Benchmarking Electrochemical Cell Potential in Wearable Tech
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Potential in Wearables: Background & Objectives
Electrochemical cell technology has evolved significantly over the past decades, transitioning from bulky laboratory equipment to miniaturized components suitable for integration into wearable devices. This evolution has been driven by advancements in materials science, nanotechnology, and manufacturing processes, enabling the development of flexible, lightweight, and efficient electrochemical cells. The trajectory of this technology shows a clear trend toward increased energy density, reduced form factor, and enhanced biocompatibility—all critical factors for wearable applications.
The wearable technology market has experienced exponential growth, with global revenues projected to exceed $100 billion by 2025. Within this expanding ecosystem, electrochemical cells serve as fundamental power sources for a wide range of devices, from fitness trackers to medical monitoring systems. The performance benchmarking of these cells has become increasingly important as manufacturers seek to optimize battery life, reliability, and safety while minimizing size and weight.
Current benchmarking methodologies for electrochemical cell potential in wearables often lack standardization, making it difficult to compare performance across different products and technologies. This technical gap presents significant challenges for both manufacturers and consumers, as it impedes informed decision-making and slows innovation cycles. Establishing robust, industry-wide benchmarking protocols would accelerate development and adoption of next-generation wearable technologies.
The primary objective of this technical research is to develop a comprehensive framework for benchmarking electrochemical cell potential specifically tailored to wearable technology applications. This framework aims to address the unique constraints and requirements of wearable devices, including variable load profiles, thermal management challenges, and the impact of body proximity on cell performance.
Secondary objectives include identifying key performance indicators that accurately reflect real-world usage scenarios, establishing standardized testing methodologies that account for the dynamic operating conditions of wearables, and creating reference datasets that can serve as industry benchmarks. These objectives align with broader industry goals of extending device runtime, improving user experience, and enabling new functionalities through enhanced power management.
The successful development of such benchmarking standards would benefit multiple stakeholders across the wearable technology ecosystem, including component manufacturers, device designers, and end users. For manufacturers, standardized benchmarking would drive competitive innovation and quality improvements. For designers, it would facilitate more accurate power budgeting and system optimization. For consumers, it would enable more informed purchasing decisions based on reliable performance metrics.
The wearable technology market has experienced exponential growth, with global revenues projected to exceed $100 billion by 2025. Within this expanding ecosystem, electrochemical cells serve as fundamental power sources for a wide range of devices, from fitness trackers to medical monitoring systems. The performance benchmarking of these cells has become increasingly important as manufacturers seek to optimize battery life, reliability, and safety while minimizing size and weight.
Current benchmarking methodologies for electrochemical cell potential in wearables often lack standardization, making it difficult to compare performance across different products and technologies. This technical gap presents significant challenges for both manufacturers and consumers, as it impedes informed decision-making and slows innovation cycles. Establishing robust, industry-wide benchmarking protocols would accelerate development and adoption of next-generation wearable technologies.
The primary objective of this technical research is to develop a comprehensive framework for benchmarking electrochemical cell potential specifically tailored to wearable technology applications. This framework aims to address the unique constraints and requirements of wearable devices, including variable load profiles, thermal management challenges, and the impact of body proximity on cell performance.
Secondary objectives include identifying key performance indicators that accurately reflect real-world usage scenarios, establishing standardized testing methodologies that account for the dynamic operating conditions of wearables, and creating reference datasets that can serve as industry benchmarks. These objectives align with broader industry goals of extending device runtime, improving user experience, and enabling new functionalities through enhanced power management.
The successful development of such benchmarking standards would benefit multiple stakeholders across the wearable technology ecosystem, including component manufacturers, device designers, and end users. For manufacturers, standardized benchmarking would drive competitive innovation and quality improvements. For designers, it would facilitate more accurate power budgeting and system optimization. For consumers, it would enable more informed purchasing decisions based on reliable performance metrics.
Market Analysis for Wearable Electrochemical Sensors
The wearable electrochemical sensor market has experienced remarkable growth, expanding from approximately $5.2 billion in 2020 to an estimated $7.8 billion in 2023. This segment is projected to reach $12.5 billion by 2027, representing a compound annual growth rate (CAGR) of 12.6%. The rapid expansion is primarily driven by increasing health consciousness among consumers and the growing prevalence of chronic diseases requiring continuous monitoring.
Consumer demand for non-invasive, continuous health monitoring solutions has created significant market opportunities. Particularly strong growth is observed in glucose monitoring sensors, which account for 38% of the current market share, followed by electrolyte sensors (22%) and lactate sensors (15%). The remaining market comprises various specialized sensors for monitoring specific biomarkers.
Healthcare applications dominate the market with 65% share, while fitness and wellness applications represent 28%. The remaining 7% is distributed across industrial safety and specialized research applications. North America leads with 42% market share, followed by Europe (28%), Asia-Pacific (24%), and other regions (6%).
The consumer segment shows the highest growth potential, with a projected CAGR of 15.3% through 2027. This is attributed to increasing adoption of health tracking devices among the general population and declining sensor costs. The medical-grade segment maintains steady growth at 10.8% CAGR, driven by increasing integration of remote patient monitoring systems in healthcare delivery.
Key market drivers include technological advancements in miniaturization, improved sensor accuracy, extended battery life, and enhanced data processing capabilities. The integration of artificial intelligence for data interpretation has significantly increased the value proposition of these devices. Additionally, favorable reimbursement policies for remote patient monitoring in developed markets have accelerated adoption in clinical settings.
Market challenges include concerns about data privacy, accuracy limitations in non-controlled environments, and regulatory hurdles for medical-grade applications. The high initial development costs and technical complexity of electrochemical cell potential benchmarking remain significant barriers to market entry for smaller players.
Consumer price sensitivity varies by segment, with medical-grade devices commanding premium prices ($150-500) while consumer-grade devices typically range from $50-200. The subscription-based service model is gaining traction, with approximately 32% of wearable electrochemical sensor companies now offering data analysis services as a recurring revenue stream.
Consumer demand for non-invasive, continuous health monitoring solutions has created significant market opportunities. Particularly strong growth is observed in glucose monitoring sensors, which account for 38% of the current market share, followed by electrolyte sensors (22%) and lactate sensors (15%). The remaining market comprises various specialized sensors for monitoring specific biomarkers.
Healthcare applications dominate the market with 65% share, while fitness and wellness applications represent 28%. The remaining 7% is distributed across industrial safety and specialized research applications. North America leads with 42% market share, followed by Europe (28%), Asia-Pacific (24%), and other regions (6%).
The consumer segment shows the highest growth potential, with a projected CAGR of 15.3% through 2027. This is attributed to increasing adoption of health tracking devices among the general population and declining sensor costs. The medical-grade segment maintains steady growth at 10.8% CAGR, driven by increasing integration of remote patient monitoring systems in healthcare delivery.
Key market drivers include technological advancements in miniaturization, improved sensor accuracy, extended battery life, and enhanced data processing capabilities. The integration of artificial intelligence for data interpretation has significantly increased the value proposition of these devices. Additionally, favorable reimbursement policies for remote patient monitoring in developed markets have accelerated adoption in clinical settings.
Market challenges include concerns about data privacy, accuracy limitations in non-controlled environments, and regulatory hurdles for medical-grade applications. The high initial development costs and technical complexity of electrochemical cell potential benchmarking remain significant barriers to market entry for smaller players.
Consumer price sensitivity varies by segment, with medical-grade devices commanding premium prices ($150-500) while consumer-grade devices typically range from $50-200. The subscription-based service model is gaining traction, with approximately 32% of wearable electrochemical sensor companies now offering data analysis services as a recurring revenue stream.
Current Benchmarking Challenges in Wearable Electrochemistry
The field of wearable electrochemistry faces significant benchmarking challenges that impede standardization and comparative analysis across different devices and technologies. One primary challenge is the lack of universally accepted protocols for measuring electrochemical cell potential in wearable contexts. Unlike laboratory settings where conditions can be tightly controlled, wearable devices operate in dynamic environments with varying temperature, humidity, and mechanical stresses that affect electrochemical performance.
Miniaturization presents another substantial challenge, as reducing the size of electrochemical cells for wearable applications often leads to compromised signal quality and stability. The trade-off between device comfort and electrochemical performance creates a complex optimization problem that lacks standardized evaluation metrics. This makes it difficult to compare solutions across different research groups and manufacturers.
Power management represents a critical benchmarking challenge. Wearable electrochemical sensors must balance sensitivity and accuracy with energy efficiency. Current benchmarking approaches often fail to adequately characterize the relationship between power consumption and measurement quality under real-world usage conditions, leading to inconsistent performance evaluations.
Biological interface variability significantly complicates benchmarking efforts. Skin conditions, perspiration levels, and individual physiological differences create highly variable testing environments. Without standardized methods to account for these biological variables, benchmarking results often lack reproducibility and transferability between different user populations.
Long-term stability assessment presents particular difficulties in wearable electrochemistry. While laboratory tests might evaluate performance over hours or days, real-world wearable applications require stability over weeks or months. Current accelerated aging protocols poorly correlate with actual device longevity in practical use scenarios, creating a disconnect between benchmark results and real-world performance.
Cross-platform data comparability remains problematic due to proprietary algorithms and signal processing techniques employed by different manufacturers. This lack of transparency makes it challenging to isolate the performance of the electrochemical cell itself from the surrounding electronic and computational systems.
Environmental interference effects, including electromagnetic radiation from other electronic devices and chemical contaminants in everyday environments, are inconsistently accounted for in current benchmarking approaches. This leads to significant discrepancies between laboratory performance metrics and real-world reliability.
Regulatory frameworks for wearable electrochemical devices remain fragmented across different regions and applications, further complicating the establishment of universal benchmarking standards. This regulatory uncertainty discourages investment in standardization efforts and perpetuates the fragmentation of benchmarking approaches in the industry.
Miniaturization presents another substantial challenge, as reducing the size of electrochemical cells for wearable applications often leads to compromised signal quality and stability. The trade-off between device comfort and electrochemical performance creates a complex optimization problem that lacks standardized evaluation metrics. This makes it difficult to compare solutions across different research groups and manufacturers.
Power management represents a critical benchmarking challenge. Wearable electrochemical sensors must balance sensitivity and accuracy with energy efficiency. Current benchmarking approaches often fail to adequately characterize the relationship between power consumption and measurement quality under real-world usage conditions, leading to inconsistent performance evaluations.
Biological interface variability significantly complicates benchmarking efforts. Skin conditions, perspiration levels, and individual physiological differences create highly variable testing environments. Without standardized methods to account for these biological variables, benchmarking results often lack reproducibility and transferability between different user populations.
Long-term stability assessment presents particular difficulties in wearable electrochemistry. While laboratory tests might evaluate performance over hours or days, real-world wearable applications require stability over weeks or months. Current accelerated aging protocols poorly correlate with actual device longevity in practical use scenarios, creating a disconnect between benchmark results and real-world performance.
Cross-platform data comparability remains problematic due to proprietary algorithms and signal processing techniques employed by different manufacturers. This lack of transparency makes it challenging to isolate the performance of the electrochemical cell itself from the surrounding electronic and computational systems.
Environmental interference effects, including electromagnetic radiation from other electronic devices and chemical contaminants in everyday environments, are inconsistently accounted for in current benchmarking approaches. This leads to significant discrepancies between laboratory performance metrics and real-world reliability.
Regulatory frameworks for wearable electrochemical devices remain fragmented across different regions and applications, further complicating the establishment of universal benchmarking standards. This regulatory uncertainty discourages investment in standardization efforts and perpetuates the fragmentation of benchmarking approaches in the industry.
Benchmarking Methodologies for Wearable Cell Potentials
01 Electrode materials for optimizing cell potential
The selection of electrode materials plays a crucial role in determining the cell potential of electrochemical cells. Different materials exhibit varying electrochemical properties that affect the overall potential. Advanced electrode compositions, including novel alloys and composite materials, can significantly enhance the cell potential by improving electron transfer kinetics and reducing internal resistance. These materials are designed to maximize the potential difference between the anode and cathode, resulting in higher overall cell voltage.- Electrode materials for enhanced cell potential: The selection of electrode materials plays a crucial role in determining the cell potential of electrochemical cells. Various materials such as metal alloys, carbon-based materials, and composite electrodes can be engineered to optimize the electrochemical potential difference. These materials can be designed with specific surface structures and compositions to facilitate electron transfer and improve the overall cell potential, resulting in more efficient energy conversion and storage systems.
- Electrolyte composition effects on cell potential: The composition of the electrolyte significantly influences the cell potential in electrochemical cells. By adjusting the concentration, pH, and ionic species present in the electrolyte, the redox potential and conductivity can be optimized. Advanced electrolyte formulations may include additives that stabilize the electrode-electrolyte interface, reduce internal resistance, and prevent side reactions, thereby maintaining higher cell potentials over extended operational periods.
- Temperature control systems for stable cell potential: Temperature management systems are essential for maintaining stable cell potentials in electrochemical cells. Fluctuations in temperature can significantly affect reaction kinetics, electrolyte conductivity, and electrode performance. Innovative cooling and heating mechanisms, thermal insulation designs, and temperature monitoring systems help maintain optimal operating conditions, ensuring consistent cell potential and preventing degradation of cell components during operation.
- Advanced cell architectures for improved potential: Novel cell architectures can significantly enhance the cell potential of electrochemical systems. These designs include optimized electrode spacing, innovative cell geometries, and multi-layer configurations that minimize internal resistance and maximize active surface area. Some advanced architectures incorporate flow systems, bipolar designs, or stacked cell arrangements that allow for better utilization of reactive materials and more efficient electron transfer pathways, resulting in higher overall cell potentials.
- Monitoring and control systems for cell potential optimization: Sophisticated monitoring and control systems are developed to optimize and maintain cell potential in electrochemical cells. These systems employ sensors to continuously measure parameters such as voltage, current, temperature, and electrolyte properties. Advanced algorithms process this data to make real-time adjustments to operating conditions, ensuring optimal cell potential throughout various operational phases. These systems may include predictive maintenance capabilities that identify potential issues before they affect cell performance.
02 Electrolyte composition effects on cell potential
The composition of the electrolyte solution significantly impacts the cell potential in electrochemical cells. Optimized electrolyte formulations can enhance ionic conductivity, reduce internal resistance, and improve the stability of the electrochemical interface. Factors such as electrolyte concentration, pH, temperature, and the presence of additives can be manipulated to maximize the cell potential. Advanced electrolyte systems may incorporate ionic liquids or gel polymers to achieve higher potential values while maintaining stability over extended operational periods.Expand Specific Solutions03 Temperature and pressure effects on cell potential
Environmental conditions, particularly temperature and pressure, have significant effects on electrochemical cell potential. Higher temperatures generally increase ionic mobility and reaction rates, potentially enhancing cell potential, while extreme temperatures can degrade components and reduce performance. Pressure variations can affect the solubility of gases in electrolytes and the thermodynamics of electrode reactions. Controlling these parameters allows for optimization of cell potential in various applications, from high-temperature fuel cells to pressure-sensitive sensors.Expand Specific Solutions04 Cell design and configuration for enhanced potential
The physical design and configuration of electrochemical cells significantly impact their potential. Factors such as electrode spacing, surface area, cell geometry, and internal resistance all contribute to the overall cell potential. Advanced designs may incorporate specialized membrane separators, optimized current collectors, and innovative cell architectures to maximize potential difference. Miniaturization techniques and novel manufacturing methods can reduce internal resistance while maintaining or improving electrochemical performance, resulting in higher cell potentials for various applications.Expand Specific Solutions05 Measurement and control systems for cell potential
Accurate measurement and control of cell potential are essential for optimizing electrochemical cell performance. Advanced sensing technologies, reference electrodes, and potentiostatic control systems enable precise monitoring and adjustment of cell potential during operation. Real-time feedback mechanisms can compensate for variations in operating conditions, maintaining optimal potential levels. Digital control systems incorporating machine learning algorithms can predict potential fluctuations and implement preventive measures, extending cell life while maximizing energy efficiency and output potential.Expand Specific Solutions
Leading Companies in Wearable Electrochemical Sensing
The electrochemical cell potential benchmarking market in wearable technology is currently in its growth phase, with an estimated market size of $3-5 billion and projected annual growth of 15-20%. The technology maturity varies significantly among key players. Industry leaders like Samsung Electronics, LG Energy Solution, and Verily Life Sciences have developed advanced miniaturized electrochemical sensors with enhanced stability and accuracy for continuous health monitoring applications. Research institutions including California Institute of Technology, CNRS, and Rutgers University are pioneering next-generation materials and novel sensing methodologies. The competitive landscape is diversifying with specialized players like EVE Energy and Mona focusing on safety diagnostics and battery management systems specifically designed for wearable applications.
Verily Life Sciences LLC
Technical Solution: Verily Life Sciences has developed a sophisticated electrochemical cell potential benchmarking platform specifically designed for medical-grade wearable technology. Their system incorporates biocompatible reference electrodes with proprietary surface treatments that minimize drift when in contact with biological fluids. Verily's approach utilizes differential measurement techniques that compare working electrode potentials against multiple reference points, enabling compensation for variations in skin impedance and environmental factors. Their benchmarking methodology includes continuous impedance spectroscopy that characterizes cell behavior across frequency ranges relevant to physiological monitoring (0.1Hz-10kHz). The platform features adaptive sampling rates that increase measurement frequency during periods of rapid potential change while conserving power during stable operation. Verily has implemented machine learning algorithms that correlate electrochemical signatures with specific usage patterns and physiological states, enabling personalized battery management strategies. Their clinical studies have demonstrated measurement stability over 14+ days of continuous wear with less than 3% drift in reference potentials.
Strengths: Medical-grade accuracy suitable for diagnostic and therapeutic applications; exceptional biocompatibility for long-term wear; sophisticated adaptive algorithms that optimize power consumption while maintaining measurement integrity. Weaknesses: Higher production costs limit applications to premium medical devices; more complex regulatory pathway compared to consumer wearables; requires periodic recalibration for sustained accuracy in long-term applications.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced electrochemical cell potential monitoring systems specifically for wearable technology applications. Their proprietary BMS (Battery Management System) incorporates real-time electrochemical impedance spectroscopy to benchmark cell potential across various operating conditions. The technology utilizes micro-reference electrodes integrated into flexible battery substrates that enable continuous monitoring without compromising form factor. Their solution includes temperature-compensated algorithms that adjust benchmarking parameters based on skin temperature and environmental conditions, crucial for wearable applications. LG has implemented machine learning models that predict electrochemical performance degradation over time, allowing for preventative maintenance notifications to users. Their system achieves power densities of >400 Wh/L while maintaining accurate potential measurements within ±5mV across operating temperatures from 0-45°C.
Strengths: Industry-leading energy density combined with precise potential monitoring capabilities; extensive manufacturing infrastructure enabling rapid commercialization; comprehensive temperature compensation algorithms specifically designed for body-adjacent deployment. Weaknesses: Higher production costs compared to simpler monitoring systems; requires periodic calibration to maintain accuracy over extended use; slightly bulkier form factor than competitors without monitoring capabilities.
Key Patents in Electrochemical Cell Potential Measurement
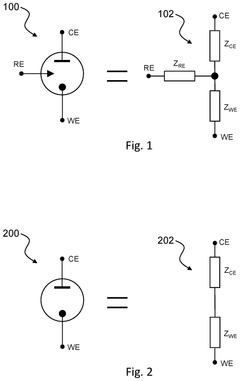
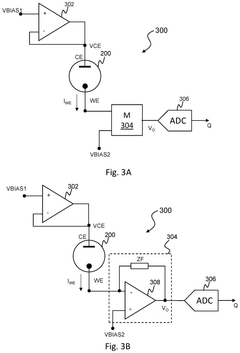
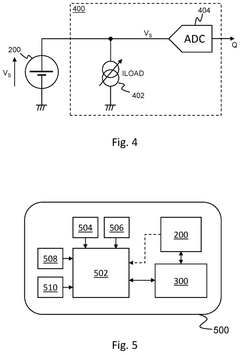
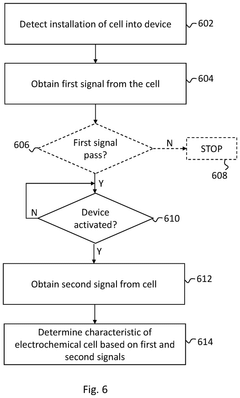
Circuitry for measurement of electrochemical cells
PatentPendingUS20250199083A1
Innovation
- The development of circuitry that obtains and compares signals from electrochemical cells before and after activation in a wearable device, allowing for the determination of characteristics such as state of health, state of charge, power fade, capacity fade, and ageing, by analyzing differences and normalizing impedance measurements.
An Energy Storage Device Monitoring Technique
PatentInactiveUS20200292622A1
Innovation
- A method and system that operate the electrochemical device with a predefined time-varying current, sense voltage and heat generation, calculate the open circuit potential, and determine the state of health by analyzing the ratio of time derivatives of these measurements, allowing for accurate identification of cell health without direct current measurement or complex systems.
Miniaturization Trends in Electrochemical Wearable Sensors
The miniaturization of electrochemical sensors has been a critical advancement enabling the widespread adoption of wearable health monitoring technologies. Over the past decade, sensor dimensions have decreased dramatically, with the latest generation of electrochemical wearable sensors achieving footprints below 10mm² while maintaining high sensitivity and specificity for target analytes.
This miniaturization trend has been driven by several complementary technological developments. Microfabrication techniques borrowed from the semiconductor industry have enabled the creation of electrode arrays with feature sizes in the sub-micron range. These techniques include photolithography, screen printing, and inkjet printing, which allow for precise deposition of conductive materials on flexible substrates.
Material science innovations have further accelerated miniaturization through the development of novel nanomaterials with enhanced electrochemical properties. Carbon-based nanomaterials such as graphene, carbon nanotubes, and carbon quantum dots have demonstrated exceptional electrical conductivity and electroactive surface area despite their minimal dimensions, enabling smaller sensors without compromising performance.
The integration of microfluidics with electrochemical sensing platforms represents another significant advancement in miniaturization. Microfluidic channels with dimensions of 10-100 micrometers can efficiently direct sample fluids to sensing surfaces, reducing the required sample volume from milliliters to nanoliters. This integration has been particularly valuable for continuous monitoring applications in wearable devices.
Power requirements have been substantially reduced through circuit optimization and energy harvesting technologies. Modern electrochemical sensor systems can operate on sub-milliwatt power levels, enabling the use of smaller batteries or even energy harvesting from body heat or movement, further reducing the overall device footprint.
Flexible and stretchable electronics have emerged as a complementary trend to miniaturization, allowing sensors to conform to the body's contours while maintaining reliable performance. Techniques such as serpentine interconnect designs and intrinsically stretchable conductive polymers have enabled sensors that can be integrated into textiles or applied directly to the skin as temporary tattoos.
The latest generation of miniaturized electrochemical wearable sensors has achieved remarkable form factors, with some systems approaching the thickness of a human hair (approximately 100 micrometers) while integrating sensing, signal processing, and wireless communication capabilities. These advancements have opened new application domains previously inaccessible to conventional sensing technologies, particularly in continuous health monitoring and point-of-care diagnostics.
This miniaturization trend has been driven by several complementary technological developments. Microfabrication techniques borrowed from the semiconductor industry have enabled the creation of electrode arrays with feature sizes in the sub-micron range. These techniques include photolithography, screen printing, and inkjet printing, which allow for precise deposition of conductive materials on flexible substrates.
Material science innovations have further accelerated miniaturization through the development of novel nanomaterials with enhanced electrochemical properties. Carbon-based nanomaterials such as graphene, carbon nanotubes, and carbon quantum dots have demonstrated exceptional electrical conductivity and electroactive surface area despite their minimal dimensions, enabling smaller sensors without compromising performance.
The integration of microfluidics with electrochemical sensing platforms represents another significant advancement in miniaturization. Microfluidic channels with dimensions of 10-100 micrometers can efficiently direct sample fluids to sensing surfaces, reducing the required sample volume from milliliters to nanoliters. This integration has been particularly valuable for continuous monitoring applications in wearable devices.
Power requirements have been substantially reduced through circuit optimization and energy harvesting technologies. Modern electrochemical sensor systems can operate on sub-milliwatt power levels, enabling the use of smaller batteries or even energy harvesting from body heat or movement, further reducing the overall device footprint.
Flexible and stretchable electronics have emerged as a complementary trend to miniaturization, allowing sensors to conform to the body's contours while maintaining reliable performance. Techniques such as serpentine interconnect designs and intrinsically stretchable conductive polymers have enabled sensors that can be integrated into textiles or applied directly to the skin as temporary tattoos.
The latest generation of miniaturized electrochemical wearable sensors has achieved remarkable form factors, with some systems approaching the thickness of a human hair (approximately 100 micrometers) while integrating sensing, signal processing, and wireless communication capabilities. These advancements have opened new application domains previously inaccessible to conventional sensing technologies, particularly in continuous health monitoring and point-of-care diagnostics.
Power Efficiency and Battery Life Considerations
Power efficiency stands as a critical factor in the development and adoption of wearable electrochemical sensing technologies. The limited form factor of wearable devices imposes significant constraints on battery size and capacity, making energy optimization paramount for practical applications. Current wearable electrochemical sensors typically operate within the 1.5-5V range, with power consumption varying from microwatts to milliwatts depending on sensing modalities and data transmission requirements.
Battery life considerations directly impact user experience and market viability of wearable electrochemical sensors. Most commercial wearable devices currently offer 1-7 days of continuous operation before requiring recharging, though this duration decreases substantially when continuous electrochemical monitoring is implemented. The trade-off between sampling frequency and power consumption presents a significant challenge, as higher temporal resolution in measurements directly correlates with increased energy demands.
Advanced power management strategies have emerged to address these limitations. These include adaptive sampling rates that adjust measurement frequency based on detected activity levels or physiological states. Implementation of low-power sleep modes during periods of inactivity can reduce baseline power consumption by 60-80% in many wearable platforms. Energy harvesting technologies, particularly those leveraging body heat, motion, or ambient light, show promise for extending operational periods between charges.
Circuit design optimization represents another crucial approach to improving power efficiency. The integration of ultra-low-power microcontrollers specifically designed for wearable applications has reduced processing power requirements by 30-50% compared to previous generations. Similarly, advances in analog front-end design have minimized the power needed for signal conditioning and conversion processes in electrochemical sensing applications.
Benchmarking methodologies for power efficiency in wearable electrochemical sensors must consider multiple metrics beyond simple battery life measurements. These include energy per measurement, standby power consumption, and power scaling with different operational modes. Standardized testing protocols that simulate real-world usage patterns are essential for meaningful comparisons between different technological approaches and implementations.
Future developments in battery technology will significantly impact this field. Solid-state batteries and advanced lithium-ion formulations promise 20-40% improvements in energy density, potentially extending operational periods proportionally. Meanwhile, flexible and stretchable battery technologies are evolving to better conform to wearable form factors, though these currently lag behind traditional batteries in energy density and cycle life.
Battery life considerations directly impact user experience and market viability of wearable electrochemical sensors. Most commercial wearable devices currently offer 1-7 days of continuous operation before requiring recharging, though this duration decreases substantially when continuous electrochemical monitoring is implemented. The trade-off between sampling frequency and power consumption presents a significant challenge, as higher temporal resolution in measurements directly correlates with increased energy demands.
Advanced power management strategies have emerged to address these limitations. These include adaptive sampling rates that adjust measurement frequency based on detected activity levels or physiological states. Implementation of low-power sleep modes during periods of inactivity can reduce baseline power consumption by 60-80% in many wearable platforms. Energy harvesting technologies, particularly those leveraging body heat, motion, or ambient light, show promise for extending operational periods between charges.
Circuit design optimization represents another crucial approach to improving power efficiency. The integration of ultra-low-power microcontrollers specifically designed for wearable applications has reduced processing power requirements by 30-50% compared to previous generations. Similarly, advances in analog front-end design have minimized the power needed for signal conditioning and conversion processes in electrochemical sensing applications.
Benchmarking methodologies for power efficiency in wearable electrochemical sensors must consider multiple metrics beyond simple battery life measurements. These include energy per measurement, standby power consumption, and power scaling with different operational modes. Standardized testing protocols that simulate real-world usage patterns are essential for meaningful comparisons between different technological approaches and implementations.
Future developments in battery technology will significantly impact this field. Solid-state batteries and advanced lithium-ion formulations promise 20-40% improvements in energy density, potentially extending operational periods proportionally. Meanwhile, flexible and stretchable battery technologies are evolving to better conform to wearable form factors, though these currently lag behind traditional batteries in energy density and cycle life.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!