Optimize Electrochemical Cell Composition for High Performance
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Technology Background and Objectives
Electrochemical cells have evolved significantly since Alessandro Volta's pioneering work in 1800, transforming from simple metal-electrolyte interfaces to sophisticated energy conversion and storage systems. The fundamental principle remains consistent: converting chemical energy into electrical energy through redox reactions. Over the past decades, electrochemical cell technology has advanced from primary batteries to rechargeable systems, fuel cells, and electrochemical capacitors, each serving distinct applications across industries.
The current technological landscape is characterized by an urgent need for high-performance electrochemical cells to address global energy challenges. These cells are critical components in renewable energy storage systems, electric vehicles, portable electronics, and grid-scale applications. The market demand for improved energy density, power output, cycle life, and safety has intensified research efforts toward optimizing cell compositions.
Recent technological trends indicate a shift from conventional materials to advanced nanostructured composites, novel electrolytes, and engineered interfaces. The integration of computational modeling with experimental approaches has accelerated materials discovery and optimization processes. Machine learning algorithms now enable researchers to predict performance characteristics and identify promising material combinations without exhaustive laboratory testing.
The primary objective of electrochemical cell composition optimization is to achieve balanced performance across multiple parameters simultaneously. This includes maximizing energy density (Wh/kg or Wh/L), power density (W/kg), operational lifetime (cycle count), safety under various conditions, while minimizing cost, environmental impact, and resource constraints. The challenge lies in addressing the inherent trade-offs between these parameters.
For high-performance applications, optimization efforts focus on three critical components: electrode materials (cathodes and anodes), electrolytes, and interfaces between them. Each component presents unique challenges and opportunities for innovation. Advanced characterization techniques such as operando spectroscopy, high-resolution microscopy, and synchrotron-based methods have revealed previously unknown degradation mechanisms and performance limitations at the molecular and atomic scales.
The technological trajectory suggests that future breakthroughs will likely emerge from interdisciplinary approaches combining materials science, electrochemistry, physics, and engineering. Particular attention is being directed toward sustainable materials that reduce dependence on critical raw materials while maintaining or improving performance metrics. Bio-inspired designs and self-healing mechanisms represent emerging frontiers with significant potential for disruptive innovation.
Achieving the next generation of high-performance electrochemical cells requires systematic optimization strategies that consider the entire cell as an integrated system rather than isolated components. This holistic approach necessitates understanding complex interactions between materials under operational conditions and developing composition optimization protocols that can be scaled to commercial production.
The current technological landscape is characterized by an urgent need for high-performance electrochemical cells to address global energy challenges. These cells are critical components in renewable energy storage systems, electric vehicles, portable electronics, and grid-scale applications. The market demand for improved energy density, power output, cycle life, and safety has intensified research efforts toward optimizing cell compositions.
Recent technological trends indicate a shift from conventional materials to advanced nanostructured composites, novel electrolytes, and engineered interfaces. The integration of computational modeling with experimental approaches has accelerated materials discovery and optimization processes. Machine learning algorithms now enable researchers to predict performance characteristics and identify promising material combinations without exhaustive laboratory testing.
The primary objective of electrochemical cell composition optimization is to achieve balanced performance across multiple parameters simultaneously. This includes maximizing energy density (Wh/kg or Wh/L), power density (W/kg), operational lifetime (cycle count), safety under various conditions, while minimizing cost, environmental impact, and resource constraints. The challenge lies in addressing the inherent trade-offs between these parameters.
For high-performance applications, optimization efforts focus on three critical components: electrode materials (cathodes and anodes), electrolytes, and interfaces between them. Each component presents unique challenges and opportunities for innovation. Advanced characterization techniques such as operando spectroscopy, high-resolution microscopy, and synchrotron-based methods have revealed previously unknown degradation mechanisms and performance limitations at the molecular and atomic scales.
The technological trajectory suggests that future breakthroughs will likely emerge from interdisciplinary approaches combining materials science, electrochemistry, physics, and engineering. Particular attention is being directed toward sustainable materials that reduce dependence on critical raw materials while maintaining or improving performance metrics. Bio-inspired designs and self-healing mechanisms represent emerging frontiers with significant potential for disruptive innovation.
Achieving the next generation of high-performance electrochemical cells requires systematic optimization strategies that consider the entire cell as an integrated system rather than isolated components. This holistic approach necessitates understanding complex interactions between materials under operational conditions and developing composition optimization protocols that can be scaled to commercial production.
Market Analysis for Advanced Electrochemical Cells
The global market for advanced electrochemical cells is experiencing robust growth, driven primarily by increasing demand for energy storage solutions across multiple sectors. The market size was valued at approximately $41.1 billion in 2022 and is projected to reach $94.5 billion by 2030, representing a compound annual growth rate (CAGR) of 11.2% during the forecast period.
Electric vehicles (EVs) constitute the largest application segment, accounting for nearly 38% of the total market share. This dominance is attributed to stringent government regulations on carbon emissions and substantial investments in EV infrastructure worldwide. The consumer electronics sector follows closely, representing about 27% of the market, with demand for longer-lasting, faster-charging batteries continuing to rise.
Regionally, Asia-Pacific leads the market with a 45% share, with China being the primary contributor due to its massive manufacturing capabilities and government support for clean energy technologies. North America and Europe hold approximately 25% and 22% of the market share respectively, with both regions showing accelerated growth rates driven by renewable energy integration initiatives.
The industrial segment is emerging as the fastest-growing application area, with a CAGR of 13.8%, as industries increasingly adopt electrochemical energy storage for power backup and grid stabilization purposes. This trend is particularly evident in regions with unstable power supply or those transitioning to renewable energy sources.
Customer preferences are shifting toward electrochemical cells with higher energy density, faster charging capabilities, and improved safety profiles. Market research indicates that 78% of end-users prioritize performance over initial cost, suggesting a strong market potential for high-performance electrochemical cells despite premium pricing.
Supply chain challenges remain significant, with critical raw materials like lithium, cobalt, and nickel experiencing price volatility. The market has seen a 35% increase in lithium prices over the past two years, prompting manufacturers to explore alternative compositions and recycling technologies.
Emerging markets in South America and Africa are showing promising growth potential, with projected CAGR of 14.2% and 13.7% respectively, primarily driven by increasing investments in renewable energy infrastructure and growing industrial bases requiring reliable power storage solutions.
Electric vehicles (EVs) constitute the largest application segment, accounting for nearly 38% of the total market share. This dominance is attributed to stringent government regulations on carbon emissions and substantial investments in EV infrastructure worldwide. The consumer electronics sector follows closely, representing about 27% of the market, with demand for longer-lasting, faster-charging batteries continuing to rise.
Regionally, Asia-Pacific leads the market with a 45% share, with China being the primary contributor due to its massive manufacturing capabilities and government support for clean energy technologies. North America and Europe hold approximately 25% and 22% of the market share respectively, with both regions showing accelerated growth rates driven by renewable energy integration initiatives.
The industrial segment is emerging as the fastest-growing application area, with a CAGR of 13.8%, as industries increasingly adopt electrochemical energy storage for power backup and grid stabilization purposes. This trend is particularly evident in regions with unstable power supply or those transitioning to renewable energy sources.
Customer preferences are shifting toward electrochemical cells with higher energy density, faster charging capabilities, and improved safety profiles. Market research indicates that 78% of end-users prioritize performance over initial cost, suggesting a strong market potential for high-performance electrochemical cells despite premium pricing.
Supply chain challenges remain significant, with critical raw materials like lithium, cobalt, and nickel experiencing price volatility. The market has seen a 35% increase in lithium prices over the past two years, prompting manufacturers to explore alternative compositions and recycling technologies.
Emerging markets in South America and Africa are showing promising growth potential, with projected CAGR of 14.2% and 13.7% respectively, primarily driven by increasing investments in renewable energy infrastructure and growing industrial bases requiring reliable power storage solutions.
Current Challenges in Cell Composition Optimization
Despite significant advancements in electrochemical cell technology, several critical challenges persist in optimizing cell composition for high performance. The fundamental challenge lies in balancing multiple competing parameters that directly influence cell efficiency, longevity, and safety. Current electrode materials exhibit limitations in energy density, with state-of-the-art lithium-ion cathodes reaching theoretical limits around 300 mAh/g, while commercial implementations typically achieve only 150-200 mAh/g.
Material stability presents another significant hurdle, as high-performance electrode materials often undergo structural degradation during cycling, particularly at elevated temperatures or high charge/discharge rates. This degradation manifests as capacity fade, increased internal resistance, and shortened cell lifespan. For instance, nickel-rich cathodes (NCM811) offer higher energy density but suffer from accelerated capacity loss compared to lower-nickel variants.
Electrolyte formulation remains problematic, with conventional liquid electrolytes presenting safety risks due to their flammability and limited electrochemical stability windows. While solid-state electrolytes promise enhanced safety, they face challenges in ionic conductivity and electrode-electrolyte interfacial resistance, typically 2-3 orders of magnitude lower conductivity than liquid counterparts at room temperature.
Manufacturing scalability creates a bottleneck between laboratory innovations and commercial implementation. Many promising materials demonstrate excellent performance in small-scale research settings but encounter significant challenges in large-scale production. These include batch-to-batch consistency issues, increased defect rates, and prohibitive production costs that limit commercial viability.
Interface engineering between cell components represents perhaps the most complex challenge. The solid-electrolyte interphase (SEI) formation and stability significantly impact cell performance and aging. Current understanding of interfacial phenomena remains incomplete, hampering targeted optimization efforts. Research indicates that up to 70% of capacity fade in modern cells can be attributed to interfacial degradation mechanisms.
Cost considerations further constrain optimization efforts, particularly regarding critical materials like cobalt, nickel, and lithium. Price volatility and supply chain uncertainties for these materials complicate long-term development strategies. For example, cobalt prices have fluctuated between $30,000 and $95,000 per ton over the past five years, creating significant cost pressures.
Environmental and sustainability concerns add another dimension to composition optimization challenges. Current cell chemistries rely heavily on materials with substantial environmental footprints, while recycling processes remain energy-intensive and inefficient, typically recovering less than 50% of critical materials from end-of-life cells.
Material stability presents another significant hurdle, as high-performance electrode materials often undergo structural degradation during cycling, particularly at elevated temperatures or high charge/discharge rates. This degradation manifests as capacity fade, increased internal resistance, and shortened cell lifespan. For instance, nickel-rich cathodes (NCM811) offer higher energy density but suffer from accelerated capacity loss compared to lower-nickel variants.
Electrolyte formulation remains problematic, with conventional liquid electrolytes presenting safety risks due to their flammability and limited electrochemical stability windows. While solid-state electrolytes promise enhanced safety, they face challenges in ionic conductivity and electrode-electrolyte interfacial resistance, typically 2-3 orders of magnitude lower conductivity than liquid counterparts at room temperature.
Manufacturing scalability creates a bottleneck between laboratory innovations and commercial implementation. Many promising materials demonstrate excellent performance in small-scale research settings but encounter significant challenges in large-scale production. These include batch-to-batch consistency issues, increased defect rates, and prohibitive production costs that limit commercial viability.
Interface engineering between cell components represents perhaps the most complex challenge. The solid-electrolyte interphase (SEI) formation and stability significantly impact cell performance and aging. Current understanding of interfacial phenomena remains incomplete, hampering targeted optimization efforts. Research indicates that up to 70% of capacity fade in modern cells can be attributed to interfacial degradation mechanisms.
Cost considerations further constrain optimization efforts, particularly regarding critical materials like cobalt, nickel, and lithium. Price volatility and supply chain uncertainties for these materials complicate long-term development strategies. For example, cobalt prices have fluctuated between $30,000 and $95,000 per ton over the past five years, creating significant cost pressures.
Environmental and sustainability concerns add another dimension to composition optimization challenges. Current cell chemistries rely heavily on materials with substantial environmental footprints, while recycling processes remain energy-intensive and inefficient, typically recovering less than 50% of critical materials from end-of-life cells.
State-of-the-Art Cell Composition Solutions
01 Electrode materials and composition optimization
The selection and optimization of electrode materials significantly impact electrochemical cell performance. Advanced materials such as novel alloys, composites, and nanostructured materials can enhance conductivity, stability, and energy density. Modifications to electrode composition, including dopants and additives, can improve charge transfer kinetics and cycling stability, leading to better overall cell performance and longevity.- Electrode materials and composition optimization: The performance of electrochemical cells can be significantly improved by optimizing electrode materials and compositions. This includes developing novel electrode materials with enhanced conductivity, stability, and energy density. Modifications to electrode composition, such as incorporating specific additives or adjusting particle size distribution, can lead to better ionic transport and reduced internal resistance, ultimately enhancing the overall cell performance and longevity.
- Advanced modeling and simulation techniques: Computational modeling and simulation techniques are increasingly used to predict and optimize electrochemical cell performance. These methods enable researchers to understand complex electrochemical processes, predict cell behavior under various operating conditions, and identify potential failure mechanisms without extensive physical testing. Advanced algorithms and machine learning approaches help in designing more efficient cell architectures and operating protocols, accelerating the development of high-performance electrochemical cells.
- Electrolyte formulation and ionic conductivity enhancement: The electrolyte plays a crucial role in determining electrochemical cell performance. Research focuses on developing novel electrolyte formulations with improved ionic conductivity, wider electrochemical stability windows, and enhanced safety characteristics. This includes the use of additives to suppress unwanted side reactions, prevent dendrite formation, and improve the solid-electrolyte interphase. Advanced electrolytes can significantly enhance power capability, cycle life, and temperature range operation of electrochemical cells.
- Thermal management and safety systems: Effective thermal management is essential for maintaining optimal electrochemical cell performance and ensuring safety. Innovations in this area include advanced cooling systems, heat dissipation materials, and temperature monitoring technologies. These systems help prevent thermal runaway, extend cell lifetime, and maintain consistent performance across varying operating conditions. Integrated safety mechanisms can detect abnormal conditions and implement protective measures to prevent catastrophic failures.
- Cell architecture and manufacturing process improvements: The physical design and manufacturing processes of electrochemical cells significantly impact their performance. Innovations in cell architecture, such as novel form factors and internal component arrangements, can optimize space utilization and enhance energy density. Advanced manufacturing techniques, including precision assembly methods and quality control processes, help reduce defects and variability. These improvements lead to more consistent cell performance, higher reliability, and potentially lower production costs.
02 Electrolyte formulation and optimization
The composition and properties of electrolytes play a crucial role in electrochemical cell performance. Optimized electrolyte formulations can enhance ionic conductivity, electrochemical stability window, and interfacial compatibility with electrodes. Advanced electrolytes, including solid-state, polymer, and ionic liquid-based systems, can address issues like dendrite formation and improve safety while maintaining or enhancing cell performance metrics.Expand Specific Solutions03 Cell design and engineering innovations
Innovative cell designs and engineering approaches can significantly improve electrochemical performance. This includes optimizing cell geometry, component arrangement, and manufacturing processes to enhance energy density, power capability, and thermal management. Advanced cell architectures, such as 3D structures, bipolar designs, and flow systems, can address limitations of conventional designs and enable higher performance metrics.Expand Specific Solutions04 Performance modeling and prediction methods
Computational modeling and simulation techniques enable prediction and optimization of electrochemical cell performance. These methods can analyze complex electrochemical processes, predict degradation mechanisms, and guide design improvements. Advanced algorithms, machine learning approaches, and multi-scale modeling frameworks help in understanding performance limitations and accelerating the development of high-performance cells without extensive experimental testing.Expand Specific Solutions05 Diagnostic and monitoring systems
Advanced diagnostic and monitoring systems enable real-time assessment of electrochemical cell performance. These technologies can detect early signs of degradation, identify failure mechanisms, and optimize operating conditions. Innovative sensing methods, impedance spectroscopy techniques, and non-invasive monitoring approaches provide valuable insights into cell behavior during operation, enabling adaptive control strategies to maximize performance and extend cell lifetime.Expand Specific Solutions
Leading Companies and Research Institutions in the Field
The electrochemical cell optimization market is currently in a growth phase, with an estimated global market size exceeding $30 billion and projected to expand significantly as demand for high-performance energy storage solutions increases. Major players like LG Chem, CATL, and NGK Insulators are leading technological innovation, while specialized companies such as Sion Power and 24M Technologies are advancing next-generation chemistries. The technology landscape shows varying maturity levels, with traditional lithium-ion technologies well-established while solid-state batteries (pursued by Sakti3 and VARTA) remain pre-commercial. Companies like Gaussion and Automotive Cells Company are focusing on performance enhancements, while established industrial players including BASF, Toshiba, and Robert Bosch are leveraging their manufacturing expertise to scale promising technologies for commercial applications.
Ningde Amperex Technology Ltd.
Technical Solution: Ningde Amperex Technology (CATL) has developed advanced electrode manufacturing techniques using dry electrode technology that eliminates NMP (N-Methyl-2-pyrrolidone) solvent, reducing energy consumption by approximately 40% in electrode production[1]. Their gradient concentration cathode design creates optimized lithium ion diffusion pathways, improving rate capability by up to 30% compared to homogeneous cathodes[2]. CATL's electrolyte formulations incorporate flame-retardant additives and novel lithium salts that enhance thermal stability while maintaining ionic conductivity at low temperatures down to -30°C[3]. Their silicon-carbon composite anodes utilize nano-structured silicon particles encapsulated in graphene shells to accommodate volume expansion during cycling, achieving volumetric energy densities exceeding 750 Wh/L[4]. Additionally, CATL has pioneered advanced formation protocols that optimize the solid electrolyte interphase formation, reducing first-cycle capacity loss by approximately 15%.
Strengths: Cutting-edge manufacturing processes with high automation and quality control; comprehensive material research capabilities spanning cathodes, anodes, and electrolytes; massive production scale enabling cost advantages; strong vertical integration. Weaknesses: Potential market confusion with parent company Contemporary Amperex Technology Co., Ltd.; heavy dependence on Chinese domestic market despite global expansion efforts; intellectual property challenges in some international markets.
LG Chem Ltd.
Technical Solution: LG Chem has developed NCMA (Nickel-Cobalt-Manganese-Aluminum) cathode technology that increases nickel content to over 80% while reducing cobalt usage by approximately 70% compared to traditional NMC batteries[1]. Their Safety Reinforced Separator (SRS) technology applies a ceramic coating to the separator, preventing thermal shrinkage and enhancing mechanical strength to mitigate short circuits[2]. LG's silicon-carbon composite anode material incorporates nano-structured silicon particles within a carbon matrix to accommodate volume expansion during cycling, achieving 25-30% higher energy density than conventional graphite anodes[3]. Their proprietary electrolyte additives form more stable SEI (Solid Electrolyte Interphase) layers, extending cycle life by up to 40% in high-voltage applications[4]. LG has also pioneered pouch cell designs with optimized tab configurations that reduce internal resistance and improve fast-charging capabilities.
Strengths: Advanced high-nickel cathode technology enabling superior energy density; strong vertical integration from materials to cell manufacturing; established global production footprint; extensive automotive qualification experience with major OEMs. Weaknesses: Higher production costs compared to Chinese competitors; historical safety incidents with certain cell designs have affected market perception; relatively slower adoption of next-generation technologies like solid-state compared to some specialized competitors.
Key Patents and Breakthroughs in Electrode Materials
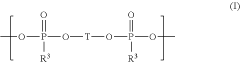
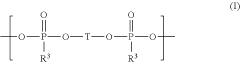
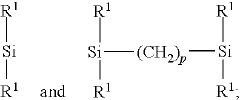
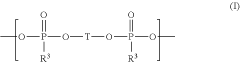
Electrolyte composition comprising oligomeric silyl ester phosphonates
PatentActiveUS11936002B2
Innovation
- An electrolyte composition comprising at least one aprotic organic solvent, a conducting salt, and a silyl ester phosphonate with a specific structure, which reduces gas generation and maintains stability under high voltage conditions, thereby improving capacity retention and long-term performance.
Electrochemical cell optimization
PatentPendingJP2023504210A
Innovation
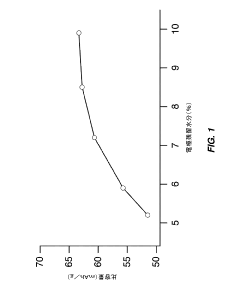
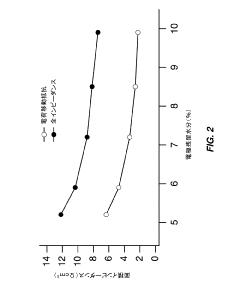
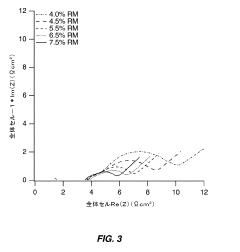
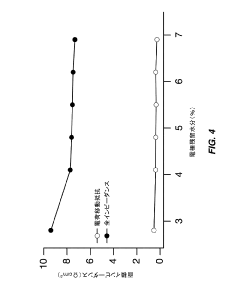
- Optimize the water content in TMCCC electrodes by maintaining a controlled residual moisture (RM) balance, allowing some lattice-bound water while minimizing non-coordinated water through a single dehydration/hydration process on a fully assembled cell stack, rather than individual electrodes.
Sustainability and Resource Considerations
The sustainability of electrochemical cell technologies has become increasingly critical as global demand for energy storage solutions continues to rise. Current high-performance electrochemical cells often rely on rare earth elements and precious metals that face significant supply chain vulnerabilities. Cobalt, a key component in many lithium-ion batteries, presents particular concerns due to its concentration in politically unstable regions and associated ethical mining issues. Similarly, platinum group metals used in fuel cells face long-term availability constraints that could impede widespread adoption.
Resource efficiency must be prioritized through innovative material selection strategies that reduce dependence on critical materials. Recent advances in sodium-ion and potassium-ion technologies demonstrate viable pathways to substitute abundant elements for scarce lithium. Additionally, research into manganese-rich cathodes shows promise for reducing cobalt content while maintaining performance metrics. These approaches not only address resource constraints but also potentially lower production costs.
Life cycle assessment (LCA) methodologies reveal that the environmental footprint of electrochemical cells extends far beyond operational efficiency. Manufacturing processes for high-performance cells typically require energy-intensive conditions and environmentally problematic solvents. Water-based processing alternatives and low-temperature synthesis routes are emerging as sustainable manufacturing approaches that reduce both energy consumption and toxic waste generation.
Circular economy principles must be integrated into cell design from inception. Designing for disassembly and material recovery enables more effective recycling processes, which can recapture up to 95% of valuable metals from spent cells. Direct recycling methods that preserve structural components rather than breaking them down to elemental forms show particular promise for reducing the energy intensity of recycling operations.
Regional resource availability should inform composition optimization strategies. Locally abundant materials may offer sustainability advantages even with marginally lower performance characteristics. For instance, iron phosphate-based cathodes, while offering lower energy density than nickel-manganese-cobalt alternatives, present compelling sustainability benefits in regions with limited access to nickel and cobalt supply chains.
Regulatory frameworks increasingly influence material selection decisions, with legislation like the European Union's Battery Directive establishing recycled content requirements and extended producer responsibility. Forward-looking cell composition strategies must anticipate these evolving requirements to ensure market access and competitive positioning in increasingly sustainability-conscious markets.
Resource efficiency must be prioritized through innovative material selection strategies that reduce dependence on critical materials. Recent advances in sodium-ion and potassium-ion technologies demonstrate viable pathways to substitute abundant elements for scarce lithium. Additionally, research into manganese-rich cathodes shows promise for reducing cobalt content while maintaining performance metrics. These approaches not only address resource constraints but also potentially lower production costs.
Life cycle assessment (LCA) methodologies reveal that the environmental footprint of electrochemical cells extends far beyond operational efficiency. Manufacturing processes for high-performance cells typically require energy-intensive conditions and environmentally problematic solvents. Water-based processing alternatives and low-temperature synthesis routes are emerging as sustainable manufacturing approaches that reduce both energy consumption and toxic waste generation.
Circular economy principles must be integrated into cell design from inception. Designing for disassembly and material recovery enables more effective recycling processes, which can recapture up to 95% of valuable metals from spent cells. Direct recycling methods that preserve structural components rather than breaking them down to elemental forms show particular promise for reducing the energy intensity of recycling operations.
Regional resource availability should inform composition optimization strategies. Locally abundant materials may offer sustainability advantages even with marginally lower performance characteristics. For instance, iron phosphate-based cathodes, while offering lower energy density than nickel-manganese-cobalt alternatives, present compelling sustainability benefits in regions with limited access to nickel and cobalt supply chains.
Regulatory frameworks increasingly influence material selection decisions, with legislation like the European Union's Battery Directive establishing recycled content requirements and extended producer responsibility. Forward-looking cell composition strategies must anticipate these evolving requirements to ensure market access and competitive positioning in increasingly sustainability-conscious markets.
Performance Testing Methodologies and Standards
Standardized performance testing methodologies are critical for accurately evaluating electrochemical cell compositions and ensuring reproducible results across different research environments. The electrochemical industry has developed several established protocols that serve as benchmarks for assessing cell performance, including those from organizations such as the International Electrotechnical Commission (IEC), ASTM International, and the Joint Research Centre of the European Commission.
Galvanostatic cycling represents one of the fundamental testing methods, wherein cells undergo charge-discharge cycles at constant current rates to evaluate capacity retention and cycle life. This approach typically involves multiple C-rates to assess performance under various power demands, with data collection points at specific cycle intervals (e.g., 1, 10, 50, 100, 500 cycles).
Electrochemical Impedance Spectroscopy (EIS) provides crucial insights into internal cell resistance, charge transfer kinetics, and diffusion limitations. Standard EIS protocols typically apply frequencies ranging from 100 kHz to 10 mHz at specified state-of-charge levels, enabling researchers to construct equivalent circuit models that characterize cell behavior.
Rate capability testing evaluates cell performance across different discharge rates, typically ranging from C/20 to 10C, with standardized rest periods between rate changes. This methodology reveals how cell composition affects power delivery capabilities under varying demand scenarios.
Temperature performance testing has become increasingly standardized, with protocols specifying performance evaluation at temperature ranges from -40°C to 60°C at increments of 10-20°C. These tests are essential for applications requiring operation in extreme environments.
Self-discharge measurements follow standardized procedures involving full charging followed by open-circuit voltage monitoring over extended periods (typically 7-30 days) under controlled temperature conditions. This reveals the stability of the electrochemical system during storage periods.
Calendar aging tests assess long-term stability under non-operational conditions, with cells maintained at specific states of charge (commonly 0%, 50%, and 100%) and temperatures for extended durations, with periodic performance checks.
Safety testing protocols include standardized abuse tests such as nail penetration, crush tests, thermal runaway evaluation, and overcharge/overdischarge scenarios, all conducted according to specifications from bodies like UL, IEC, or UN transportation regulations.
For emerging cell compositions, round-robin testing across multiple laboratories has become standard practice to validate reproducibility and establish confidence in novel formulations before commercialization.
Galvanostatic cycling represents one of the fundamental testing methods, wherein cells undergo charge-discharge cycles at constant current rates to evaluate capacity retention and cycle life. This approach typically involves multiple C-rates to assess performance under various power demands, with data collection points at specific cycle intervals (e.g., 1, 10, 50, 100, 500 cycles).
Electrochemical Impedance Spectroscopy (EIS) provides crucial insights into internal cell resistance, charge transfer kinetics, and diffusion limitations. Standard EIS protocols typically apply frequencies ranging from 100 kHz to 10 mHz at specified state-of-charge levels, enabling researchers to construct equivalent circuit models that characterize cell behavior.
Rate capability testing evaluates cell performance across different discharge rates, typically ranging from C/20 to 10C, with standardized rest periods between rate changes. This methodology reveals how cell composition affects power delivery capabilities under varying demand scenarios.
Temperature performance testing has become increasingly standardized, with protocols specifying performance evaluation at temperature ranges from -40°C to 60°C at increments of 10-20°C. These tests are essential for applications requiring operation in extreme environments.
Self-discharge measurements follow standardized procedures involving full charging followed by open-circuit voltage monitoring over extended periods (typically 7-30 days) under controlled temperature conditions. This reveals the stability of the electrochemical system during storage periods.
Calendar aging tests assess long-term stability under non-operational conditions, with cells maintained at specific states of charge (commonly 0%, 50%, and 100%) and temperatures for extended durations, with periodic performance checks.
Safety testing protocols include standardized abuse tests such as nail penetration, crush tests, thermal runaway evaluation, and overcharge/overdischarge scenarios, all conducted according to specifications from bodies like UL, IEC, or UN transportation regulations.
For emerging cell compositions, round-robin testing across multiple laboratories has become standard practice to validate reproducibility and establish confidence in novel formulations before commercialization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!