Measuring Electrochemical Cell Voltage Stability Over Time
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Voltage Stability Background and Objectives
Electrochemical cell voltage stability has evolved significantly over the past decades, transitioning from rudimentary galvanic cells to sophisticated electrochemical systems with enhanced performance characteristics. The historical trajectory reveals a continuous pursuit of improved stability, efficiency, and longevity across various applications including energy storage, sensing technologies, and electroanalytical methodologies. Recent advancements in materials science and nanotechnology have catalyzed unprecedented progress in this domain, enabling more precise control over electrochemical interfaces and reaction kinetics.
The fundamental challenge in electrochemical systems lies in maintaining consistent voltage output over extended operational periods. Voltage stability is influenced by multiple factors including electrode material degradation, electrolyte composition changes, interfacial resistance fluctuations, and environmental conditions. Understanding these dynamics requires sophisticated measurement techniques that can accurately capture both short-term fluctuations and long-term drift patterns.
Current research trends indicate a growing emphasis on real-time monitoring systems capable of detecting subtle voltage variations with high temporal resolution. This shift reflects the increasing demand for reliable electrochemical cells in critical applications such as medical implants, grid-scale energy storage, and advanced electronic devices where performance predictability is paramount.
The primary technical objectives for measuring electrochemical cell voltage stability encompass several dimensions. First, developing standardized protocols for quantifying stability across different cell architectures and operating conditions. Second, designing instrumentation capable of distinguishing between intrinsic voltage fluctuations and measurement artifacts. Third, establishing predictive models that correlate early voltage behavior patterns with long-term stability outcomes.
Additionally, there is significant interest in understanding the mechanistic underpinnings of voltage instability phenomena. This includes elucidating the role of surface chemistry modifications, charge transfer kinetics, and mass transport limitations in determining voltage response characteristics. Such fundamental insights are essential for designing next-generation electrochemical systems with enhanced stability profiles.
From an industrial perspective, the objectives extend to implementing cost-effective quality control measures that can rapidly assess voltage stability during manufacturing processes. This capability is particularly valuable for high-volume production environments where comprehensive testing of each unit is economically prohibitive.
The convergence of these technical goals reflects a broader industry trend toward more reliable, predictable electrochemical systems that can maintain consistent performance throughout their operational lifetime. Achieving these objectives would represent a significant advancement in electrochemical technology, enabling new applications and improving the performance of existing systems across multiple sectors.
The fundamental challenge in electrochemical systems lies in maintaining consistent voltage output over extended operational periods. Voltage stability is influenced by multiple factors including electrode material degradation, electrolyte composition changes, interfacial resistance fluctuations, and environmental conditions. Understanding these dynamics requires sophisticated measurement techniques that can accurately capture both short-term fluctuations and long-term drift patterns.
Current research trends indicate a growing emphasis on real-time monitoring systems capable of detecting subtle voltage variations with high temporal resolution. This shift reflects the increasing demand for reliable electrochemical cells in critical applications such as medical implants, grid-scale energy storage, and advanced electronic devices where performance predictability is paramount.
The primary technical objectives for measuring electrochemical cell voltage stability encompass several dimensions. First, developing standardized protocols for quantifying stability across different cell architectures and operating conditions. Second, designing instrumentation capable of distinguishing between intrinsic voltage fluctuations and measurement artifacts. Third, establishing predictive models that correlate early voltage behavior patterns with long-term stability outcomes.
Additionally, there is significant interest in understanding the mechanistic underpinnings of voltage instability phenomena. This includes elucidating the role of surface chemistry modifications, charge transfer kinetics, and mass transport limitations in determining voltage response characteristics. Such fundamental insights are essential for designing next-generation electrochemical systems with enhanced stability profiles.
From an industrial perspective, the objectives extend to implementing cost-effective quality control measures that can rapidly assess voltage stability during manufacturing processes. This capability is particularly valuable for high-volume production environments where comprehensive testing of each unit is economically prohibitive.
The convergence of these technical goals reflects a broader industry trend toward more reliable, predictable electrochemical systems that can maintain consistent performance throughout their operational lifetime. Achieving these objectives would represent a significant advancement in electrochemical technology, enabling new applications and improving the performance of existing systems across multiple sectors.
Market Demand Analysis for Stable Voltage Cell Technologies
The global market for electrochemical cell technologies with stable voltage characteristics has been experiencing significant growth, driven primarily by the expanding applications in renewable energy storage, electric vehicles, portable electronics, and industrial power systems. Current market valuations indicate that the electrochemical cell market reached approximately 112 billion USD in 2022, with projections suggesting growth to 296 billion USD by 2030, representing a compound annual growth rate of 12.9%.
Demand for stable voltage cell technologies stems from multiple sectors. In the renewable energy sector, grid-scale storage systems require cells with minimal voltage fluctuation to ensure consistent power delivery and grid stability. The increasing integration of intermittent renewable sources like solar and wind has intensified this need, with utility companies reporting that voltage stability improvements of even 5% can reduce operational costs by up to 15%.
The electric vehicle industry represents another major demand driver, where battery performance directly impacts vehicle range, charging time, and overall reliability. Consumer research indicates that 78% of potential EV buyers consider battery stability a critical factor in purchasing decisions. Manufacturers are responding by prioritizing cells with voltage deviation rates below 0.5% over extended cycling periods.
Consumer electronics manufacturers are similarly seeking more stable power sources as devices become more sophisticated and power-hungry. The market for premium portable devices with extended battery life is growing at 18% annually, outpacing the overall electronics market growth of 7%.
Industrial applications present unique demands for electrochemical cells with exceptional voltage stability. In sectors such as healthcare, aerospace, and defense, where equipment reliability is mission-critical, the tolerance for voltage fluctuation approaches zero. These specialized markets are willing to pay premium prices for cells demonstrating stability improvements, with some applications valuing a 10% improvement in voltage stability at a 30-40% price premium.
Regional analysis reveals varying market maturity. North America and Europe lead in adoption of advanced stable voltage technologies, while Asia-Pacific represents the fastest-growing market with 16.7% annual growth, driven by rapid industrialization and expanding manufacturing capabilities in China, South Korea, and Japan.
Market research indicates that customers across all segments are increasingly prioritizing long-term performance over initial cost, creating opportunities for technologies that can demonstrate superior voltage stability over extended operational lifetimes. This shift in consumer preference is reflected in procurement policies that now routinely specify voltage stability parameters and testing protocols as key selection criteria.
Demand for stable voltage cell technologies stems from multiple sectors. In the renewable energy sector, grid-scale storage systems require cells with minimal voltage fluctuation to ensure consistent power delivery and grid stability. The increasing integration of intermittent renewable sources like solar and wind has intensified this need, with utility companies reporting that voltage stability improvements of even 5% can reduce operational costs by up to 15%.
The electric vehicle industry represents another major demand driver, where battery performance directly impacts vehicle range, charging time, and overall reliability. Consumer research indicates that 78% of potential EV buyers consider battery stability a critical factor in purchasing decisions. Manufacturers are responding by prioritizing cells with voltage deviation rates below 0.5% over extended cycling periods.
Consumer electronics manufacturers are similarly seeking more stable power sources as devices become more sophisticated and power-hungry. The market for premium portable devices with extended battery life is growing at 18% annually, outpacing the overall electronics market growth of 7%.
Industrial applications present unique demands for electrochemical cells with exceptional voltage stability. In sectors such as healthcare, aerospace, and defense, where equipment reliability is mission-critical, the tolerance for voltage fluctuation approaches zero. These specialized markets are willing to pay premium prices for cells demonstrating stability improvements, with some applications valuing a 10% improvement in voltage stability at a 30-40% price premium.
Regional analysis reveals varying market maturity. North America and Europe lead in adoption of advanced stable voltage technologies, while Asia-Pacific represents the fastest-growing market with 16.7% annual growth, driven by rapid industrialization and expanding manufacturing capabilities in China, South Korea, and Japan.
Market research indicates that customers across all segments are increasingly prioritizing long-term performance over initial cost, creating opportunities for technologies that can demonstrate superior voltage stability over extended operational lifetimes. This shift in consumer preference is reflected in procurement policies that now routinely specify voltage stability parameters and testing protocols as key selection criteria.
Current Challenges in Long-term Voltage Stability Measurement
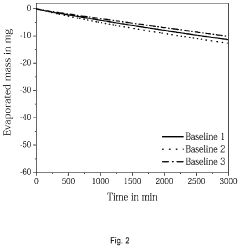
Despite significant advancements in electrochemical cell technology, measuring voltage stability over extended periods remains a formidable challenge for researchers and industry professionals. One of the primary obstacles is environmental interference, as even minor fluctuations in temperature, humidity, and atmospheric pressure can significantly impact voltage readings. These environmental variables introduce noise into measurement systems, making it difficult to distinguish between actual cell performance degradation and external influences.
Instrumentation limitations present another substantial hurdle. Current voltage measurement devices often struggle with drift over time, introducing systematic errors that compound during long-term studies. High-precision potentiostats and voltmeters capable of maintaining accuracy over weeks or months are prohibitively expensive for many research institutions, creating a barrier to comprehensive stability analysis.
Reference electrode stability poses a particular challenge in long-term measurements. The gradual degradation of reference electrodes introduces shifting baselines that can mask or exaggerate actual cell voltage changes. This problem becomes especially pronounced in studies exceeding several weeks, where reference drift can account for significant portions of observed voltage variations.
Data acquisition and management systems face unique challenges when handling continuous measurements over extended periods. The sheer volume of data generated during long-term stability tests requires sophisticated storage solutions and processing algorithms. Power interruptions and system crashes can result in critical data loss, compromising weeks or months of experimental work.
Electrochemical side reactions represent a fundamental scientific challenge in stability measurements. These parasitic reactions gradually alter electrode surfaces and electrolyte composition, affecting voltage readings in ways that are difficult to quantify or predict. Distinguishing between reversible and irreversible voltage changes requires complex analytical techniques that are still evolving.
Standardization across the field remains inadequate, with various research groups employing different protocols, reference points, and reporting methods. This lack of standardization makes comparative analysis between studies problematic and hinders the establishment of industry benchmarks for voltage stability.
Accelerated testing methodologies, while promising for expediting research, introduce their own set of challenges. The correlation between accelerated test results and real-world performance remains contentious, with questions about whether mechanisms of degradation under accelerated conditions accurately reflect those occurring during normal operation.
Instrumentation limitations present another substantial hurdle. Current voltage measurement devices often struggle with drift over time, introducing systematic errors that compound during long-term studies. High-precision potentiostats and voltmeters capable of maintaining accuracy over weeks or months are prohibitively expensive for many research institutions, creating a barrier to comprehensive stability analysis.
Reference electrode stability poses a particular challenge in long-term measurements. The gradual degradation of reference electrodes introduces shifting baselines that can mask or exaggerate actual cell voltage changes. This problem becomes especially pronounced in studies exceeding several weeks, where reference drift can account for significant portions of observed voltage variations.
Data acquisition and management systems face unique challenges when handling continuous measurements over extended periods. The sheer volume of data generated during long-term stability tests requires sophisticated storage solutions and processing algorithms. Power interruptions and system crashes can result in critical data loss, compromising weeks or months of experimental work.
Electrochemical side reactions represent a fundamental scientific challenge in stability measurements. These parasitic reactions gradually alter electrode surfaces and electrolyte composition, affecting voltage readings in ways that are difficult to quantify or predict. Distinguishing between reversible and irreversible voltage changes requires complex analytical techniques that are still evolving.
Standardization across the field remains inadequate, with various research groups employing different protocols, reference points, and reporting methods. This lack of standardization makes comparative analysis between studies problematic and hinders the establishment of industry benchmarks for voltage stability.
Accelerated testing methodologies, while promising for expediting research, introduce their own set of challenges. The correlation between accelerated test results and real-world performance remains contentious, with questions about whether mechanisms of degradation under accelerated conditions accurately reflect those occurring during normal operation.
Contemporary Approaches to Cell Voltage Stability Monitoring
01 Electrolyte composition for voltage stability
The composition of electrolytes plays a crucial role in maintaining voltage stability in electrochemical cells. Specific electrolyte formulations can minimize voltage fluctuations during charging and discharging cycles. These formulations may include additives that prevent side reactions at electrode surfaces, reduce internal resistance, and enhance ionic conductivity, all contributing to more stable cell voltage over time.- Electrolyte composition for voltage stability: The composition of electrolytes plays a crucial role in maintaining voltage stability in electrochemical cells. Specific electrolyte formulations can minimize voltage fluctuations during charging and discharging cycles. These formulations may include additives that prevent side reactions at electrode surfaces, reduce internal resistance, and enhance ionic conductivity, all contributing to more stable cell voltage over time.
- Electrode material selection for voltage stability: The choice of electrode materials significantly impacts the voltage stability of electrochemical cells. Materials with consistent redox potentials and minimal structural changes during cycling help maintain stable cell voltages. Advanced electrode materials can reduce polarization effects and voltage hysteresis, leading to more reliable and consistent cell performance across multiple charge-discharge cycles.
- Battery management systems for voltage regulation: Battery management systems (BMS) employ sophisticated control algorithms to monitor and regulate cell voltage. These systems can detect voltage anomalies, balance cells in multi-cell configurations, and implement protective measures to prevent over-voltage or under-voltage conditions. Advanced BMS technologies incorporate temperature compensation and adaptive control strategies to maintain voltage stability under varying operational conditions.
- Temperature control methods for voltage stabilization: Temperature fluctuations can significantly impact electrochemical cell voltage stability. Various thermal management techniques, including active cooling systems, phase change materials, and insulation designs, help maintain optimal operating temperatures. Controlling the cell temperature within a narrow range minimizes internal resistance variations and prevents accelerated degradation mechanisms that affect voltage stability.
- Novel cell designs for enhanced voltage stability: Innovative electrochemical cell architectures incorporate structural features that promote voltage stability. These designs may include modified separator configurations, optimized electrode geometries, and improved current collector designs. Some approaches focus on minimizing internal impedance variations, reducing concentration gradients within the cell, and ensuring uniform current distribution, all of which contribute to more stable cell voltage during operation.
02 Electrode material selection for stable voltage
The choice of electrode materials significantly impacts voltage stability in electrochemical cells. Materials with consistent redox potentials and minimal structural changes during cycling help maintain stable cell voltage. Advanced electrode materials can reduce voltage hysteresis, prevent capacity fading, and ensure reliable performance across a wide range of operating conditions, resulting in improved voltage stability throughout the cell's lifetime.Expand Specific Solutions03 Battery management systems for voltage regulation
Battery management systems (BMS) employ sophisticated control algorithms to monitor and regulate cell voltage. These systems can detect voltage anomalies, balance cells within a battery pack, and implement protective measures to prevent over-voltage or under-voltage conditions. Advanced BMS technologies incorporate temperature compensation, state-of-charge estimation, and adaptive control strategies to maintain voltage stability under varying operational demands.Expand Specific Solutions04 Temperature control methods for voltage stabilization
Temperature management is essential for maintaining voltage stability in electrochemical cells. Extreme temperatures can cause significant voltage fluctuations and accelerate degradation mechanisms. Thermal management solutions including active cooling systems, phase change materials, and insulation techniques help maintain optimal operating temperatures. Controlling temperature gradients within cells and across battery packs ensures more uniform voltage behavior and extends cell life.Expand Specific Solutions05 Novel cell designs for enhanced voltage stability
Innovative electrochemical cell designs incorporate structural features that promote voltage stability. These designs may include modified current collectors, optimized electrode geometries, and improved separator technologies. Some approaches focus on reducing internal impedance, minimizing concentration gradients, and ensuring uniform current distribution. Advanced manufacturing techniques enable precise control of cell components, resulting in more consistent and stable voltage performance throughout the cell's operational life.Expand Specific Solutions
Leading Companies and Research Institutions in Electrochemical Testing
The electrochemical cell voltage stability measurement market is currently in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. The competitive landscape features a mix of established players and emerging specialists across three main segments: academic research institutions (CNRS, Caltech, NTU), automotive manufacturers (Renault, Audi, Mercedes-Benz, BYD), and battery technology companies (CATL, LG Energy Solution, SVOLT, 24M). Technical maturity varies significantly, with companies like CATL, LG Energy Solution, and BYD leading commercial deployment while research institutions focus on fundamental innovations. Automotive manufacturers are increasingly investing in proprietary measurement technologies to support their electrification strategies. The market is characterized by growing collaboration between academic and industrial players to address challenges in long-term voltage stability measurement for next-generation battery technologies.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed an advanced Battery Management System (BMS) with high-precision voltage monitoring capabilities for measuring electrochemical cell voltage stability. Their system employs a multi-layered approach combining hardware and software solutions. The hardware includes precision voltage sensors with accuracy up to ±0.5mV and temperature compensation mechanisms to account for environmental variations. Their proprietary algorithm uses statistical methods to filter noise and detect minute voltage fluctuations that might indicate degradation patterns. The system incorporates real-time impedance spectroscopy techniques that can measure internal resistance changes without disrupting normal battery operation[1]. LG's solution also features long-term data logging capabilities that store voltage profiles over extended periods, enabling trend analysis and predictive maintenance. Their cloud-based analytics platform processes this data to generate stability reports and lifetime predictions based on voltage decay patterns observed during cycling tests.
Strengths: Superior accuracy in voltage measurements with advanced noise filtering; comprehensive data analytics for predictive maintenance; seamless integration with existing battery systems. Weaknesses: Higher implementation cost compared to conventional monitoring systems; requires significant computing resources for complex algorithms; proprietary nature limits compatibility with third-party systems.
Robert Bosch GmbH
Technical Solution: Bosch has developed an integrated Battery Management System (BMS) with advanced voltage stability monitoring capabilities for automotive and industrial applications. Their system features a distributed architecture with smart cell monitoring units that provide individual cell measurements with accuracy up to ±1mV. The solution incorporates proprietary signal processing algorithms that can detect and compensate for measurement drift over time, ensuring long-term data reliability. Bosch's approach includes dynamic calibration procedures that periodically verify measurement accuracy against internal reference voltages[4]. Their system employs machine learning algorithms trained on extensive historical data to identify patterns indicative of cell aging or impending failure based on voltage stability characteristics. The BMS includes load-dependent compensation that accounts for IR drop effects during high current operations, providing more accurate assessment of true electrochemical voltage stability. Bosch's solution also features redundant measurement paths and fault detection mechanisms to ensure reliability in safety-critical applications. Their comprehensive data management system allows for detailed analysis of voltage stability trends across different operational conditions and throughout the battery lifecycle.
Strengths: Robust design suitable for harsh automotive environments; excellent long-term measurement stability through dynamic calibration; sophisticated machine learning capabilities for predictive diagnostics. Weaknesses: Relatively high system complexity requiring specialized integration expertise; significant computational requirements for advanced analytics features; primarily optimized for automotive rather than general research applications.
Key Technical Innovations in Stability Measurement Systems
Method and apparatus for characterising an electrochemical device during operation
PatentWO2024003202A2
Innovation
- A method and apparatus that connect an electrochemical device to a power source and use an automated liquid sampler or autosampler system to probe and analyze the liquid electrolyte in-operando, detecting and characterizing liquid decomposition products via GC-MS, allowing for time-resolved measurements of aging processes and differentiation between positive and negative electrode aging.
In-situ electrochemical cell with simultaneous thermal analysis
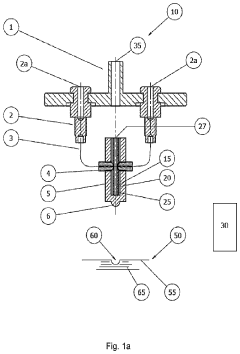
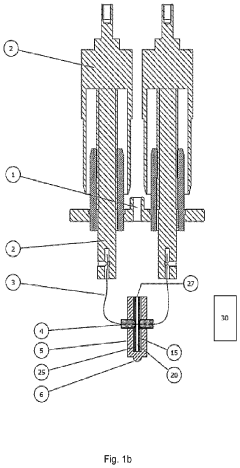
PatentPendingUS20240151780A1
Innovation
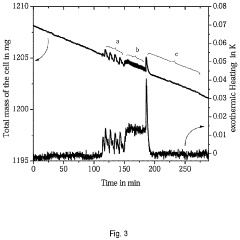
- An electrochemical cell design that allows simultaneous measurement of heat, mass change, and gas evolution during operation, using a specially adapted lid to minimize external interference and connect cables, enabling integration with thermal analyzers like TGA, DTA, and STA systems for precise analysis of heat flow, mass retention, and gas composition.
Standardization and Calibration Protocols for Stability Testing
Standardized protocols for electrochemical cell voltage stability testing are essential for ensuring reproducibility and reliability of results across different laboratories and research institutions. The development of these protocols requires careful consideration of multiple factors that can influence measurement accuracy and consistency. Currently, several international organizations, including ASTM International, IEC, and ISO, have established guidelines that serve as foundational frameworks for stability testing.
Calibration procedures must be implemented before initiating any long-term voltage stability measurements. This typically involves using certified reference materials with known electrochemical properties to verify instrument accuracy. For voltage measurements specifically, potentiostats should be calibrated using standard reference electrodes such as the Standard Hydrogen Electrode (SHE), Silver/Silver Chloride (Ag/AgCl), or Saturated Calomel Electrode (SCE). The calibration frequency should be determined based on the testing duration, with more frequent calibrations recommended for extended stability studies.
Temperature control represents a critical aspect of standardized testing protocols, as electrochemical reactions are highly temperature-dependent. Stability tests should be conducted in temperature-controlled environments, typically maintained within ±0.5°C of the target temperature. Additionally, protocols must specify whether testing occurs at ambient conditions or elevated temperatures that simulate real-world applications.
Data acquisition parameters require standardization to ensure meaningful comparisons between different studies. This includes sampling rates, filtering methods, and signal processing techniques. For long-term stability measurements, continuous data logging may generate excessive data volumes; therefore, protocols often recommend strategic sampling intervals that capture both short-term fluctuations and long-term trends without overwhelming data management systems.
Statistical analysis methods must be standardized to properly interpret voltage stability data. This includes defining acceptable drift parameters, methods for outlier identification, and statistical significance thresholds. Most protocols recommend reporting both absolute voltage values and percentage changes relative to initial readings, along with standard deviations to quantify measurement uncertainty.
Environmental factors such as humidity, atmospheric pressure, and exposure to light can significantly impact electrochemical cell performance. Standardized protocols should specify controlled environmental conditions and include procedures for documenting any deviations that might affect measurement outcomes. For cells sensitive to atmospheric contaminants, testing in inert atmospheres or sealed environments may be prescribed.
Reporting requirements constitute the final critical component of standardization protocols. These typically mandate documentation of all testing parameters, calibration records, environmental conditions, and raw data preservation methods to ensure full reproducibility and traceability of results.
Calibration procedures must be implemented before initiating any long-term voltage stability measurements. This typically involves using certified reference materials with known electrochemical properties to verify instrument accuracy. For voltage measurements specifically, potentiostats should be calibrated using standard reference electrodes such as the Standard Hydrogen Electrode (SHE), Silver/Silver Chloride (Ag/AgCl), or Saturated Calomel Electrode (SCE). The calibration frequency should be determined based on the testing duration, with more frequent calibrations recommended for extended stability studies.
Temperature control represents a critical aspect of standardized testing protocols, as electrochemical reactions are highly temperature-dependent. Stability tests should be conducted in temperature-controlled environments, typically maintained within ±0.5°C of the target temperature. Additionally, protocols must specify whether testing occurs at ambient conditions or elevated temperatures that simulate real-world applications.
Data acquisition parameters require standardization to ensure meaningful comparisons between different studies. This includes sampling rates, filtering methods, and signal processing techniques. For long-term stability measurements, continuous data logging may generate excessive data volumes; therefore, protocols often recommend strategic sampling intervals that capture both short-term fluctuations and long-term trends without overwhelming data management systems.
Statistical analysis methods must be standardized to properly interpret voltage stability data. This includes defining acceptable drift parameters, methods for outlier identification, and statistical significance thresholds. Most protocols recommend reporting both absolute voltage values and percentage changes relative to initial readings, along with standard deviations to quantify measurement uncertainty.
Environmental factors such as humidity, atmospheric pressure, and exposure to light can significantly impact electrochemical cell performance. Standardized protocols should specify controlled environmental conditions and include procedures for documenting any deviations that might affect measurement outcomes. For cells sensitive to atmospheric contaminants, testing in inert atmospheres or sealed environments may be prescribed.
Reporting requirements constitute the final critical component of standardization protocols. These typically mandate documentation of all testing parameters, calibration records, environmental conditions, and raw data preservation methods to ensure full reproducibility and traceability of results.
Environmental Factors Affecting Long-term Cell Performance
Environmental conditions play a critical role in determining the long-term voltage stability of electrochemical cells. Temperature variations represent one of the most significant factors, with both high and low extremes causing accelerated degradation. Elevated temperatures typically increase reaction rates within the cell, potentially leading to faster electrolyte decomposition, electrode material degradation, and increased internal resistance. Conversely, extremely low temperatures can reduce ionic conductivity, impair charge transfer processes, and in some cases cause physical damage to cell components through thermal contraction.
Humidity and moisture exposure constitute another major environmental challenge. Water ingress into cell components can trigger parasitic reactions, particularly in lithium-based systems where moisture reacts with electrolytes to form compounds that increase internal resistance. Even sealed cells may experience gradual moisture penetration over extended periods, making this a significant consideration for long-term stability measurements.
Atmospheric pressure fluctuations, while often overlooked, can affect cell performance by altering gas solubility within electrolytes and potentially stressing physical seals. This becomes particularly relevant for cells deployed in aerospace applications or other environments with significant pressure variations.
Electromagnetic interference (EMI) represents a growing concern as electrochemical cells are increasingly deployed in environments with complex electronic systems. Strong electromagnetic fields can potentially influence electron transfer processes at electrode surfaces and interfere with measurement equipment, complicating accurate long-term stability assessments.
Mechanical factors including vibration, shock, and physical orientation also impact voltage stability. Continuous vibration can accelerate electrode material shedding, while orientation changes may affect electrolyte distribution within the cell. These mechanical stresses often manifest as gradual capacity fade and increased internal resistance over time.
Radiation exposure presents unique challenges in specialized applications such as space systems and nuclear environments. Ionizing radiation can degrade organic electrolyte components, alter separator properties, and induce defects in crystalline electrode materials, all contributing to accelerated voltage decay.
Atmospheric contaminants like sulfur compounds, carbon dioxide, and volatile organic compounds can penetrate cell housings over time, potentially reacting with internal components. These slow chemical processes may not be apparent in short-term testing but can significantly impact long-term voltage stability, particularly in industrial or urban deployment scenarios.
Humidity and moisture exposure constitute another major environmental challenge. Water ingress into cell components can trigger parasitic reactions, particularly in lithium-based systems where moisture reacts with electrolytes to form compounds that increase internal resistance. Even sealed cells may experience gradual moisture penetration over extended periods, making this a significant consideration for long-term stability measurements.
Atmospheric pressure fluctuations, while often overlooked, can affect cell performance by altering gas solubility within electrolytes and potentially stressing physical seals. This becomes particularly relevant for cells deployed in aerospace applications or other environments with significant pressure variations.
Electromagnetic interference (EMI) represents a growing concern as electrochemical cells are increasingly deployed in environments with complex electronic systems. Strong electromagnetic fields can potentially influence electron transfer processes at electrode surfaces and interfere with measurement equipment, complicating accurate long-term stability assessments.
Mechanical factors including vibration, shock, and physical orientation also impact voltage stability. Continuous vibration can accelerate electrode material shedding, while orientation changes may affect electrolyte distribution within the cell. These mechanical stresses often manifest as gradual capacity fade and increased internal resistance over time.
Radiation exposure presents unique challenges in specialized applications such as space systems and nuclear environments. Ionizing radiation can degrade organic electrolyte components, alter separator properties, and induce defects in crystalline electrode materials, all contributing to accelerated voltage decay.
Atmospheric contaminants like sulfur compounds, carbon dioxide, and volatile organic compounds can penetrate cell housings over time, potentially reacting with internal components. These slow chemical processes may not be apparent in short-term testing but can significantly impact long-term voltage stability, particularly in industrial or urban deployment scenarios.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!