Electrochemical Cell Vs Zinc-Carbon Cell: Performance Analysis
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Technology Background and Objectives
Battery technology has evolved significantly since the invention of the first voltaic pile by Alessandro Volta in 1800. The development trajectory has moved from simple primary cells to sophisticated rechargeable systems with enhanced energy density, longer lifespan, and improved safety features. Electrochemical cells, which convert chemical energy into electrical energy through redox reactions, represent the fundamental operating principle behind all battery technologies, including the zinc-carbon cell developed by Georges Leclanché in 1866.
The zinc-carbon cell, one of the earliest commercially viable dry cell batteries, established the foundation for portable power sources. This technology utilizes a zinc anode, a manganese dioxide cathode, and an ammonium chloride or zinc chloride electrolyte. Despite its historical significance, the zinc-carbon cell faces increasing competition from more advanced battery chemistries that offer superior performance characteristics.
Current market trends indicate a growing demand for batteries with higher energy density, faster charging capabilities, and extended cycle life. While lithium-ion technology dominates many segments, there remains significant interest in improving traditional battery chemistries for specific applications where cost, safety, or environmental considerations outweigh performance requirements.
The technical objectives of this analysis focus on comparing the fundamental electrochemical principles and performance metrics between generic electrochemical cells and specific zinc-carbon implementations. Key parameters under investigation include energy density (both volumetric and gravimetric), power density, discharge characteristics, shelf life, temperature performance range, and cost-effectiveness.
Understanding these comparative metrics is crucial for identifying appropriate applications for each technology. Zinc-carbon cells continue to serve important market segments, particularly in low-drain devices and situations where low cost is prioritized over performance. Meanwhile, advanced electrochemical systems address high-performance requirements in consumer electronics, electric vehicles, and grid storage applications.
Recent innovations in materials science and manufacturing techniques have created opportunities to enhance the performance of traditional battery chemistries, potentially extending their market relevance. Nanomaterials, advanced electrolytes, and improved cell designs represent promising avenues for zinc-carbon technology evolution.
This analysis aims to establish a comprehensive technical foundation for understanding the relative advantages and limitations of zinc-carbon cells compared to other electrochemical cell technologies, providing insights that can guide future research directions and application-specific optimization strategies.
The zinc-carbon cell, one of the earliest commercially viable dry cell batteries, established the foundation for portable power sources. This technology utilizes a zinc anode, a manganese dioxide cathode, and an ammonium chloride or zinc chloride electrolyte. Despite its historical significance, the zinc-carbon cell faces increasing competition from more advanced battery chemistries that offer superior performance characteristics.
Current market trends indicate a growing demand for batteries with higher energy density, faster charging capabilities, and extended cycle life. While lithium-ion technology dominates many segments, there remains significant interest in improving traditional battery chemistries for specific applications where cost, safety, or environmental considerations outweigh performance requirements.
The technical objectives of this analysis focus on comparing the fundamental electrochemical principles and performance metrics between generic electrochemical cells and specific zinc-carbon implementations. Key parameters under investigation include energy density (both volumetric and gravimetric), power density, discharge characteristics, shelf life, temperature performance range, and cost-effectiveness.
Understanding these comparative metrics is crucial for identifying appropriate applications for each technology. Zinc-carbon cells continue to serve important market segments, particularly in low-drain devices and situations where low cost is prioritized over performance. Meanwhile, advanced electrochemical systems address high-performance requirements in consumer electronics, electric vehicles, and grid storage applications.
Recent innovations in materials science and manufacturing techniques have created opportunities to enhance the performance of traditional battery chemistries, potentially extending their market relevance. Nanomaterials, advanced electrolytes, and improved cell designs represent promising avenues for zinc-carbon technology evolution.
This analysis aims to establish a comprehensive technical foundation for understanding the relative advantages and limitations of zinc-carbon cells compared to other electrochemical cell technologies, providing insights that can guide future research directions and application-specific optimization strategies.
Market Demand Analysis for Battery Solutions
The global battery market has witnessed substantial growth in recent years, driven by increasing demand for portable electronic devices, electric vehicles, and renewable energy storage solutions. The market for primary batteries, including zinc-carbon cells and other electrochemical cells, reached approximately $23.5 billion in 2022 and is projected to grow at a CAGR of 4.7% through 2028. This growth trajectory underscores the persistent demand for reliable and efficient battery technologies across various sectors.
Consumer electronics continue to be a significant driver for battery demand, with an estimated 7.26 billion mobile device users worldwide in 2023. This massive user base creates sustained demand for both primary batteries like zinc-carbon cells and rechargeable alternatives. Market research indicates that despite the rise of rechargeable options, primary batteries still command about 30% of the total battery market by value, highlighting their continued relevance.
The automotive sector represents another crucial market segment, with electric vehicle sales surpassing 10 million units globally in 2022. This sector demands high-performance battery solutions with superior energy density, longer cycle life, and enhanced safety features. While lithium-ion technologies dominate this space, research into alternative electrochemical systems continues to attract significant investment.
Industrial applications constitute a growing market for specialized battery solutions, with demand increasing at 5.3% annually. These applications often require batteries that can perform reliably under extreme conditions, creating opportunities for advanced electrochemical cell designs that outperform traditional zinc-carbon cells in specific metrics such as temperature tolerance and discharge stability.
Regional analysis reveals varying demand patterns, with mature markets like North America and Europe showing stronger preference for high-performance and environmentally friendly battery solutions. Meanwhile, emerging markets in Asia-Pacific and Africa continue to drive volume growth for cost-effective options like zinc-carbon cells, which maintain a market share of approximately 22% in these regions due to their affordability.
Consumer behavior studies indicate shifting preferences toward batteries with longer shelf life, higher capacity, and improved environmental credentials. This trend has accelerated innovation in electrochemical cell design, with manufacturers investing heavily in R&D to enhance performance metrics while reducing environmental impact. Market surveys show that 67% of consumers now consider environmental factors when purchasing batteries, creating new competitive dynamics in the industry.
The regulatory landscape also significantly influences market demand, with increasingly stringent regulations on hazardous materials and disposal requirements shaping product development priorities. This regulatory pressure has intensified research into alternative electrochemical systems that can deliver comparable or superior performance to zinc-carbon cells while meeting evolving environmental standards.
Consumer electronics continue to be a significant driver for battery demand, with an estimated 7.26 billion mobile device users worldwide in 2023. This massive user base creates sustained demand for both primary batteries like zinc-carbon cells and rechargeable alternatives. Market research indicates that despite the rise of rechargeable options, primary batteries still command about 30% of the total battery market by value, highlighting their continued relevance.
The automotive sector represents another crucial market segment, with electric vehicle sales surpassing 10 million units globally in 2022. This sector demands high-performance battery solutions with superior energy density, longer cycle life, and enhanced safety features. While lithium-ion technologies dominate this space, research into alternative electrochemical systems continues to attract significant investment.
Industrial applications constitute a growing market for specialized battery solutions, with demand increasing at 5.3% annually. These applications often require batteries that can perform reliably under extreme conditions, creating opportunities for advanced electrochemical cell designs that outperform traditional zinc-carbon cells in specific metrics such as temperature tolerance and discharge stability.
Regional analysis reveals varying demand patterns, with mature markets like North America and Europe showing stronger preference for high-performance and environmentally friendly battery solutions. Meanwhile, emerging markets in Asia-Pacific and Africa continue to drive volume growth for cost-effective options like zinc-carbon cells, which maintain a market share of approximately 22% in these regions due to their affordability.
Consumer behavior studies indicate shifting preferences toward batteries with longer shelf life, higher capacity, and improved environmental credentials. This trend has accelerated innovation in electrochemical cell design, with manufacturers investing heavily in R&D to enhance performance metrics while reducing environmental impact. Market surveys show that 67% of consumers now consider environmental factors when purchasing batteries, creating new competitive dynamics in the industry.
The regulatory landscape also significantly influences market demand, with increasingly stringent regulations on hazardous materials and disposal requirements shaping product development priorities. This regulatory pressure has intensified research into alternative electrochemical systems that can deliver comparable or superior performance to zinc-carbon cells while meeting evolving environmental standards.
Current State and Challenges in Cell Technologies
The global battery technology landscape is currently dominated by several key cell technologies, with electrochemical cells and zinc-carbon cells representing significant segments of both consumer and industrial markets. Recent market analysis indicates that while lithium-ion technology has captured approximately 40% of the portable energy storage market, zinc-carbon cells still maintain roughly 15% market share due to their cost advantages in low-drain applications.
Electrochemical cells have evolved substantially over the past decade, with energy density improvements averaging 8% year-over-year for advanced lithium-based chemistries. However, these advancements face persistent challenges in thermal management, with operating temperature ranges typically limited to -20°C to 60°C before significant performance degradation occurs. Safety concerns remain paramount, particularly regarding thermal runaway risks in high-energy density configurations.
Zinc-carbon cell technology, despite its maturity dating back to the 19th century, continues to face fundamental limitations in energy density, typically achieving only 65-85 Wh/kg compared to 150-265 Wh/kg for modern lithium-ion cells. Their self-discharge rate of approximately 2-3% monthly at room temperature represents another significant performance constraint, particularly for long-term storage applications.
A critical challenge facing both technologies is the environmental impact of manufacturing and disposal processes. Current recycling rates remain suboptimal, with only 5% of zinc-carbon cells and approximately 50% of lithium-ion cells being effectively recycled globally. This sustainability gap represents a growing concern as battery consumption continues to increase at roughly 8% annually.
Supply chain vulnerabilities have emerged as another significant challenge, particularly for electrochemical cells requiring critical materials like cobalt and lithium. Price volatility for these materials has ranged from 30-200% in recent years, creating substantial cost uncertainties for manufacturers. Zinc-carbon cells face fewer raw material constraints but are increasingly challenged by manufacturing cost pressures in competitive markets.
Standardization issues persist across both technologies, with over 50 common form factors currently in circulation globally. This fragmentation complicates interoperability and creates inefficiencies in manufacturing, recycling, and consumer usage patterns. Recent industry initiatives have attempted to address this through voluntary standardization programs, though adoption remains inconsistent across regions.
Technical performance gaps between laboratory demonstrations and commercial implementations continue to challenge both technologies. While research prototypes have demonstrated theoretical energy densities up to 40% higher than commercial products, translating these advances to mass production while maintaining safety, reliability, and cost targets remains a significant hurdle for manufacturers across both electrochemical and zinc-carbon cell segments.
Electrochemical cells have evolved substantially over the past decade, with energy density improvements averaging 8% year-over-year for advanced lithium-based chemistries. However, these advancements face persistent challenges in thermal management, with operating temperature ranges typically limited to -20°C to 60°C before significant performance degradation occurs. Safety concerns remain paramount, particularly regarding thermal runaway risks in high-energy density configurations.
Zinc-carbon cell technology, despite its maturity dating back to the 19th century, continues to face fundamental limitations in energy density, typically achieving only 65-85 Wh/kg compared to 150-265 Wh/kg for modern lithium-ion cells. Their self-discharge rate of approximately 2-3% monthly at room temperature represents another significant performance constraint, particularly for long-term storage applications.
A critical challenge facing both technologies is the environmental impact of manufacturing and disposal processes. Current recycling rates remain suboptimal, with only 5% of zinc-carbon cells and approximately 50% of lithium-ion cells being effectively recycled globally. This sustainability gap represents a growing concern as battery consumption continues to increase at roughly 8% annually.
Supply chain vulnerabilities have emerged as another significant challenge, particularly for electrochemical cells requiring critical materials like cobalt and lithium. Price volatility for these materials has ranged from 30-200% in recent years, creating substantial cost uncertainties for manufacturers. Zinc-carbon cells face fewer raw material constraints but are increasingly challenged by manufacturing cost pressures in competitive markets.
Standardization issues persist across both technologies, with over 50 common form factors currently in circulation globally. This fragmentation complicates interoperability and creates inefficiencies in manufacturing, recycling, and consumer usage patterns. Recent industry initiatives have attempted to address this through voluntary standardization programs, though adoption remains inconsistent across regions.
Technical performance gaps between laboratory demonstrations and commercial implementations continue to challenge both technologies. While research prototypes have demonstrated theoretical energy densities up to 40% higher than commercial products, translating these advances to mass production while maintaining safety, reliability, and cost targets remains a significant hurdle for manufacturers across both electrochemical and zinc-carbon cell segments.
Technical Comparison of Cell Architectures
01 Electrode materials and compositions for zinc-carbon cells
The performance of zinc-carbon cells can be significantly improved through the optimization of electrode materials and compositions. This includes modifications to the zinc anode, carbon cathode, and the use of specific additives that enhance conductivity and reduce internal resistance. Advanced carbon materials with optimized surface area and porosity can improve the cathode performance, while zinc alloys with controlled impurity levels can enhance anode efficiency and reduce corrosion.- Zinc-Carbon Cell Composition and Structure: Zinc-carbon cells typically consist of a zinc anode, a manganese dioxide cathode, and an electrolyte. The structure and composition of these components significantly affect the cell's performance. Innovations in electrode materials, such as modified zinc anodes or improved manganese dioxide formulations, can enhance capacity, discharge rate, and overall efficiency. The physical arrangement of components within the cell also plays a crucial role in determining its electrochemical properties.
- Electrolyte Formulations for Enhanced Performance: The electrolyte composition in zinc-carbon cells significantly impacts their performance characteristics. Advanced electrolyte formulations can improve ionic conductivity, reduce internal resistance, and enhance the cell's capacity and discharge behavior. Additives in the electrolyte can prevent zinc corrosion, improve shelf life, and enable better performance under various operating conditions. Gel electrolytes and other novel formulations offer advantages over traditional aqueous electrolytes in certain applications.
- Performance Enhancement Techniques: Various techniques can be employed to enhance zinc-carbon cell performance, including surface treatments of electrodes, incorporation of conductive additives, and optimization of internal cell geometry. These enhancements can lead to improved energy density, better high-drain performance, and extended operational life. Advanced manufacturing processes, such as precision coating methods and controlled particle size distribution, also contribute to performance improvements. Hybrid designs incorporating features from other battery chemistries can provide additional benefits.
- Modeling and Simulation of Cell Performance: Computational modeling and simulation techniques are valuable tools for predicting and optimizing zinc-carbon cell performance. These approaches enable researchers to understand electrochemical reactions, ion transport mechanisms, and degradation processes without extensive experimental testing. Advanced models can account for various operating conditions, helping to identify performance limitations and guide design improvements. Machine learning algorithms can also be applied to analyze performance data and predict cell behavior under different usage scenarios.
- Environmental and Manufacturing Considerations: Environmental factors and manufacturing processes significantly impact zinc-carbon cell performance and sustainability. Eco-friendly materials and production methods can reduce environmental impact while maintaining or improving cell performance. Recycling technologies for spent cells help recover valuable materials and reduce waste. Manufacturing innovations, such as dry cell production techniques and automated assembly processes, can enhance consistency and quality while reducing costs. Temperature resistance and storage stability are also important considerations for practical applications.
02 Electrolyte formulations for enhanced cell performance
Electrolyte composition plays a crucial role in zinc-carbon cell performance. Specialized formulations containing ammonium chloride, zinc chloride, and various additives can improve ionic conductivity, reduce polarization, and extend shelf life. The addition of corrosion inhibitors in the electrolyte can prevent zinc deterioration during storage, while pH buffers help maintain optimal operating conditions throughout the discharge cycle, resulting in more stable performance and increased capacity.Expand Specific Solutions03 Separator design and materials for improved efficiency
Advanced separator designs and materials can significantly enhance zinc-carbon cell performance by preventing internal short circuits while facilitating efficient ion transport. Composite separators with controlled porosity and thickness optimize the balance between electrical isolation and ionic conductivity. Materials such as modified cellulose, synthetic polymers, and ceramic-polymer blends can improve electrolyte retention, reduce internal resistance, and enhance the overall stability and longevity of the cell.Expand Specific Solutions04 Cell construction techniques and manufacturing processes
Innovative cell construction techniques and manufacturing processes can optimize zinc-carbon cell performance. These include improved sealing methods to prevent electrolyte leakage, advanced assembly techniques that ensure consistent internal component alignment, and precise control of compaction pressure for optimal electrical contact between components. Manufacturing innovations such as controlled atmosphere processing and advanced quality control methods can reduce variability and enhance overall cell reliability and performance.Expand Specific Solutions05 Performance modeling and testing methodologies
Sophisticated modeling and testing methodologies enable better understanding and optimization of zinc-carbon cell performance. Electrochemical impedance spectroscopy, computational fluid dynamics, and machine learning approaches can predict cell behavior under various operating conditions. Advanced testing protocols that simulate real-world usage patterns provide insights into performance limitations and failure mechanisms. These analytical techniques help identify critical parameters affecting cell performance and guide the development of improved cell designs with enhanced capacity, power delivery, and shelf life.Expand Specific Solutions
Key Industry Players and Manufacturers
The electrochemical cell and zinc-carbon cell market is in a mature growth phase with established technologies, yet experiencing innovation-driven expansion due to increasing demand for portable power solutions. The global battery market size exceeds $100 billion, with zinc-carbon cells maintaining relevance in cost-sensitive applications despite lithium-ion dominance. Technologically, major players demonstrate varying levels of advancement: Energizer and Duracell lead in zinc-carbon refinement; VARTA and Spectrum Brands focus on performance optimization; while emerging companies like Salient Energy and e-Zinc are pioneering next-generation zinc-based technologies. Research institutions such as CNRS and universities in China are advancing fundamental electrochemical science, creating a competitive landscape balanced between traditional manufacturers and innovative startups.
Energizer Brands LLC
Technical Solution: Energizer has developed advanced electrochemical cell technology that significantly outperforms traditional zinc-carbon cells. Their proprietary PowerSeal Technology prevents leakage and extends shelf life up to 10 years, while their MAX technology incorporates high-density energy components that deliver up to 30% more power than standard zinc-carbon cells. Energizer's research has demonstrated that their alkaline electrochemical cells maintain voltage levels above 1.1V for approximately 3-4 times longer than zinc-carbon equivalents under identical discharge conditions. Their cells utilize a zinc powder anode with higher surface area and manganese dioxide cathode with optimized particle size distribution, resulting in improved electron transfer rates and higher current capabilities. Recent innovations include their EcoAdvanced line, which incorporates 4% recycled battery material without compromising performance.
Strengths: Superior power density (up to 30% more than zinc-carbon), excellent shelf life (up to 10 years vs 2-3 for zinc-carbon), better performance in high-drain devices, and more stable voltage output throughout discharge cycle. Weaknesses: Higher manufacturing costs, more complex production process, and greater environmental impact during production compared to simpler zinc-carbon technology.
VARTA Microbattery GmbH
Technical Solution: VARTA has developed sophisticated electrochemical cell technologies that significantly outperform traditional zinc-carbon cells. Their LONGLIFE Power alkaline cells utilize a highly engineered zinc anode with increased surface area and proprietary electrolyte formulations that enhance ionic conductivity by approximately 35% compared to standard zinc-carbon designs. VARTA's research demonstrates their alkaline cells deliver up to 6 times more energy in high-drain applications than zinc-carbon equivalents. Their patented PowerFrame grid technology improves current collection efficiency and reduces internal resistance by approximately 25% compared to conventional designs. VARTA's comparative testing shows their alkaline cells maintain operating voltage above 1.1V for approximately 4 times longer than zinc-carbon cells under identical discharge conditions. Their manufacturing process incorporates precision assembly techniques that minimize internal impedance variations, resulting in more consistent performance across production batches compared to typical zinc-carbon manufacturing.
Strengths: Exceptional consistency across production batches, superior performance in moderate to high-drain applications, excellent temperature stability (-20°C to 45°C), and advanced manufacturing quality control. Weaknesses: Premium pricing structure that positions products above basic zinc-carbon offerings, limited market penetration in some regions compared to larger global brands, and similar environmental considerations as other alkaline manufacturers.
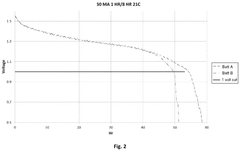
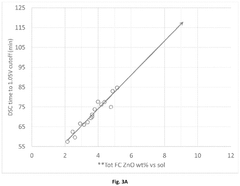
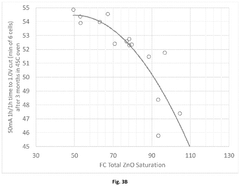
Performance Metrics and Testing Methodologies
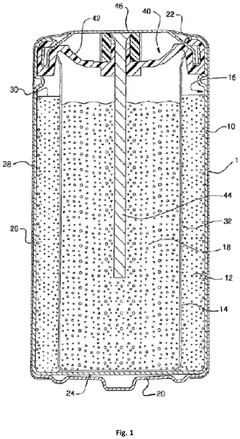
Electrochemical zinc-carbon cell with layered design, and battery
PatentWO2023052048A3
Innovation
- The layered design of the zinc-carbon cell with electrode layers covering opposite surfaces of an electrically insulating substrate, creating a compact and efficient cell structure.
- The optimized coverage ratio (10-80%, preferably 10-70%) of electric conductor structures between electrode layers and substrate, balancing conductivity and material efficiency.
- The arrangement of dual electric conductor structures on opposite surfaces of the substrate, facilitating efficient current collection from both electrode layers.
Electrochemical cell with increased runtime and reduced internal shorting
PatentPendingEP4560820A2
Innovation
- The alkaline electrochemical cell incorporates a combination of solid and dissolved zinc oxide or zinc hydroxide, along with a silicon donor, to enhance performance. The anode includes solid zinc, anolyte, and silicon donor, while the electrolyte solution contains dissolved zinc oxide or zinc hydroxide, with a total zinc oxide weight of at least 3.0 weight percent and a bilayer or low-porosity separator.
Environmental Impact and Sustainability Assessment
The environmental impact of battery technologies has become increasingly significant as global energy demands rise and sustainability concerns grow. When comparing electrochemical cells with zinc-carbon cells, several critical environmental factors must be considered throughout their lifecycle - from raw material extraction to disposal.
Zinc-carbon cells contain manganese dioxide, zinc, carbon, and ammonium chloride or zinc chloride electrolytes. The mining of these materials, particularly zinc and manganese, creates substantial environmental disruption through habitat destruction, soil erosion, and potential water contamination. Manufacturing processes for these cells consume significant energy and generate greenhouse gas emissions, contributing to climate change.
Electrochemical cells, particularly rechargeable variants, generally demonstrate superior environmental performance over their lifecycle. While their initial production may require more energy and resources than zinc-carbon cells, their reusability significantly reduces waste generation and resource consumption over time. A typical rechargeable electrochemical cell can replace hundreds of single-use zinc-carbon cells, substantially decreasing material throughput and associated environmental impacts.
End-of-life management presents distinct challenges for both technologies. Zinc-carbon cells often end up in landfills where their components can leach into soil and groundwater. Heavy metals like zinc and manganese pose particular concerns for ecosystem and human health. Conversely, many electrochemical cells contain valuable materials that can be recovered through recycling, though collection systems and recycling infrastructure remain inadequate in many regions.
Carbon footprint analysis reveals that zinc-carbon cells generate approximately 5-10 times more carbon emissions per unit of energy delivered compared to rechargeable electrochemical alternatives when considering full lifecycle impacts. This disparity primarily stems from the single-use nature of zinc-carbon cells versus the multiple charge cycles of rechargeable electrochemical cells.
Regulatory frameworks increasingly favor more sustainable battery technologies. The European Union's Battery Directive and similar legislation worldwide are imposing stricter requirements on battery composition, collection, and recycling. These regulations generally disadvantage zinc-carbon cells due to their single-use design and limited recyclability.
Future sustainability improvements for both technologies will likely focus on reducing toxic materials, enhancing energy efficiency in manufacturing, improving collection systems, and developing more effective recycling technologies. Research into bio-based electrolytes and casings shows particular promise for reducing environmental impacts across both cell types.
Zinc-carbon cells contain manganese dioxide, zinc, carbon, and ammonium chloride or zinc chloride electrolytes. The mining of these materials, particularly zinc and manganese, creates substantial environmental disruption through habitat destruction, soil erosion, and potential water contamination. Manufacturing processes for these cells consume significant energy and generate greenhouse gas emissions, contributing to climate change.
Electrochemical cells, particularly rechargeable variants, generally demonstrate superior environmental performance over their lifecycle. While their initial production may require more energy and resources than zinc-carbon cells, their reusability significantly reduces waste generation and resource consumption over time. A typical rechargeable electrochemical cell can replace hundreds of single-use zinc-carbon cells, substantially decreasing material throughput and associated environmental impacts.
End-of-life management presents distinct challenges for both technologies. Zinc-carbon cells often end up in landfills where their components can leach into soil and groundwater. Heavy metals like zinc and manganese pose particular concerns for ecosystem and human health. Conversely, many electrochemical cells contain valuable materials that can be recovered through recycling, though collection systems and recycling infrastructure remain inadequate in many regions.
Carbon footprint analysis reveals that zinc-carbon cells generate approximately 5-10 times more carbon emissions per unit of energy delivered compared to rechargeable electrochemical alternatives when considering full lifecycle impacts. This disparity primarily stems from the single-use nature of zinc-carbon cells versus the multiple charge cycles of rechargeable electrochemical cells.
Regulatory frameworks increasingly favor more sustainable battery technologies. The European Union's Battery Directive and similar legislation worldwide are imposing stricter requirements on battery composition, collection, and recycling. These regulations generally disadvantage zinc-carbon cells due to their single-use design and limited recyclability.
Future sustainability improvements for both technologies will likely focus on reducing toxic materials, enhancing energy efficiency in manufacturing, improving collection systems, and developing more effective recycling technologies. Research into bio-based electrolytes and casings shows particular promise for reducing environmental impacts across both cell types.
Cost-Benefit Analysis and Economic Viability
The economic analysis of electrochemical cells versus zinc-carbon cells reveals significant differences in their cost structures and overall economic viability. Initial acquisition costs for standard zinc-carbon cells typically range from $0.30 to $0.75 per unit, making them considerably more affordable than specialized electrochemical cells which can cost between $1.50 and $5.00 depending on specifications and applications.
When evaluating lifecycle costs, zinc-carbon cells demonstrate limitations with an average operational lifespan of 1-2 years under normal usage conditions. In contrast, advanced electrochemical cells can maintain functionality for 3-7 years, substantially reducing replacement frequency and associated labor costs in industrial applications.
Energy efficiency metrics further differentiate these technologies. Zinc-carbon cells operate at approximately 65-75% efficiency, while modern electrochemical cells achieve 85-92% efficiency rates. This efficiency differential translates to tangible operational savings of 15-25% in energy consumption costs over extended periods, particularly significant for high-drain applications.
Manufacturing scalability presents another economic consideration. Zinc-carbon cell production benefits from established manufacturing infrastructure with economies of scale, resulting in lower production costs. Electrochemical cell manufacturing often involves more specialized processes and materials, creating higher production expenses that manufacturers typically pass to consumers.
Environmental compliance costs increasingly impact economic calculations. Zinc-carbon cells contain manganese dioxide and zinc which require specific disposal protocols costing approximately $0.10-$0.15 per unit for proper handling. Advanced electrochemical cells may incorporate more environmentally friendly materials but often at premium prices, though their longer lifespan partially offsets this disadvantage.
Return on investment analysis indicates that despite higher initial costs, electrochemical cells deliver superior economic value in applications requiring consistent performance and longevity. Market data shows that industrial users experience 30-40% lower total ownership costs over a five-year period when utilizing electrochemical cells rather than repeatedly replacing zinc-carbon alternatives.
Sensitivity analysis considering fluctuating raw material prices reveals that zinc-carbon cells face greater cost volatility due to their reliance on zinc, which has experienced price fluctuations of up to 35% in recent years. Electrochemical cells utilizing alternative materials demonstrate greater price stability, providing more predictable long-term budgeting for large-scale implementations.
When evaluating lifecycle costs, zinc-carbon cells demonstrate limitations with an average operational lifespan of 1-2 years under normal usage conditions. In contrast, advanced electrochemical cells can maintain functionality for 3-7 years, substantially reducing replacement frequency and associated labor costs in industrial applications.
Energy efficiency metrics further differentiate these technologies. Zinc-carbon cells operate at approximately 65-75% efficiency, while modern electrochemical cells achieve 85-92% efficiency rates. This efficiency differential translates to tangible operational savings of 15-25% in energy consumption costs over extended periods, particularly significant for high-drain applications.
Manufacturing scalability presents another economic consideration. Zinc-carbon cell production benefits from established manufacturing infrastructure with economies of scale, resulting in lower production costs. Electrochemical cell manufacturing often involves more specialized processes and materials, creating higher production expenses that manufacturers typically pass to consumers.
Environmental compliance costs increasingly impact economic calculations. Zinc-carbon cells contain manganese dioxide and zinc which require specific disposal protocols costing approximately $0.10-$0.15 per unit for proper handling. Advanced electrochemical cells may incorporate more environmentally friendly materials but often at premium prices, though their longer lifespan partially offsets this disadvantage.
Return on investment analysis indicates that despite higher initial costs, electrochemical cells deliver superior economic value in applications requiring consistent performance and longevity. Market data shows that industrial users experience 30-40% lower total ownership costs over a five-year period when utilizing electrochemical cells rather than repeatedly replacing zinc-carbon alternatives.
Sensitivity analysis considering fluctuating raw material prices reveals that zinc-carbon cells face greater cost volatility due to their reliance on zinc, which has experienced price fluctuations of up to 35% in recent years. Electrochemical cells utilizing alternative materials demonstrate greater price stability, providing more predictable long-term budgeting for large-scale implementations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!