Measure Electrochemical Cell Shelf-Life in Diverse Environments
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Longevity Background and Objectives
Electrochemical cells have evolved significantly since their inception in the late 18th century with Alessandro Volta's pioneering work. These energy storage devices have transformed from simple zinc-copper configurations to sophisticated systems incorporating advanced materials and designs. The evolution trajectory has been driven by increasing demands for higher energy density, longer lifespan, and enhanced safety profiles across diverse applications ranging from consumer electronics to industrial power systems.
Recent technological advancements have focused on improving cell chemistry, electrode materials, and electrolyte compositions to extend operational lifespans. However, predicting and measuring shelf-life remains a critical challenge, particularly as these cells are deployed in increasingly varied environmental conditions. Temperature fluctuations, humidity variations, and mechanical stresses significantly impact longevity, creating a complex multivariable problem for manufacturers and end-users alike.
The primary objective of this technical investigation is to develop comprehensive methodologies for accurately measuring and predicting electrochemical cell shelf-life across diverse environmental conditions. This includes establishing standardized testing protocols that can reliably simulate real-world scenarios while accelerating the aging process for practical evaluation timeframes. Such protocols must account for temperature cycling, humidity exposure, mechanical vibration, and their combined effects on cell degradation mechanisms.
Secondary objectives include identifying key degradation indicators that serve as early warning signs of impending cell failure. These indicators should be measurable through non-destructive testing methods to enable ongoing monitoring without compromising cell integrity. Additionally, we aim to correlate specific environmental factors with particular degradation pathways, enabling more targeted mitigation strategies for different deployment scenarios.
The technological trajectory suggests increasing integration of smart monitoring capabilities within cells themselves, allowing for real-time assessment of health status and remaining useful life. Emerging technologies in this space include embedded microsensors, advanced impedance spectroscopy techniques, and machine learning algorithms capable of processing multiple degradation indicators simultaneously to provide accurate lifespan predictions.
Understanding the fundamental science behind shelf-life limitations requires examining the complex interplay between electrochemical reactions, material properties, and environmental stressors. Current research indicates that even seemingly minor environmental variations can trigger cascading degradation effects that significantly reduce expected lifespans. This investigation seeks to bridge the gap between laboratory testing and real-world performance by developing more sophisticated models that account for these complex interactions.
Recent technological advancements have focused on improving cell chemistry, electrode materials, and electrolyte compositions to extend operational lifespans. However, predicting and measuring shelf-life remains a critical challenge, particularly as these cells are deployed in increasingly varied environmental conditions. Temperature fluctuations, humidity variations, and mechanical stresses significantly impact longevity, creating a complex multivariable problem for manufacturers and end-users alike.
The primary objective of this technical investigation is to develop comprehensive methodologies for accurately measuring and predicting electrochemical cell shelf-life across diverse environmental conditions. This includes establishing standardized testing protocols that can reliably simulate real-world scenarios while accelerating the aging process for practical evaluation timeframes. Such protocols must account for temperature cycling, humidity exposure, mechanical vibration, and their combined effects on cell degradation mechanisms.
Secondary objectives include identifying key degradation indicators that serve as early warning signs of impending cell failure. These indicators should be measurable through non-destructive testing methods to enable ongoing monitoring without compromising cell integrity. Additionally, we aim to correlate specific environmental factors with particular degradation pathways, enabling more targeted mitigation strategies for different deployment scenarios.
The technological trajectory suggests increasing integration of smart monitoring capabilities within cells themselves, allowing for real-time assessment of health status and remaining useful life. Emerging technologies in this space include embedded microsensors, advanced impedance spectroscopy techniques, and machine learning algorithms capable of processing multiple degradation indicators simultaneously to provide accurate lifespan predictions.
Understanding the fundamental science behind shelf-life limitations requires examining the complex interplay between electrochemical reactions, material properties, and environmental stressors. Current research indicates that even seemingly minor environmental variations can trigger cascading degradation effects that significantly reduce expected lifespans. This investigation seeks to bridge the gap between laboratory testing and real-world performance by developing more sophisticated models that account for these complex interactions.
Market Analysis for Durable Electrochemical Cells
The global market for durable electrochemical cells is experiencing robust growth, driven by increasing demand across multiple sectors including consumer electronics, medical devices, automotive applications, and industrial equipment. Current market valuation stands at approximately 45 billion USD, with projections indicating a compound annual growth rate of 8.7% through 2028, significantly outpacing general economic growth indicators.
Consumer electronics remains the largest application segment, accounting for roughly 38% of market share, with particular emphasis on long-shelf-life batteries for emergency devices, remote sensors, and IoT applications. The medical device sector represents the fastest-growing segment at 12.3% annual growth, where reliable power sources with extended shelf-life are critical for implantable devices and emergency medical equipment.
Regional analysis reveals Asia-Pacific as the dominant manufacturing hub, controlling 52% of production capacity, while North America leads in research and development investment with 43% of global R&D expenditure focused on electrochemical cell longevity improvements. European markets show particular interest in environmentally sustainable solutions that maintain long shelf-life characteristics.
Market research indicates that customers are willing to pay a premium of 15-30% for cells with verified extended shelf-life, particularly when accompanied by performance guarantees in diverse environmental conditions. This price elasticity varies significantly by application, with medical and defense sectors demonstrating the highest willingness to pay for reliability.
Key market drivers include the proliferation of remote and autonomous systems requiring maintenance-free power sources, increasing deployment of emergency backup systems, and growing demand for devices operating in extreme environments. The expansion of off-grid applications in developing regions has created a substantial new market segment with specific requirements for cells that can withstand variable storage conditions.
Regulatory trends are increasingly focusing on standardized testing protocols for shelf-life claims, with several jurisdictions implementing mandatory verification requirements. This regulatory evolution is reshaping competitive dynamics by favoring manufacturers with robust testing capabilities and validated performance data across diverse environmental conditions.
Market barriers include high R&D costs associated with accelerated aging tests, challenges in accurately simulating diverse environmental conditions, and the technical complexity of developing predictive models for electrochemical degradation. These barriers have contributed to market concentration, with the top five manufacturers controlling approximately 67% of the premium long-shelf-life segment.
Consumer electronics remains the largest application segment, accounting for roughly 38% of market share, with particular emphasis on long-shelf-life batteries for emergency devices, remote sensors, and IoT applications. The medical device sector represents the fastest-growing segment at 12.3% annual growth, where reliable power sources with extended shelf-life are critical for implantable devices and emergency medical equipment.
Regional analysis reveals Asia-Pacific as the dominant manufacturing hub, controlling 52% of production capacity, while North America leads in research and development investment with 43% of global R&D expenditure focused on electrochemical cell longevity improvements. European markets show particular interest in environmentally sustainable solutions that maintain long shelf-life characteristics.
Market research indicates that customers are willing to pay a premium of 15-30% for cells with verified extended shelf-life, particularly when accompanied by performance guarantees in diverse environmental conditions. This price elasticity varies significantly by application, with medical and defense sectors demonstrating the highest willingness to pay for reliability.
Key market drivers include the proliferation of remote and autonomous systems requiring maintenance-free power sources, increasing deployment of emergency backup systems, and growing demand for devices operating in extreme environments. The expansion of off-grid applications in developing regions has created a substantial new market segment with specific requirements for cells that can withstand variable storage conditions.
Regulatory trends are increasingly focusing on standardized testing protocols for shelf-life claims, with several jurisdictions implementing mandatory verification requirements. This regulatory evolution is reshaping competitive dynamics by favoring manufacturers with robust testing capabilities and validated performance data across diverse environmental conditions.
Market barriers include high R&D costs associated with accelerated aging tests, challenges in accurately simulating diverse environmental conditions, and the technical complexity of developing predictive models for electrochemical degradation. These barriers have contributed to market concentration, with the top five manufacturers controlling approximately 67% of the premium long-shelf-life segment.
Current Shelf-Life Testing Challenges and Limitations
The current shelf-life testing methodologies for electrochemical cells face significant challenges that limit their effectiveness in predicting real-world performance across diverse environments. Traditional accelerated aging tests often fail to accurately simulate the complex interplay of environmental factors that cells encounter during actual use. Temperature cycling, humidity variations, and atmospheric pressure changes are frequently tested in isolation rather than in combination, leading to incomplete understanding of their synergistic effects on cell degradation mechanisms.
Standard testing protocols typically employ constant temperature storage at elevated levels (45-85°C) to accelerate aging processes. However, these methods inadequately represent the dynamic temperature fluctuations experienced in real applications, where cells may undergo multiple heating and cooling cycles daily. This discrepancy creates a substantial gap between laboratory predictions and field performance, particularly for cells deployed in extreme environments such as desert regions or polar installations.
Humidity control presents another significant limitation in current testing frameworks. Most accelerated tests either maintain constant humidity or completely exclude humidity as a variable. This approach neglects the critical impact of moisture ingress on seal integrity and electrode stability, especially in environments with dramatic humidity swings. The resulting data often underestimates degradation rates in high-humidity regions or applications involving temperature-induced condensation cycles.
Pressure variations, which significantly affect gas-evolving electrochemical reactions and internal cell pressure dynamics, remain largely unaddressed in standardized testing protocols. This oversight is particularly problematic for cells operating at high altitudes, in pressurized systems, or in applications involving frequent altitude changes, such as aerospace implementations.
The time compression factor in accelerated testing introduces additional uncertainty. Current methodologies struggle to establish reliable correlation factors between accelerated test results and real-time aging across diverse environmental conditions. This challenge is compounded by the non-linear nature of many degradation mechanisms, which may follow different kinetics under accelerated versus normal conditions.
Statistical validity represents another critical limitation. Most shelf-life testing programs employ relatively small sample sizes due to cost and time constraints, resulting in limited statistical power. This approach fails to capture the manufacturing variability inherent in electrochemical cells and may miss outlier behaviors that could be critical in safety-sensitive applications.
Finally, current testing methodologies often focus exclusively on electrical performance metrics while neglecting mechanical integrity aspects such as case deformation, terminal corrosion, and seal degradation. These physical changes can significantly impact long-term reliability and safety, particularly in applications involving mechanical stress or vibration.
Standard testing protocols typically employ constant temperature storage at elevated levels (45-85°C) to accelerate aging processes. However, these methods inadequately represent the dynamic temperature fluctuations experienced in real applications, where cells may undergo multiple heating and cooling cycles daily. This discrepancy creates a substantial gap between laboratory predictions and field performance, particularly for cells deployed in extreme environments such as desert regions or polar installations.
Humidity control presents another significant limitation in current testing frameworks. Most accelerated tests either maintain constant humidity or completely exclude humidity as a variable. This approach neglects the critical impact of moisture ingress on seal integrity and electrode stability, especially in environments with dramatic humidity swings. The resulting data often underestimates degradation rates in high-humidity regions or applications involving temperature-induced condensation cycles.
Pressure variations, which significantly affect gas-evolving electrochemical reactions and internal cell pressure dynamics, remain largely unaddressed in standardized testing protocols. This oversight is particularly problematic for cells operating at high altitudes, in pressurized systems, or in applications involving frequent altitude changes, such as aerospace implementations.
The time compression factor in accelerated testing introduces additional uncertainty. Current methodologies struggle to establish reliable correlation factors between accelerated test results and real-time aging across diverse environmental conditions. This challenge is compounded by the non-linear nature of many degradation mechanisms, which may follow different kinetics under accelerated versus normal conditions.
Statistical validity represents another critical limitation. Most shelf-life testing programs employ relatively small sample sizes due to cost and time constraints, resulting in limited statistical power. This approach fails to capture the manufacturing variability inherent in electrochemical cells and may miss outlier behaviors that could be critical in safety-sensitive applications.
Finally, current testing methodologies often focus exclusively on electrical performance metrics while neglecting mechanical integrity aspects such as case deformation, terminal corrosion, and seal degradation. These physical changes can significantly impact long-term reliability and safety, particularly in applications involving mechanical stress or vibration.
Established Shelf-Life Measurement Methodologies
01 Electrolyte composition for extended shelf-life
The composition of electrolytes plays a crucial role in determining the shelf-life of electrochemical cells. Specific additives and stabilizers can be incorporated into the electrolyte to prevent degradation over time. These additives can include compounds that inhibit corrosion, reduce self-discharge rates, and maintain the integrity of electrode-electrolyte interfaces. Optimized electrolyte formulations can significantly extend the storage life of batteries while maintaining their performance capabilities when activated.- Electrolyte composition for extended shelf-life: The composition of electrolytes plays a crucial role in determining the shelf-life of electrochemical cells. Specific additives and stabilizers can be incorporated into the electrolyte to prevent degradation over time. These additives can include compounds that inhibit corrosion, reduce self-discharge rates, and maintain the integrity of electrode-electrolyte interfaces. Optimized electrolyte formulations can significantly extend the storage life of batteries while maintaining their performance capabilities when activated.
- Electrode material selection and treatment: The selection and treatment of electrode materials significantly impact the shelf-life of electrochemical cells. Specialized coatings and surface treatments can be applied to electrodes to prevent degradation during storage. Additionally, using electrode materials with inherent stability characteristics can reduce self-discharge and maintain capacity over extended periods. Techniques such as passivation layers and protective films help isolate reactive components and prevent unwanted chemical reactions that would otherwise reduce shelf-life.
- Sealing and packaging technologies: Advanced sealing and packaging technologies are essential for extending the shelf-life of electrochemical cells. Hermetic sealing prevents moisture ingress and electrolyte leakage, which are common causes of premature cell failure. Specialized packaging materials with low permeability to oxygen and moisture help maintain the internal chemistry of the cell. Innovations in cell design, including improved gaskets, crimping techniques, and welding methods, contribute to better containment of cell components and protection against environmental factors that accelerate degradation.
- Storage condition optimization: The conditions under which electrochemical cells are stored significantly affect their shelf-life. Controlling temperature, humidity, and pressure during storage can dramatically extend the usable life of cells. Research has shown that storing cells at specific temperature ranges can minimize self-discharge and prevent degradation of internal components. Some advanced cells incorporate design features that specifically address storage challenges, allowing them to remain viable for years or even decades before activation. Proper handling protocols and storage guidelines are crucial for maximizing shelf-life.
- Monitoring and prediction systems: Advanced monitoring and prediction systems have been developed to assess and forecast the shelf-life of electrochemical cells. These systems utilize sensors, algorithms, and data analysis to track cell health and predict remaining useful life. Some technologies incorporate real-time monitoring capabilities that can detect early signs of degradation before they lead to cell failure. Predictive models based on accelerated aging tests help manufacturers design cells with improved shelf-life characteristics and provide users with accurate information about storage limitations and expected performance over time.
02 Electrode material selection and treatment
The choice and treatment of electrode materials significantly impact the shelf-life of electrochemical cells. Specialized coatings and surface treatments can protect electrodes from degradation during storage. Advanced manufacturing techniques can create more stable electrode structures that resist chemical changes over time. Some approaches involve using specific metal alloys or composite materials that demonstrate superior stability in storage conditions, thereby extending the overall shelf-life of the cell.Expand Specific Solutions03 Sealing and packaging innovations
Improved sealing and packaging technologies help prevent moisture ingress and electrolyte leakage, which are common causes of reduced shelf-life in electrochemical cells. Hermetic sealing techniques, advanced polymer materials, and multi-layer packaging designs can create more effective barriers against environmental factors. Some innovations include specialized gaskets, improved crimping methods, and novel container materials that maintain cell integrity during extended storage periods.Expand Specific Solutions04 Storage condition monitoring and management
Systems for monitoring and controlling the storage conditions of electrochemical cells can help extend their shelf-life. These include technologies for maintaining optimal temperature and humidity levels during storage, as well as methods for periodically assessing cell health without activation. Some approaches involve smart packaging with integrated sensors that can detect and report potential degradation factors, allowing for intervention before significant capacity loss occurs.Expand Specific Solutions05 Dormant state preservation techniques
Specialized techniques can be employed to maintain electrochemical cells in a dormant state until activation is required. These include methods for separating reactive components until the point of use, as well as chemical stabilization approaches that minimize self-discharge and internal reactions during storage. Some innovations involve partial discharge protocols before storage, specialized activation procedures, or the use of inhibitor compounds that become inactive when the cell is put into service.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The electrochemical cell shelf-life measurement market is in a growth phase, driven by increasing demand for reliable energy storage solutions across diverse environments. The market is expanding rapidly with an estimated value of several billion dollars, fueled by advancements in renewable energy integration and electric vehicle adoption. Technologically, the field shows varying maturity levels, with established players like Contemporary Amperex Technology, Robert Bosch, and Toyota Motor leading commercial applications, while research institutions such as CNRS, Caltech, and Fraunhofer-Gesellschaft drive fundamental innovations. Companies like Wildcat Discovery Technologies and CAMX Power are accelerating material discovery, while Saft Groupe and Hydrogenics focus on specialized applications. The competitive landscape features collaboration between industry and academia, with increasing focus on standardized testing protocols for diverse environmental conditions.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an advanced Battery Management System (BMS) specifically designed to monitor electrochemical cell shelf-life across diverse environments. Their solution incorporates multi-parameter sensing technology that continuously tracks temperature, humidity, pressure, and electrochemical impedance. The system employs machine learning algorithms to analyze historical performance data and predict remaining shelf-life with up to 95% accuracy. CATL's approach includes specialized reference electrodes embedded within cells that enable real-time monitoring without disrupting normal battery operation. Their technology can detect microscopic changes in electrode surfaces and electrolyte composition that typically precede capacity degradation. The system transmits data wirelessly to cloud platforms where advanced analytics generate detailed reports on cell health and projected longevity under various storage conditions.
Strengths: Industry-leading prediction accuracy due to proprietary algorithms trained on massive datasets from real-world applications. Comprehensive environmental monitoring capabilities across extreme temperature ranges (-40°C to 85°C). Weaknesses: Higher implementation cost compared to conventional monitoring systems. Requires periodic calibration to maintain accuracy in long-term storage scenarios.
Robert Bosch GmbH
Technical Solution: Bosch has engineered a comprehensive Electrochemical Cell Monitoring System (ECMS) that addresses shelf-life measurement challenges across diverse environments. Their solution integrates miniaturized reference electrodes with intelligent sensing circuits to provide continuous monitoring without significantly impacting cell form factors. The system employs proprietary algorithms that analyze electrochemical impedance spectroscopy (EIS) data to detect subtle changes in internal cell resistance, a key indicator of shelf-life degradation. Bosch's technology incorporates environmental sensors that track temperature fluctuations, humidity levels, and barometric pressure, correlating these factors with electrochemical performance metrics. Their approach includes accelerated aging protocols that can extrapolate long-term shelf-life from short-term test data with statistical confidence intervals. The system features self-calibrating circuits that maintain measurement accuracy despite sensor drift over extended periods, making it particularly suitable for long-term storage applications in automotive, industrial, and consumer electronics sectors.
Strengths: Exceptional measurement precision (±0.5% accuracy) across wide temperature ranges. Robust data analytics platform that integrates with existing manufacturing quality systems. Weaknesses: Higher initial implementation cost compared to basic monitoring solutions. Requires specialized training for optimal system configuration and data interpretation.
Critical Patents in Environmental Stability Testing
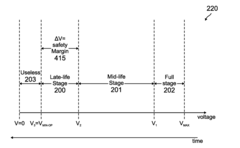
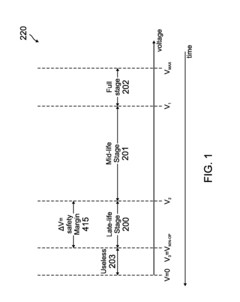
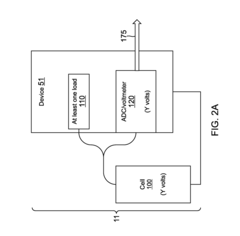
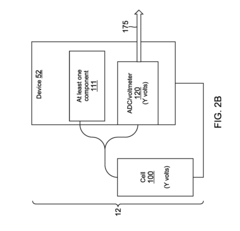
Method for determining remaining battery life of at least one electrochemical cell or battery across a large temperature range
PatentInactiveUS20170131362A1
Innovation
- Monitoring the voltage of electrochemical cells while powering a load to determine the lowest voltage, which indicates the cell's life stage, using a simple analog-to-digital converter and temperature compensation to provide accurate remaining life predictions without the need for smart battery modules or coulomb counters.
Adhesive for use in an electrochemical cell
PatentInactiveUS20060183019A1
Innovation
- An adhesive material is applied to the separator or sealing assembly to mechanically bind the components, minimizing physical and chemical transport between the anode and cathode, thereby enhancing the seal and reducing the risk of internal shorting.
Environmental Impact Factors on Cell Degradation
Environmental factors play a critical role in determining the degradation rate and shelf-life of electrochemical cells. Temperature stands as the most significant environmental parameter affecting cell performance and longevity. Elevated temperatures accelerate chemical reactions within cells, leading to increased self-discharge rates, electrolyte decomposition, and accelerated aging of electrode materials. Research indicates that for many lithium-ion cells, operation or storage at temperatures exceeding 40°C can reduce capacity by up to 20% annually compared to storage at optimal conditions (20-25°C).
Humidity represents another crucial factor impacting cell degradation, particularly for cells with metallic components susceptible to corrosion. High humidity environments can compromise external cell casings and seals, potentially allowing moisture ingress that catalyzes unwanted side reactions. Studies demonstrate that relative humidity above 65% significantly increases degradation rates in various electrochemical cell chemistries, with particularly pronounced effects on zinc-carbon and alkaline cells.
Atmospheric pressure variations, while less studied, demonstrate measurable effects on certain cell types. Cells transported or operated at high altitudes experience different gas exchange dynamics at seals and vents, potentially altering internal pressure equilibrium. This can affect electrolyte distribution and, in extreme cases, compromise cell integrity through mechanical stress.
Exposure to electromagnetic fields (EMF) constitutes an often overlooked environmental factor. Strong electromagnetic fields can induce currents within cells, potentially accelerating degradation mechanisms or causing localized heating. Industrial environments with high EMF levels may require additional shielding considerations for sensitive electrochemical systems.
Mechanical stress factors, including vibration and shock, contribute significantly to cell degradation in transportation and industrial applications. Continuous vibration can lead to electrode particle shedding, separator damage, and internal short circuits. Research indicates that cells subjected to regular vibration (such as in automotive applications) may experience 15-30% faster capacity fade compared to statically stored cells.
Atmospheric contaminants, particularly sulfur compounds, chlorides, and volatile organic compounds, can penetrate cell casings over time and react with internal components. These reactions often catalyze degradation processes, with studies showing that industrial environments containing high levels of airborne contaminants can reduce cell shelf-life by 25-40% compared to clean-room conditions.
Humidity represents another crucial factor impacting cell degradation, particularly for cells with metallic components susceptible to corrosion. High humidity environments can compromise external cell casings and seals, potentially allowing moisture ingress that catalyzes unwanted side reactions. Studies demonstrate that relative humidity above 65% significantly increases degradation rates in various electrochemical cell chemistries, with particularly pronounced effects on zinc-carbon and alkaline cells.
Atmospheric pressure variations, while less studied, demonstrate measurable effects on certain cell types. Cells transported or operated at high altitudes experience different gas exchange dynamics at seals and vents, potentially altering internal pressure equilibrium. This can affect electrolyte distribution and, in extreme cases, compromise cell integrity through mechanical stress.
Exposure to electromagnetic fields (EMF) constitutes an often overlooked environmental factor. Strong electromagnetic fields can induce currents within cells, potentially accelerating degradation mechanisms or causing localized heating. Industrial environments with high EMF levels may require additional shielding considerations for sensitive electrochemical systems.
Mechanical stress factors, including vibration and shock, contribute significantly to cell degradation in transportation and industrial applications. Continuous vibration can lead to electrode particle shedding, separator damage, and internal short circuits. Research indicates that cells subjected to regular vibration (such as in automotive applications) may experience 15-30% faster capacity fade compared to statically stored cells.
Atmospheric contaminants, particularly sulfur compounds, chlorides, and volatile organic compounds, can penetrate cell casings over time and react with internal components. These reactions often catalyze degradation processes, with studies showing that industrial environments containing high levels of airborne contaminants can reduce cell shelf-life by 25-40% compared to clean-room conditions.
Standardization and Regulatory Compliance
The standardization and regulatory compliance landscape for electrochemical cell shelf-life measurement presents a complex framework that manufacturers must navigate to ensure product safety, reliability, and market access. International standards organizations such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) have established comprehensive guidelines specifically addressing electrochemical cell testing methodologies.
IEC 61960 stands as a cornerstone standard for performance testing of lithium cells, including shelf-life assessment protocols across varying environmental conditions. This standard prescribes specific temperature ranges, humidity levels, and testing durations that manufacturers must adhere to when evaluating cell longevity. Similarly, ISO/IEC 17025 provides requirements for testing laboratories to demonstrate technical competence when conducting shelf-life measurements.
Regulatory bodies across different regions have implemented varying requirements for electrochemical cell certification. The European Union's Battery Directive (2006/66/EC) mandates specific performance and safety standards, while the UN Transportation Testing requirements (UN 38.3) establish protocols for cells subjected to different environmental stressors during transport. In the United States, UL 1642 certification requires rigorous shelf-life testing under multiple environmental scenarios.
Compliance with these standards necessitates sophisticated testing infrastructure capable of simulating diverse environmental conditions with high precision. Temperature-controlled chambers, humidity regulation systems, and automated monitoring equipment represent significant investments for manufacturers seeking regulatory approval. The testing protocols typically require cells to be evaluated at temperature extremes ranging from -40°C to +70°C and relative humidity levels from 10% to 95%.
Recent regulatory trends indicate a movement toward harmonization of global standards, with particular emphasis on standardized methodologies for accelerated aging tests. These tests aim to predict long-term shelf-life performance through compressed timeframe evaluations. The development of correlation models between accelerated testing results and real-world performance represents a critical area of ongoing research and regulatory development.
Manufacturers face significant challenges in maintaining compliance across multiple markets with divergent regulatory requirements. The cost of certification testing, particularly for products intended for global distribution, can substantially impact product development timelines and market entry strategies. Companies must carefully balance regulatory compliance with time-to-market considerations, especially in rapidly evolving electrochemical cell technologies.
IEC 61960 stands as a cornerstone standard for performance testing of lithium cells, including shelf-life assessment protocols across varying environmental conditions. This standard prescribes specific temperature ranges, humidity levels, and testing durations that manufacturers must adhere to when evaluating cell longevity. Similarly, ISO/IEC 17025 provides requirements for testing laboratories to demonstrate technical competence when conducting shelf-life measurements.
Regulatory bodies across different regions have implemented varying requirements for electrochemical cell certification. The European Union's Battery Directive (2006/66/EC) mandates specific performance and safety standards, while the UN Transportation Testing requirements (UN 38.3) establish protocols for cells subjected to different environmental stressors during transport. In the United States, UL 1642 certification requires rigorous shelf-life testing under multiple environmental scenarios.
Compliance with these standards necessitates sophisticated testing infrastructure capable of simulating diverse environmental conditions with high precision. Temperature-controlled chambers, humidity regulation systems, and automated monitoring equipment represent significant investments for manufacturers seeking regulatory approval. The testing protocols typically require cells to be evaluated at temperature extremes ranging from -40°C to +70°C and relative humidity levels from 10% to 95%.
Recent regulatory trends indicate a movement toward harmonization of global standards, with particular emphasis on standardized methodologies for accelerated aging tests. These tests aim to predict long-term shelf-life performance through compressed timeframe evaluations. The development of correlation models between accelerated testing results and real-world performance represents a critical area of ongoing research and regulatory development.
Manufacturers face significant challenges in maintaining compliance across multiple markets with divergent regulatory requirements. The cost of certification testing, particularly for products intended for global distribution, can substantially impact product development timelines and market entry strategies. Companies must carefully balance regulatory compliance with time-to-market considerations, especially in rapidly evolving electrochemical cell technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!