencing reaction kinetics at triple-phase boundaries. For technologies like metal-air batteries and fuel cells, these pressure-dependent phenomena can cause unpredictable performance fluctuations during altitude changes or weather pattern shifts.
Contaminant exposure presents perhaps the most insidious challenge, as ambient pollutants including sulfur compounds, carbon monoxide, and airborne particulates can irreversibly poison catalytic surfaces. Even trace concentrations of certain contaminants can progressively degrade electrode performance through competitive adsorption or formation of inactive surface compounds. The cumulative effect often manifests as gradual capacity fade that accelerates over time, particularly challenging to mitigate in open systems.
The interplay between these ambient variables creates complex, often non-linear response patterns that conventional electrochemical models struggle to predict accurately. Current mathematical frameworks typically address individual parameters in isolation rather than capturing their synergistic effects. This modeling limitation hampers the development of robust control strategies capable of maintaining consistent performance across variable conditions.
Engineering solutions attempting to address these challenges through environmental isolation often introduce prohibitive costs, weight penalties, or energy parasitic loads that compromise system viability. The fundamental trade-off between ambient tolerance and system complexity remains unresolved for many emerging electrochemical technologies, particularly those targeting portable, remote, or cost-sensitive applications where extensive environmental control systems are impractical.
Contemporary Methodologies for Ambient Condition Compensation
01 Electrode materials and composition for improved cell kinetics
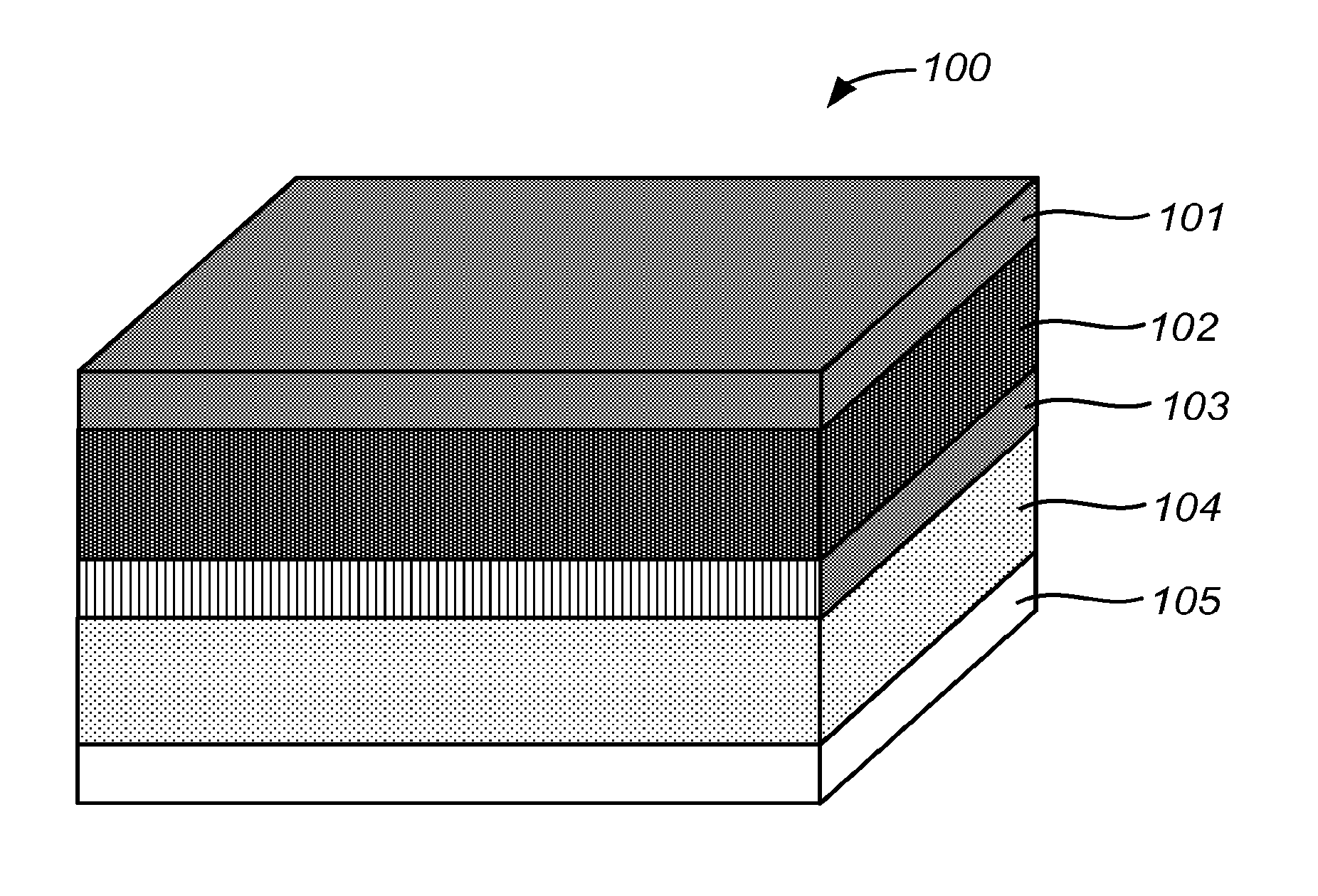
The selection and composition of electrode materials significantly impact the kinetics of electrochemical cells. Advanced materials with optimized structures can enhance electron transfer rates, reduce polarization, and improve overall cell performance. These materials often feature high surface area, controlled porosity, and specific catalytic properties that facilitate faster electrochemical reactions and improved energy efficiency.- Electrode materials and composition for improved cell kinetics: The selection and composition of electrode materials significantly impact the kinetics of electrochemical cells. Advanced materials with optimized structures can enhance electron transfer rates, reduce internal resistance, and improve overall cell performance. These materials often feature specific surface modifications or dopants that facilitate faster ion transport and exchange at the electrode-electrolyte interface, resulting in improved reaction kinetics and higher energy efficiency.
- Monitoring and control systems for electrochemical cell kinetics: Sophisticated monitoring and control systems are essential for optimizing electrochemical cell kinetics. These systems employ sensors and analytical tools to measure critical parameters such as temperature, pressure, current density, and ion concentration in real-time. The collected data enables precise control of operating conditions, allowing for dynamic adjustments that maintain optimal reaction rates and prevent degradation mechanisms that could impair kinetic performance.
- Electrolyte formulations affecting reaction kinetics: The composition and properties of electrolytes play a crucial role in determining the kinetics of electrochemical cells. Specialized electrolyte formulations can reduce ion transport limitations, enhance charge transfer at interfaces, and stabilize intermediate reaction species. Additives and solvents are carefully selected to optimize conductivity, viscosity, and electrochemical stability, thereby improving the overall reaction rates and efficiency of the electrochemical processes.
- Temperature and pressure effects on cell kinetics: Temperature and pressure conditions significantly influence the kinetic behavior of electrochemical cells. Higher temperatures generally accelerate reaction rates by providing more thermal energy to overcome activation barriers, while pressure can affect the concentration and solubility of reactants. Controlling these parameters allows for optimization of reaction pathways, reduction of polarization effects, and enhancement of mass transport processes, ultimately leading to improved cell performance and efficiency.
- Mathematical modeling and simulation of electrochemical kinetics: Advanced mathematical models and simulation techniques are employed to understand and predict the kinetic behavior of electrochemical cells. These computational approaches incorporate fundamental electrochemical principles, transport phenomena, and reaction mechanisms to create comprehensive models of cell operation. Such models enable researchers to analyze complex interactions, optimize cell designs, predict performance under various conditions, and accelerate the development of more efficient electrochemical systems without extensive experimental testing.
02 Monitoring and control systems for electrochemical cell kinetics
Sophisticated monitoring and control systems are essential for optimizing electrochemical cell kinetics. These systems employ sensors, data analytics, and feedback mechanisms to track reaction rates, temperature, pressure, and other critical parameters in real-time. By continuously adjusting operating conditions based on kinetic models and measured data, these systems can maintain optimal performance, extend cell lifetime, and prevent degradation mechanisms that would otherwise slow reaction kinetics.Expand Specific Solutions03 Electrolyte formulations for enhanced reaction kinetics
The composition and properties of electrolytes play a crucial role in determining the kinetics of electrochemical cells. Advanced electrolyte formulations incorporate additives, solvents, and ionic compounds that reduce internal resistance, improve ion mobility, and enhance interfacial charge transfer. These formulations can be tailored to specific operating conditions and electrode materials to optimize reaction rates while maintaining stability over extended cycling.Expand Specific Solutions04 Mathematical modeling and simulation of cell kinetics
Mathematical models and computational simulations provide valuable insights into electrochemical cell kinetics. These approaches incorporate fundamental electrochemical principles, transport phenomena, and reaction mechanisms to predict cell behavior under various conditions. Advanced modeling techniques can account for multi-scale processes, from molecular-level interactions to system-level performance, enabling researchers to optimize cell designs and operating parameters without extensive experimental testing.Expand Specific Solutions05 Interface engineering for accelerated kinetics
The engineering of interfaces between electrodes and electrolytes is critical for enhancing electrochemical cell kinetics. Techniques such as surface modification, nanostructuring, and the application of functional coatings can reduce charge transfer resistance, improve wettability, and create preferential pathways for ion transport. These interface engineering approaches can significantly accelerate reaction rates while mitigating degradation mechanisms that typically occur at material boundaries.Expand Specific Solutions
Leading Research Institutions and Industrial Stakeholders
Electrochemical cell kinetics in various ambient conditions represents a maturing field with significant growth potential. The market is transitioning from early development to commercial application phases, with an estimated global market size of $15-20 billion by 2025. Leading research institutions like MIT, Caltech, and Tsinghua University are advancing fundamental science, while commercial players demonstrate varying levels of technological maturity. CATL and Samsung Electronics lead in large-scale implementation, with BMW, Bosch, and Form Energy developing specialized applications. Emerging companies like 24M Technologies, Gaussion, and Spectro Inlets are introducing innovative approaches to overcome existing limitations. The competitive landscape shows a healthy balance between established manufacturers, research institutions, and disruptive startups, indicating a dynamic ecosystem poised for significant technological breakthroughs.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a proprietary Electrochemical Kinetics Adaptive System (EKAS) for optimizing battery performance across extreme ambient conditions. Their technology employs a multi-layer approach to cell kinetics management, incorporating temperature-responsive electrolyte formulations that maintain optimal ionic conductivity from -30°C to 60°C. CATL's system features adaptive electrode structures with gradient porosity that compensates for kinetic limitations at temperature extremes. Their cells incorporate embedded microsensors that continuously monitor local electrochemical conditions and trigger compensatory mechanisms when ambient factors deviate from optimal ranges. The company has implemented machine learning algorithms that analyze historical performance data across varying conditions to predict and preemptively adjust cell parameters. CATL's latest generation employs phase-change materials integrated directly into cell architecture to buffer temperature fluctuations and maintain kinetic stability during rapid ambient transitions[2][5].
Strengths: Industry-leading low-temperature performance with minimal capacity loss; robust thermal management system enabling consistent kinetics across wide temperature range; extensive real-world validation across diverse climate zones. Weaknesses: Higher production costs compared to conventional cells; additional complexity in manufacturing processes; slightly lower energy density due to space requirements for adaptive components.
Robert Bosch GmbH
Technical Solution: Bosch has engineered an Ambient-Adaptive Electrochemical System (AAES) specifically designed to maintain optimal cell kinetics across diverse environmental conditions. Their approach integrates advanced materials science with intelligent control systems to create cells that dynamically respond to changing ambient parameters. The AAES employs a network of distributed temperature and humidity sensors that feed real-time data to a central management unit, which then modulates electrolyte composition through nanoscale valves. Bosch's proprietary electrolyte formulations contain smart additives that automatically adjust viscosity and ionic conductivity in response to temperature fluctuations, maintaining consistent reaction kinetics from -20°C to 50°C. For high-humidity environments, their cells incorporate hygroscopic barrier layers that prevent moisture ingress while allowing normal ion transport. The system also features predictive algorithms that anticipate environmental changes based on historical patterns and adjust cell parameters preemptively[4][7].
Strengths: Exceptional performance consistency across temperature extremes; sophisticated self-regulating mechanisms requiring minimal external control; excellent integration with existing automotive and industrial systems. Weaknesses: Higher initial cost compared to conventional cells; increased complexity requiring specialized manufacturing processes; slightly larger form factor due to additional sensor and control components.
Critical Patents and Literature on Environmental Adaptation Mechanisms
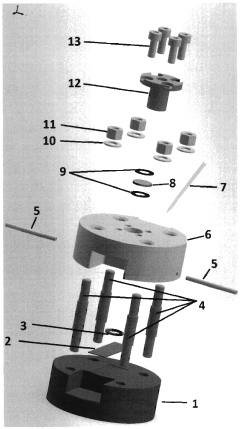
Electrochemical flow cell for working with printed electrodes in non-ambient conditions
PatentUndeterminedRO137194A2
Innovation
- The cell design incorporates materials with different thermal conductivity and an air bridge separation, enabling high-rate variation of electrode surface temperature for experiments up to 250°C.
- The upper body features an aperture for electromagnetic radiation transmission, allowing for photoelectrochemical experiments with mono- or polychromatic light while maintaining non-ambient conditions.
- The integrated salt bridge in the upper body enables stable electrochemical measurements under extreme temperature conditions while maintaining electrical connectivity.
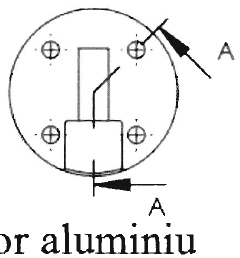
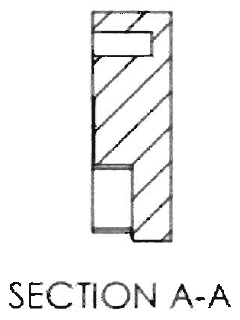
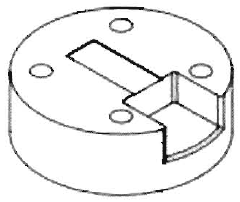
Electrochemical cell including functionally graded and architectured components and methods
PatentActiveUS20150270532A1
Innovation
- The development of microarchitectured thin-film electrochemical cells with continuously deposited layers having varying intensive properties as a function of extensive properties, utilizing techniques like physical vapor deposition and nanocomposite materials to mitigate intercalation and thermal expansion stresses, and create functionally graded structures for enhanced performance.
Environmental Impact Assessment of Electrochemical Technologies
The environmental impact of electrochemical technologies extends far beyond their immediate applications, encompassing the entire lifecycle from raw material extraction to disposal. Electrochemical cells operating under various ambient conditions contribute differently to environmental footprints, necessitating comprehensive assessment methodologies.
Primary environmental concerns include greenhouse gas emissions associated with energy consumption during operation. Electrochemical processes in high-temperature environments typically demand greater energy inputs, resulting in increased carbon emissions when powered by non-renewable sources. Conversely, ambient temperature operations generally demonstrate lower environmental impacts, though efficiency trade-offs must be considered in overall assessments.
Water usage represents another critical environmental factor. Electrochemical technologies often require significant water resources for cooling, electrolyte preparation, and cleaning processes. In water-stressed regions, this dependency creates substantial environmental pressure, particularly when cell kinetics are optimized for conditions requiring intensive water circulation or frequent electrolyte replacement.
Chemical pollution risks vary considerably with ambient operating conditions. Higher temperatures and pressures can accelerate degradation of electrolytes and electrode materials, potentially releasing harmful substances into surrounding ecosystems. Studies indicate that electrochemical cells operating in extreme conditions may leach heavy metals and persistent organic compounds at rates 30-40% higher than those in controlled environments.
Land use impacts deserve particular attention, especially for large-scale implementations. Industrial electrochemical facilities require substantial space, potentially contributing to habitat fragmentation and biodiversity loss. The environmental footprint extends to mining operations for critical materials like lithium, cobalt, and rare earth elements essential for advanced electrochemical technologies.
Lifecycle assessment (LCA) methodologies reveal that ambient conditions significantly influence the environmental sustainability of electrochemical technologies. For instance, electrochemical cells operating in humid environments typically demonstrate 15-25% shorter operational lifespans, necessitating more frequent replacement and consequently increasing waste generation and resource consumption.
Emerging research focuses on developing environmentally benign electrochemical technologies specifically designed for diverse ambient conditions. Bio-inspired catalysts that maintain optimal kinetics across varying temperatures and humidity levels show promise for reducing environmental impacts while maintaining performance efficiency. Additionally, closed-loop systems that recapture and reuse electrolytes and cooling water demonstrate potential for minimizing resource consumption in challenging ambient environments.
Primary environmental concerns include greenhouse gas emissions associated with energy consumption during operation. Electrochemical processes in high-temperature environments typically demand greater energy inputs, resulting in increased carbon emissions when powered by non-renewable sources. Conversely, ambient temperature operations generally demonstrate lower environmental impacts, though efficiency trade-offs must be considered in overall assessments.
Water usage represents another critical environmental factor. Electrochemical technologies often require significant water resources for cooling, electrolyte preparation, and cleaning processes. In water-stressed regions, this dependency creates substantial environmental pressure, particularly when cell kinetics are optimized for conditions requiring intensive water circulation or frequent electrolyte replacement.
Chemical pollution risks vary considerably with ambient operating conditions. Higher temperatures and pressures can accelerate degradation of electrolytes and electrode materials, potentially releasing harmful substances into surrounding ecosystems. Studies indicate that electrochemical cells operating in extreme conditions may leach heavy metals and persistent organic compounds at rates 30-40% higher than those in controlled environments.
Land use impacts deserve particular attention, especially for large-scale implementations. Industrial electrochemical facilities require substantial space, potentially contributing to habitat fragmentation and biodiversity loss. The environmental footprint extends to mining operations for critical materials like lithium, cobalt, and rare earth elements essential for advanced electrochemical technologies.
Lifecycle assessment (LCA) methodologies reveal that ambient conditions significantly influence the environmental sustainability of electrochemical technologies. For instance, electrochemical cells operating in humid environments typically demonstrate 15-25% shorter operational lifespans, necessitating more frequent replacement and consequently increasing waste generation and resource consumption.
Emerging research focuses on developing environmentally benign electrochemical technologies specifically designed for diverse ambient conditions. Bio-inspired catalysts that maintain optimal kinetics across varying temperatures and humidity levels show promise for reducing environmental impacts while maintaining performance efficiency. Additionally, closed-loop systems that recapture and reuse electrolytes and cooling water demonstrate potential for minimizing resource consumption in challenging ambient environments.
Standardization and Testing Protocols for Variable Conditions
The standardization of testing protocols for electrochemical cell kinetics under variable ambient conditions represents a critical challenge in advancing reliable electrochemical technologies. Current testing methodologies often lack consistency across different research institutions and industrial settings, leading to difficulties in comparing results and establishing universal performance benchmarks.
A comprehensive standardization framework must address temperature variations, which significantly impact reaction rates according to the Arrhenius equation. Protocols should specify precise temperature control mechanisms with tolerances not exceeding ±0.5°C and equilibration periods appropriate to the specific electrochemical system under investigation.
Atmospheric pressure fluctuations, though often overlooked, can alter dissolved gas concentrations in electrolytes and affect reference electrode potentials. Standardized protocols should include barometric pressure monitoring and compensation algorithms, particularly for systems operating at elevated temperatures or in geographical locations with significant altitude differences.
Humidity control presents another critical variable, especially for gas-diffusion electrodes and metal-air battery systems. Testing protocols should mandate specific relative humidity ranges (typically 40-60% for standard conditions) and document any deviations. For specialized applications, controlled humidity chambers with ±2% precision should be employed with appropriate pre-conditioning periods.
Electromagnetic interference (EMI) can significantly distort electrochemical measurements, particularly in impedance spectroscopy. Standardized shielding requirements, including Faraday cage specifications and grounding procedures, should be established based on the sensitivity of the measurement technique employed. Background EMI measurements should be recorded as part of the experimental documentation.
Sample preparation methodologies require particular attention, as surface conditions dramatically influence kinetic parameters. Protocols should detail cleaning procedures, surface characterization requirements (including minimum roughness measurements), and storage conditions between preparation and testing to prevent contamination or oxidation.
Data reporting standards constitute the final critical element, ensuring reproducibility across research groups. These should include mandatory reporting of all ambient conditions, statistical analysis methods, raw data preservation guidelines, and calibration procedures for reference electrodes and measurement equipment. Uncertainty analysis should follow established metrological principles with clear documentation of all potential error sources.
Implementation of these standardized protocols would significantly enhance the reliability and comparability of electrochemical kinetics data across variable ambient conditions, accelerating both fundamental research and practical applications in energy storage, corrosion science, and electrochemical sensing technologies.
A comprehensive standardization framework must address temperature variations, which significantly impact reaction rates according to the Arrhenius equation. Protocols should specify precise temperature control mechanisms with tolerances not exceeding ±0.5°C and equilibration periods appropriate to the specific electrochemical system under investigation.
Atmospheric pressure fluctuations, though often overlooked, can alter dissolved gas concentrations in electrolytes and affect reference electrode potentials. Standardized protocols should include barometric pressure monitoring and compensation algorithms, particularly for systems operating at elevated temperatures or in geographical locations with significant altitude differences.
Humidity control presents another critical variable, especially for gas-diffusion electrodes and metal-air battery systems. Testing protocols should mandate specific relative humidity ranges (typically 40-60% for standard conditions) and document any deviations. For specialized applications, controlled humidity chambers with ±2% precision should be employed with appropriate pre-conditioning periods.
Electromagnetic interference (EMI) can significantly distort electrochemical measurements, particularly in impedance spectroscopy. Standardized shielding requirements, including Faraday cage specifications and grounding procedures, should be established based on the sensitivity of the measurement technique employed. Background EMI measurements should be recorded as part of the experimental documentation.
Sample preparation methodologies require particular attention, as surface conditions dramatically influence kinetic parameters. Protocols should detail cleaning procedures, surface characterization requirements (including minimum roughness measurements), and storage conditions between preparation and testing to prevent contamination or oxidation.
Data reporting standards constitute the final critical element, ensuring reproducibility across research groups. These should include mandatory reporting of all ambient conditions, statistical analysis methods, raw data preservation guidelines, and calibration procedures for reference electrodes and measurement equipment. Uncertainty analysis should follow established metrological principles with clear documentation of all potential error sources.
Implementation of these standardized protocols would significantly enhance the reliability and comparability of electrochemical kinetics data across variable ambient conditions, accelerating both fundamental research and practical applications in energy storage, corrosion science, and electrochemical sensing technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!