Electrochemical Cell Vs Primary Battery: Cycle Stability
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Technology Evolution and Objectives
Battery technology has evolved significantly over the past century, transitioning from simple primary cells to sophisticated rechargeable systems. The fundamental distinction between primary batteries and electrochemical cells (rechargeable batteries) lies in their ability to restore electrical capacity after discharge. Primary batteries, developed in the early 19th century, were designed for single-use applications, while rechargeable batteries emerged later to address the growing need for reusable energy storage solutions.
The evolution of battery technology has been driven by increasing demands for higher energy density, longer cycle life, and improved safety characteristics. Early rechargeable batteries, such as lead-acid cells invented in 1859, offered limited cycle stability with significant capacity degradation after repeated charge-discharge cycles. The introduction of nickel-cadmium (NiCd) batteries in the early 20th century marked a significant improvement in cycle stability, though still facing limitations in energy density and memory effect issues.
The 1990s witnessed a revolutionary advancement with the commercialization of lithium-ion batteries, which dramatically enhanced cycle stability compared to previous technologies. Modern lithium-ion cells can achieve 1,000-3,000 cycles before significant capacity degradation, whereas primary batteries are inherently limited to a single discharge cycle. This stark contrast in cycle stability represents one of the most critical differentiating factors between these battery types.
Current technological objectives in the field focus on extending cycle stability of electrochemical cells while maintaining or improving energy density, power capability, and safety profiles. Research efforts are directed toward understanding and mitigating degradation mechanisms that limit cycle life, including electrode material structural changes, electrolyte decomposition, and interfacial reactions during repeated cycling.
The development trajectory suggests a continued emphasis on novel electrode materials, advanced electrolyte formulations, and innovative cell designs to push the boundaries of cycle stability. Emerging technologies such as solid-state batteries and lithium-sulfur systems promise theoretical improvements in cycle life, potentially reaching 5,000+ cycles under optimal conditions.
The comparison of cycle stability between electrochemical cells and primary batteries remains a fundamental consideration in battery selection for various applications. While primary batteries offer simplicity and reliability for single-use scenarios, the superior cycle stability of rechargeable systems provides compelling economic and environmental advantages for applications requiring repeated use, driving the ongoing research and development in this critical technology domain.
The evolution of battery technology has been driven by increasing demands for higher energy density, longer cycle life, and improved safety characteristics. Early rechargeable batteries, such as lead-acid cells invented in 1859, offered limited cycle stability with significant capacity degradation after repeated charge-discharge cycles. The introduction of nickel-cadmium (NiCd) batteries in the early 20th century marked a significant improvement in cycle stability, though still facing limitations in energy density and memory effect issues.
The 1990s witnessed a revolutionary advancement with the commercialization of lithium-ion batteries, which dramatically enhanced cycle stability compared to previous technologies. Modern lithium-ion cells can achieve 1,000-3,000 cycles before significant capacity degradation, whereas primary batteries are inherently limited to a single discharge cycle. This stark contrast in cycle stability represents one of the most critical differentiating factors between these battery types.
Current technological objectives in the field focus on extending cycle stability of electrochemical cells while maintaining or improving energy density, power capability, and safety profiles. Research efforts are directed toward understanding and mitigating degradation mechanisms that limit cycle life, including electrode material structural changes, electrolyte decomposition, and interfacial reactions during repeated cycling.
The development trajectory suggests a continued emphasis on novel electrode materials, advanced electrolyte formulations, and innovative cell designs to push the boundaries of cycle stability. Emerging technologies such as solid-state batteries and lithium-sulfur systems promise theoretical improvements in cycle life, potentially reaching 5,000+ cycles under optimal conditions.
The comparison of cycle stability between electrochemical cells and primary batteries remains a fundamental consideration in battery selection for various applications. While primary batteries offer simplicity and reliability for single-use scenarios, the superior cycle stability of rechargeable systems provides compelling economic and environmental advantages for applications requiring repeated use, driving the ongoing research and development in this critical technology domain.
Market Analysis for Rechargeable vs Primary Battery Solutions
The global battery market is experiencing significant growth, with a clear divergence in demand patterns between rechargeable (secondary) and primary battery solutions. The market for rechargeable batteries has been expanding at a compound annual growth rate of approximately 8.1% since 2018, driven primarily by the proliferation of portable electronic devices, electric vehicles, and renewable energy storage systems. In contrast, primary batteries maintain a steady but slower growth rate of about 2.3% annually, primarily serving traditional consumer electronics, medical devices, and emergency backup applications.
Consumer electronics continue to be the largest application segment for both battery types, accounting for over 40% of the total battery market. However, the automotive sector has emerged as the fastest-growing segment for rechargeable batteries, with electric vehicle adoption rates accelerating globally, particularly in China, Europe, and North America.
Market research indicates that cycle stability has become a critical differentiating factor influencing purchasing decisions. In industrial applications, where operational reliability is paramount, consumers demonstrate willingness to pay premium prices for batteries with superior cycle stability. A recent industry survey revealed that 78% of industrial battery purchasers ranked cycle life as their top consideration, ahead of initial cost and energy density.
The healthcare sector represents another high-value market segment where the reliability advantages of rechargeable systems with proven cycle stability are increasingly recognized. Medical device manufacturers are transitioning from primary to rechargeable solutions for implantable devices, patient monitoring equipment, and portable diagnostic tools, citing lifecycle cost benefits and reduced environmental impact as key drivers.
Regional market analysis shows Asia-Pacific dominating battery production and consumption, accounting for approximately 65% of global manufacturing capacity. North America and Europe follow with sophisticated research capabilities and growing demand for high-performance energy storage solutions. Emerging markets in South America and Africa present significant growth opportunities, particularly for affordable rechargeable solutions that can address energy access challenges in off-grid communities.
Price sensitivity varies significantly across market segments. Consumer applications remain highly price-competitive, with rechargeable solutions needing to demonstrate clear lifetime value advantages to overcome higher initial costs. Enterprise and industrial segments show greater willingness to invest in premium battery solutions with proven cycle stability, particularly when total cost of ownership calculations demonstrate long-term economic benefits.
Market forecasts project the performance gap between primary and rechargeable batteries will continue to narrow as technological innovations improve cycle stability in rechargeable systems. This convergence is expected to accelerate market share shifts toward rechargeable solutions across most application categories, with primary batteries increasingly confined to niche applications where their specific advantages remain relevant.
Consumer electronics continue to be the largest application segment for both battery types, accounting for over 40% of the total battery market. However, the automotive sector has emerged as the fastest-growing segment for rechargeable batteries, with electric vehicle adoption rates accelerating globally, particularly in China, Europe, and North America.
Market research indicates that cycle stability has become a critical differentiating factor influencing purchasing decisions. In industrial applications, where operational reliability is paramount, consumers demonstrate willingness to pay premium prices for batteries with superior cycle stability. A recent industry survey revealed that 78% of industrial battery purchasers ranked cycle life as their top consideration, ahead of initial cost and energy density.
The healthcare sector represents another high-value market segment where the reliability advantages of rechargeable systems with proven cycle stability are increasingly recognized. Medical device manufacturers are transitioning from primary to rechargeable solutions for implantable devices, patient monitoring equipment, and portable diagnostic tools, citing lifecycle cost benefits and reduced environmental impact as key drivers.
Regional market analysis shows Asia-Pacific dominating battery production and consumption, accounting for approximately 65% of global manufacturing capacity. North America and Europe follow with sophisticated research capabilities and growing demand for high-performance energy storage solutions. Emerging markets in South America and Africa present significant growth opportunities, particularly for affordable rechargeable solutions that can address energy access challenges in off-grid communities.
Price sensitivity varies significantly across market segments. Consumer applications remain highly price-competitive, with rechargeable solutions needing to demonstrate clear lifetime value advantages to overcome higher initial costs. Enterprise and industrial segments show greater willingness to invest in premium battery solutions with proven cycle stability, particularly when total cost of ownership calculations demonstrate long-term economic benefits.
Market forecasts project the performance gap between primary and rechargeable batteries will continue to narrow as technological innovations improve cycle stability in rechargeable systems. This convergence is expected to accelerate market share shifts toward rechargeable solutions across most application categories, with primary batteries increasingly confined to niche applications where their specific advantages remain relevant.
Current Limitations in Battery Cycle Stability
Despite significant advancements in battery technology, several critical limitations continue to impede the cycle stability of both electrochemical cells and primary batteries. The fundamental challenge lies in the degradation mechanisms that occur during charge-discharge cycles, particularly in rechargeable systems. Material fatigue at the electrode-electrolyte interface represents one of the most significant barriers, where repeated ion insertion and extraction leads to structural deterioration over time.
For lithium-ion batteries, which dominate the rechargeable market, capacity fade typically manifests through several pathways. Solid-electrolyte interphase (SEI) layer growth consumes active lithium irreversibly, while electrode particle cracking creates disconnected regions that no longer participate in electrochemical reactions. These issues are exacerbated at higher charge-discharge rates and elevated temperatures, conditions increasingly demanded by modern applications.
Primary batteries, though not designed for recharging, face their own stability challenges. Self-discharge rates, particularly in alkaline and zinc-carbon chemistries, limit shelf life and reliability. Environmental factors such as temperature fluctuations accelerate internal chemical reactions that deplete capacity even during storage periods.
The electrolyte component presents limitations across all battery types. Liquid electrolytes are prone to leakage and decomposition at voltage extremes, while solid electrolytes often suffer from insufficient ionic conductivity or mechanical integrity issues during cycling. The trade-off between high energy density and long-term stability remains a persistent engineering challenge.
Manufacturing inconsistencies further compound cycle stability issues. Microscopic impurities introduced during production can serve as nucleation sites for dendrite formation or catalyze unwanted side reactions. The precision required for uniform electrode coating and electrolyte distribution becomes increasingly difficult to maintain at industrial scales.
Current battery management systems (BMS) provide only partial solutions to these limitations. While they can prevent operation outside safe voltage and temperature windows, they cannot fundamentally address the underlying chemical and physical degradation mechanisms. The BMS approach represents a compromise between maximizing immediate performance and preserving long-term stability.
Economic constraints also impact cycle stability advancements. The market pressure to reduce costs often leads to material substitutions that sacrifice longevity for affordability. This creates a technological gap between laboratory-demonstrated cycle life and commercially viable products, particularly evident in emerging battery chemistries like sodium-ion and solid-state systems.
For lithium-ion batteries, which dominate the rechargeable market, capacity fade typically manifests through several pathways. Solid-electrolyte interphase (SEI) layer growth consumes active lithium irreversibly, while electrode particle cracking creates disconnected regions that no longer participate in electrochemical reactions. These issues are exacerbated at higher charge-discharge rates and elevated temperatures, conditions increasingly demanded by modern applications.
Primary batteries, though not designed for recharging, face their own stability challenges. Self-discharge rates, particularly in alkaline and zinc-carbon chemistries, limit shelf life and reliability. Environmental factors such as temperature fluctuations accelerate internal chemical reactions that deplete capacity even during storage periods.
The electrolyte component presents limitations across all battery types. Liquid electrolytes are prone to leakage and decomposition at voltage extremes, while solid electrolytes often suffer from insufficient ionic conductivity or mechanical integrity issues during cycling. The trade-off between high energy density and long-term stability remains a persistent engineering challenge.
Manufacturing inconsistencies further compound cycle stability issues. Microscopic impurities introduced during production can serve as nucleation sites for dendrite formation or catalyze unwanted side reactions. The precision required for uniform electrode coating and electrolyte distribution becomes increasingly difficult to maintain at industrial scales.
Current battery management systems (BMS) provide only partial solutions to these limitations. While they can prevent operation outside safe voltage and temperature windows, they cannot fundamentally address the underlying chemical and physical degradation mechanisms. The BMS approach represents a compromise between maximizing immediate performance and preserving long-term stability.
Economic constraints also impact cycle stability advancements. The market pressure to reduce costs often leads to material substitutions that sacrifice longevity for affordability. This creates a technological gap between laboratory-demonstrated cycle life and commercially viable products, particularly evident in emerging battery chemistries like sodium-ion and solid-state systems.
Technical Approaches to Cycle Stability Enhancement
01 Electrode material composition for improved cycle stability
The composition of electrode materials significantly impacts the cycle stability of electrochemical cells and primary batteries. Advanced materials such as modified carbon structures, metal oxides, and composite electrodes can enhance the structural integrity during charge-discharge cycles. These materials minimize volume changes and prevent degradation, leading to improved cycle life and performance stability over extended periods of use.- Electrode material innovations for improved cycle stability: Advanced electrode materials play a crucial role in enhancing the cycle stability of electrochemical cells and primary batteries. These innovations include modified cathode and anode compositions, nanostructured materials, and composite electrodes that can withstand repeated charge-discharge cycles while maintaining capacity. Such materials reduce degradation mechanisms like volume expansion, structural collapse, and unwanted side reactions that typically lead to capacity fading over multiple cycles.
- Electrolyte formulations for enhanced cycle life: Specialized electrolyte formulations can significantly improve the cycle stability of electrochemical cells. These formulations may include additives that form stable solid-electrolyte interfaces, prevent dendrite formation, or reduce unwanted side reactions. Optimized electrolyte compositions help maintain ionic conductivity throughout the battery's life while minimizing degradation of electrode materials, resulting in extended cycle life and improved overall battery performance.
- Structural design and cell architecture for stability: The physical design and architecture of electrochemical cells significantly impact their cycle stability. Innovations in this area include optimized cell packaging, pressure management systems, electrode alignment techniques, and novel separator designs. These structural improvements help maintain physical integrity during cycling, manage thermal effects, and ensure uniform current distribution, all of which contribute to extended cycle life and improved reliability of primary batteries.
- Advanced manufacturing processes for cycle stability: Manufacturing techniques significantly influence the cycle stability of electrochemical cells and primary batteries. Precision coating methods, controlled particle size distribution, optimized drying processes, and advanced assembly techniques can reduce defects and ensure uniformity in cell components. These manufacturing innovations lead to more consistent performance across multiple charge-discharge cycles, reduced internal resistance over time, and overall improved cycle stability.
- Diagnostic and monitoring systems for cycle life prediction: Advanced diagnostic and monitoring systems enable better understanding and prediction of cycle stability in electrochemical cells. These technologies include in-situ measurement techniques, impedance spectroscopy, and computational models that can identify early signs of degradation. By monitoring key parameters during operation, these systems allow for adaptive control strategies that can extend cycle life by adjusting operating conditions based on the current state of the battery.
02 Electrolyte formulations for enhanced stability
Specialized electrolyte formulations play a crucial role in maintaining cycle stability. Additives that form stable solid-electrolyte interfaces (SEI) protect electrodes from continuous degradation. Advanced electrolyte compositions with optimized salt concentrations, solvents, and stabilizing compounds can suppress unwanted side reactions, reduce electrolyte decomposition, and maintain ionic conductivity throughout numerous cycles.Expand Specific Solutions03 Battery management systems for cycle life extension
Implementing sophisticated battery management systems can significantly improve cycle stability. These systems monitor and control charging/discharging parameters, temperature, and voltage limits to prevent conditions that accelerate degradation. Advanced algorithms optimize operating conditions based on real-time data, preventing overcharging, deep discharging, and thermal stress that would otherwise reduce cycle life.Expand Specific Solutions04 Structural design innovations for mechanical stability
Novel structural designs of battery components enhance mechanical stability during cycling. Engineered electrode architectures, separator modifications, and cell housing designs that accommodate volume changes during operation help maintain physical integrity. These structural innovations prevent internal short circuits, electrode delamination, and other mechanical failures that would otherwise limit cycle life.Expand Specific Solutions05 Surface modification and coating technologies
Surface treatments and protective coatings on electrode materials significantly improve cycle stability. These modifications create barriers against unwanted side reactions while maintaining essential electrochemical processes. Techniques include atomic layer deposition, polymer coatings, and functional surface groups that stabilize interfaces between electrodes and electrolytes, reducing capacity fade and extending usable battery life through multiple charge-discharge cycles.Expand Specific Solutions
Leading Battery Manufacturers and Research Institutions
The electrochemical cell and primary battery market is currently in a growth phase, with increasing demand driven by renewable energy integration and electric vehicle adoption. The global market size is projected to reach approximately $240 billion by 2027, expanding at a CAGR of 8.5%. Regarding technological maturity, established players like GM Global Technology, Contemporary Amperex Technology, and Saft Groupe demonstrate advanced capabilities in conventional lithium-ion technologies, while companies such as Sion Power, Wildcat Discovery Technologies, and OXLiD are pioneering next-generation chemistries including lithium-sulfur. Cycle stability remains a critical differentiator, with Automotive Cells Company, SK Innovation, and 24M Technologies focusing on enhanced durability solutions. The competitive landscape shows a clear division between traditional battery manufacturers and innovative startups developing breakthrough technologies to address cycle life limitations.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: Contemporary Amperex Technology (CATL) has developed advanced battery management systems that continuously monitor and optimize cell performance during cycling. Their proprietary electrolyte formulations incorporate functional additives that form stable solid-electrolyte interphase (SEI) layers, significantly reducing capacity fade during extended cycling. CATL's comparative studies between their lithium-ion cells and primary batteries demonstrate that while primary batteries offer higher initial energy density (up to 270 Wh/kg for lithium primary cells versus 260 Wh/kg for their rechargeable counterparts), their electrochemical cells maintain over 80% capacity after 2000 cycles under standardized testing protocols. Their research includes comprehensive degradation mechanism analysis using in-situ characterization techniques to identify failure modes in both battery types.
Strengths: Industry-leading cycle life in lithium-ion cells; sophisticated battery management systems that extend usable life; extensive manufacturing capacity enabling rapid implementation of research findings. Weaknesses: Higher initial cost compared to primary batteries; temperature sensitivity affecting performance in extreme conditions; limited public disclosure of proprietary electrolyte formulations.
Sion Power Corp.
Technical Solution: Sion Power has pioneered lithium-sulfur (Li-S) battery technology with their Licerion® platform, offering a unique perspective on cycle stability compared to conventional systems. Their comparative analysis between rechargeable Li-S cells and primary lithium batteries focuses on energy density retention across multiple discharge cycles. Sion's proprietary protected lithium anode technology addresses the fundamental challenge of lithium metal degradation during cycling, achieving over 500 cycles while maintaining energy densities above 400 Wh/kg - significantly outperforming primary lithium batteries in long-term applications. Their research demonstrates that while primary lithium batteries offer stable single-use performance, their Licerion® technology provides superior total energy delivery over lifetime when applications require more than 10 discharge cycles, with comprehensive testing protocols that simulate real-world usage patterns rather than idealized laboratory conditions.
Strengths: Exceptionally high energy density (400+ Wh/kg) maintained across multiple cycles; innovative protected lithium anode technology that addresses fundamental cycling limitations; potential for applications where weight is critical. Weaknesses: Higher manufacturing complexity compared to conventional lithium-ion or primary batteries; more limited commercial deployment history; potential safety concerns associated with lithium-sulfur chemistry requiring additional protection systems.
Key Patents in Electrochemical Cell Longevity
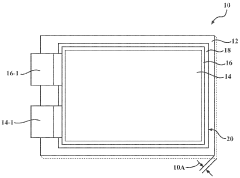
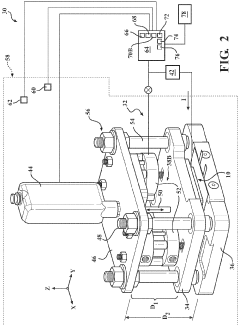
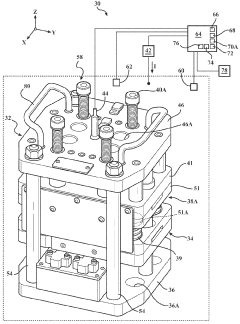
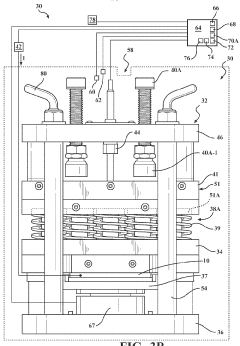
System for assessment of battery cell dimensional variation
PatentWO2023178020A2
Innovation
- A system comprising a test fixture with a pressure plate, reaction plate, elastic member assembly, electronic hardware device, contact displacement sensor, load sensor, and environmental chamber to measure and correlate battery thickness changes with applied electrical current and temperature, allowing for precise assessment of dimensional variations.
Separator for an electrochemical cell
PatentWO2008127829A2
Innovation
- A microporous membrane separator with initial porosity of at least 10% that retains 25% of its porosity after thickness compression, offsetting the volume change of the negative electrode, thereby minimizing the overall cell volume change to less than 20% during charge-discharge cycles.
Environmental Impact of Battery Lifecycle Management
The environmental footprint of battery technologies extends far beyond their operational phase, encompassing raw material extraction, manufacturing processes, usage patterns, and end-of-life management. When comparing electrochemical cells and primary batteries from an environmental perspective, cycle stability emerges as a critical differentiator in their overall ecological impact.
Electrochemical cells, particularly rechargeable lithium-ion batteries, demonstrate superior cycle stability with typical lifespans of 500-1000 complete charge-discharge cycles. This extended usability significantly reduces waste generation compared to primary batteries, which are discarded after a single use cycle. Research indicates that a single rechargeable battery can replace between 50-300 primary batteries depending on application and usage patterns, resulting in substantial waste reduction.
The manufacturing phase of both battery types carries considerable environmental burdens, including energy consumption, greenhouse gas emissions, and resource depletion. However, these environmental costs are amortized over many more usage cycles for rechargeable cells, effectively lowering their per-use environmental impact. Life Cycle Assessment (LCA) studies consistently demonstrate that despite higher initial manufacturing impacts, rechargeable batteries outperform primary batteries in environmental efficiency when used for more than 10-15 cycles.
Material recovery presents another significant environmental consideration. The recycling infrastructure for rechargeable batteries has developed more rapidly due to their higher material value and regulatory pressures. Recovery rates for cobalt, nickel, and copper from lithium-ion batteries can reach 95% in advanced recycling facilities, while primary alkaline batteries often end up in landfills due to lower economic incentives for recycling.
Energy efficiency differences between these battery types also contribute to their environmental profiles. Rechargeable lithium-ion cells typically demonstrate 80-90% charge-discharge efficiency, whereas primary batteries convert chemical energy to electrical energy with efficiencies ranging from 60-90%, depending on chemistry and discharge rate. This efficiency gap translates to different levels of resource utilization throughout the battery lifecycle.
Recent technological innovations are further widening the sustainability gap between these battery types. Advanced battery management systems have extended the useful life of rechargeable cells by optimizing charge-discharge patterns, while new electrode materials have improved cycle stability without relying on scarce resources. These developments enhance the environmental advantages of rechargeable systems over primary batteries in long-term applications.
Electrochemical cells, particularly rechargeable lithium-ion batteries, demonstrate superior cycle stability with typical lifespans of 500-1000 complete charge-discharge cycles. This extended usability significantly reduces waste generation compared to primary batteries, which are discarded after a single use cycle. Research indicates that a single rechargeable battery can replace between 50-300 primary batteries depending on application and usage patterns, resulting in substantial waste reduction.
The manufacturing phase of both battery types carries considerable environmental burdens, including energy consumption, greenhouse gas emissions, and resource depletion. However, these environmental costs are amortized over many more usage cycles for rechargeable cells, effectively lowering their per-use environmental impact. Life Cycle Assessment (LCA) studies consistently demonstrate that despite higher initial manufacturing impacts, rechargeable batteries outperform primary batteries in environmental efficiency when used for more than 10-15 cycles.
Material recovery presents another significant environmental consideration. The recycling infrastructure for rechargeable batteries has developed more rapidly due to their higher material value and regulatory pressures. Recovery rates for cobalt, nickel, and copper from lithium-ion batteries can reach 95% in advanced recycling facilities, while primary alkaline batteries often end up in landfills due to lower economic incentives for recycling.
Energy efficiency differences between these battery types also contribute to their environmental profiles. Rechargeable lithium-ion cells typically demonstrate 80-90% charge-discharge efficiency, whereas primary batteries convert chemical energy to electrical energy with efficiencies ranging from 60-90%, depending on chemistry and discharge rate. This efficiency gap translates to different levels of resource utilization throughout the battery lifecycle.
Recent technological innovations are further widening the sustainability gap between these battery types. Advanced battery management systems have extended the useful life of rechargeable cells by optimizing charge-discharge patterns, while new electrode materials have improved cycle stability without relying on scarce resources. These developments enhance the environmental advantages of rechargeable systems over primary batteries in long-term applications.
Cost-Performance Analysis of Battery Technologies
When evaluating battery technologies for various applications, cost-performance analysis becomes a critical factor in decision-making processes. The economic viability of battery solutions must be balanced against their technical capabilities, particularly cycle stability characteristics.
Electrochemical cells, specifically rechargeable batteries, demonstrate significant cost advantages over primary batteries when evaluated on a cost-per-cycle basis. While their initial acquisition costs may be higher, the ability to undergo hundreds or thousands of charge-discharge cycles distributes this cost over an extended operational lifetime. For instance, lithium-ion batteries typically achieve 500-1000 cycles at 80% depth of discharge, resulting in a substantially lower lifetime cost per kWh delivered.
Primary batteries, conversely, present lower upfront costs but higher long-term expenses due to their single-use nature. This cost structure makes them economically viable only for low-drain applications with infrequent use patterns or where recharging infrastructure is unavailable. The absence of cycle stability considerations in primary batteries simplifies their design and manufacturing processes, contributing to their lower unit costs.
The performance-to-cost ratio varies significantly across different battery chemistries. Lithium-ion technologies command premium pricing but deliver superior energy density and cycle life compared to lead-acid alternatives. This translates to better value in applications where weight, volume, and longevity are prioritized over initial investment costs.
Market trends indicate decreasing costs for rechargeable technologies, particularly lithium-ion, with prices falling approximately 85% over the past decade. This cost reduction trajectory is reshaping the economic comparison between rechargeable and primary batteries, expanding the range of applications where electrochemical cells present compelling economic advantages.
Total cost of ownership calculations must incorporate additional factors beyond purchase price, including maintenance requirements, operational efficiency, and end-of-life disposal costs. Electrochemical cells typically require battery management systems that add to system complexity and cost but extend useful life through optimized charging and discharging protocols.
Environmental considerations also factor into comprehensive cost analyses, with rechargeable systems generally offering reduced waste generation and resource consumption per unit of energy delivered. This aspect becomes increasingly relevant as regulatory frameworks evolve to incorporate extended producer responsibility and life-cycle assessment requirements.
Electrochemical cells, specifically rechargeable batteries, demonstrate significant cost advantages over primary batteries when evaluated on a cost-per-cycle basis. While their initial acquisition costs may be higher, the ability to undergo hundreds or thousands of charge-discharge cycles distributes this cost over an extended operational lifetime. For instance, lithium-ion batteries typically achieve 500-1000 cycles at 80% depth of discharge, resulting in a substantially lower lifetime cost per kWh delivered.
Primary batteries, conversely, present lower upfront costs but higher long-term expenses due to their single-use nature. This cost structure makes them economically viable only for low-drain applications with infrequent use patterns or where recharging infrastructure is unavailable. The absence of cycle stability considerations in primary batteries simplifies their design and manufacturing processes, contributing to their lower unit costs.
The performance-to-cost ratio varies significantly across different battery chemistries. Lithium-ion technologies command premium pricing but deliver superior energy density and cycle life compared to lead-acid alternatives. This translates to better value in applications where weight, volume, and longevity are prioritized over initial investment costs.
Market trends indicate decreasing costs for rechargeable technologies, particularly lithium-ion, with prices falling approximately 85% over the past decade. This cost reduction trajectory is reshaping the economic comparison between rechargeable and primary batteries, expanding the range of applications where electrochemical cells present compelling economic advantages.
Total cost of ownership calculations must incorporate additional factors beyond purchase price, including maintenance requirements, operational efficiency, and end-of-life disposal costs. Electrochemical cells typically require battery management systems that add to system complexity and cost but extend useful life through optimized charging and discharging protocols.
Environmental considerations also factor into comprehensive cost analyses, with rechargeable systems generally offering reduced waste generation and resource consumption per unit of energy delivered. This aspect becomes increasingly relevant as regulatory frameworks evolve to incorporate extended producer responsibility and life-cycle assessment requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!