Benchmarking Electrochemical Cell Acidity Impact on Performance
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Acidity Background and Objectives
Electrochemical cell technology has evolved significantly over the past century, with particular attention to the role of acidity in determining cell performance. The fundamental relationship between pH levels and electrochemical reactions was first established in the early 20th century through pioneering work by scientists such as Nernst and Sørensen. Since then, understanding of acid-base interactions within electrochemical systems has progressed from basic theoretical frameworks to sophisticated models that account for complex interfacial phenomena.
Recent technological advances have enabled more precise control and measurement of acidity in electrochemical environments, allowing researchers to investigate correlations between pH gradients and performance metrics with unprecedented accuracy. The evolution of this field has been characterized by a shift from empirical observations to mechanistic understanding, particularly regarding how proton activity influences electron transfer kinetics, electrode stability, and overall system efficiency.
Current research trends indicate growing interest in dynamic pH control strategies, localized acidity management at electrode interfaces, and the development of pH-responsive materials for advanced electrochemical applications. These developments are particularly relevant for emerging technologies such as flow batteries, fuel cells, and electrochemical CO2 reduction systems, where acidity plays a crucial role in determining reaction pathways and efficiency.
The primary objective of benchmarking electrochemical cell acidity impact on performance is to establish standardized protocols for evaluating how variations in pH affect key performance indicators across different electrochemical systems. This includes quantifying the relationship between acidity and metrics such as energy efficiency, power density, cycle life, and selectivity for specific reaction products.
Secondary objectives include identifying optimal acidity ranges for specific applications, understanding degradation mechanisms associated with pH fluctuations, and developing predictive models that can inform the design of next-generation electrochemical systems with enhanced stability and performance. These objectives align with broader industry goals of improving energy storage and conversion technologies to meet growing demands for sustainable energy solutions.
Long-term technical goals in this field include the development of self-regulating pH systems that can maintain optimal acidity levels without external intervention, as well as the creation of universal benchmarking standards that enable meaningful comparisons across different electrochemical technologies. Achievement of these goals would significantly advance our ability to design and optimize electrochemical systems for applications ranging from grid-scale energy storage to portable electronics and electrosynthesis of valuable chemicals.
Recent technological advances have enabled more precise control and measurement of acidity in electrochemical environments, allowing researchers to investigate correlations between pH gradients and performance metrics with unprecedented accuracy. The evolution of this field has been characterized by a shift from empirical observations to mechanistic understanding, particularly regarding how proton activity influences electron transfer kinetics, electrode stability, and overall system efficiency.
Current research trends indicate growing interest in dynamic pH control strategies, localized acidity management at electrode interfaces, and the development of pH-responsive materials for advanced electrochemical applications. These developments are particularly relevant for emerging technologies such as flow batteries, fuel cells, and electrochemical CO2 reduction systems, where acidity plays a crucial role in determining reaction pathways and efficiency.
The primary objective of benchmarking electrochemical cell acidity impact on performance is to establish standardized protocols for evaluating how variations in pH affect key performance indicators across different electrochemical systems. This includes quantifying the relationship between acidity and metrics such as energy efficiency, power density, cycle life, and selectivity for specific reaction products.
Secondary objectives include identifying optimal acidity ranges for specific applications, understanding degradation mechanisms associated with pH fluctuations, and developing predictive models that can inform the design of next-generation electrochemical systems with enhanced stability and performance. These objectives align with broader industry goals of improving energy storage and conversion technologies to meet growing demands for sustainable energy solutions.
Long-term technical goals in this field include the development of self-regulating pH systems that can maintain optimal acidity levels without external intervention, as well as the creation of universal benchmarking standards that enable meaningful comparisons across different electrochemical technologies. Achievement of these goals would significantly advance our ability to design and optimize electrochemical systems for applications ranging from grid-scale energy storage to portable electronics and electrosynthesis of valuable chemicals.
Market Analysis of pH-Sensitive Electrochemical Applications
The pH-sensitive electrochemical applications market has experienced significant growth over the past decade, driven primarily by advancements in healthcare diagnostics, environmental monitoring, and industrial process control. The global market value for these applications reached approximately $5.2 billion in 2022 and is projected to grow at a compound annual growth rate of 7.8% through 2028, potentially reaching $8.1 billion by that time.
Healthcare represents the largest segment of this market, accounting for roughly 42% of the total market share. Within healthcare, continuous glucose monitoring systems and point-of-care diagnostic devices that utilize pH-sensitive electrochemical sensors have seen particularly strong demand. This growth is fueled by the increasing prevalence of chronic diseases and the shift toward personalized medicine and remote patient monitoring.
Environmental monitoring applications constitute the second-largest market segment at 28%, with water quality monitoring systems being the primary driver. Stringent environmental regulations across developed economies have necessitated more accurate and continuous monitoring of pH levels in natural water bodies, wastewater treatment facilities, and industrial effluents.
Industrial process control applications represent 21% of the market, with food and beverage, chemical manufacturing, and pharmaceutical production being key industries utilizing pH-sensitive electrochemical technologies. These industries require precise pH control to ensure product quality, process efficiency, and regulatory compliance.
Regional analysis indicates North America leads the market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate of 9.3% annually, driven by rapid industrialization, increasing healthcare expenditure, and growing environmental awareness in countries like China and India.
Key market trends include miniaturization of sensors, integration with wireless communication technologies, and development of multi-parameter sensing platforms that measure pH alongside other critical parameters. The demand for real-time, continuous monitoring solutions is reshaping product development strategies across all application segments.
Customer requirements are evolving toward more durable sensors with extended calibration intervals, improved accuracy across wider pH ranges, and better resistance to biofouling and chemical interference. Price sensitivity varies significantly by application, with industrial users typically willing to pay premium prices for reliability and precision, while consumer and environmental monitoring applications face more stringent cost constraints.
Healthcare represents the largest segment of this market, accounting for roughly 42% of the total market share. Within healthcare, continuous glucose monitoring systems and point-of-care diagnostic devices that utilize pH-sensitive electrochemical sensors have seen particularly strong demand. This growth is fueled by the increasing prevalence of chronic diseases and the shift toward personalized medicine and remote patient monitoring.
Environmental monitoring applications constitute the second-largest market segment at 28%, with water quality monitoring systems being the primary driver. Stringent environmental regulations across developed economies have necessitated more accurate and continuous monitoring of pH levels in natural water bodies, wastewater treatment facilities, and industrial effluents.
Industrial process control applications represent 21% of the market, with food and beverage, chemical manufacturing, and pharmaceutical production being key industries utilizing pH-sensitive electrochemical technologies. These industries require precise pH control to ensure product quality, process efficiency, and regulatory compliance.
Regional analysis indicates North America leads the market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate of 9.3% annually, driven by rapid industrialization, increasing healthcare expenditure, and growing environmental awareness in countries like China and India.
Key market trends include miniaturization of sensors, integration with wireless communication technologies, and development of multi-parameter sensing platforms that measure pH alongside other critical parameters. The demand for real-time, continuous monitoring solutions is reshaping product development strategies across all application segments.
Customer requirements are evolving toward more durable sensors with extended calibration intervals, improved accuracy across wider pH ranges, and better resistance to biofouling and chemical interference. Price sensitivity varies significantly by application, with industrial users typically willing to pay premium prices for reliability and precision, while consumer and environmental monitoring applications face more stringent cost constraints.
Current Challenges in Acidity Control for Electrochemical Systems
Despite significant advancements in electrochemical systems, maintaining optimal acidity levels remains one of the most persistent challenges in the field. The pH environment directly influences reaction kinetics, electrode stability, and overall system efficiency. Current control methods often struggle with real-time monitoring and adjustment capabilities, particularly in dynamic operating conditions where rapid pH fluctuations occur. This limitation significantly impacts the reproducibility of results across different research groups and manufacturing facilities.
Traditional pH monitoring techniques rely heavily on discrete sampling methods that fail to capture transient acidity changes during operation. The integration of continuous monitoring systems has been attempted, but issues with sensor fouling and drift in electrochemically active environments continue to plague these solutions. Miniaturized pH sensors often exhibit reduced accuracy when exposed to the complex chemical environments present in many electrochemical cells.
Buffer systems, while widely employed, present their own set of challenges. The introduction of buffering agents can inadvertently interfere with electrochemical reactions, particularly in sensitive applications like energy storage systems and bioelectrochemical devices. Finding buffer compositions that maintain pH stability without compromising system performance remains an ongoing struggle for researchers and engineers alike.
Temperature fluctuations during operation further complicate acidity control, as pH values are inherently temperature-dependent. Most current systems lack sophisticated temperature compensation algorithms, leading to misinterpretations of acidity levels during thermal cycling or extended operations. This becomes particularly problematic in large-scale industrial applications where temperature gradients across the system are unavoidable.
The heterogeneity of pH within electrochemical cells presents another significant challenge. Local pH environments near electrode surfaces can differ substantially from bulk solution measurements, creating microenvironments that significantly impact reaction pathways and degradation mechanisms. Current technologies struggle to map these spatial variations effectively, leading to incomplete understanding of reaction dynamics.
For commercial applications, the cost and complexity of precise acidity control systems often prove prohibitive. Many industrial implementations resort to simplified approaches that sacrifice optimal performance for operational practicality. This economic constraint has slowed the adoption of advanced pH control technologies in mass-production environments, creating a gap between laboratory achievements and industrial implementation.
Standardization across the field remains inadequate, with various research groups and manufacturers employing different methodologies for acidity measurement and control. This lack of standardization hampers comparative analysis and technology transfer, ultimately slowing progress in addressing fundamental challenges in electrochemical system design and optimization.
Traditional pH monitoring techniques rely heavily on discrete sampling methods that fail to capture transient acidity changes during operation. The integration of continuous monitoring systems has been attempted, but issues with sensor fouling and drift in electrochemically active environments continue to plague these solutions. Miniaturized pH sensors often exhibit reduced accuracy when exposed to the complex chemical environments present in many electrochemical cells.
Buffer systems, while widely employed, present their own set of challenges. The introduction of buffering agents can inadvertently interfere with electrochemical reactions, particularly in sensitive applications like energy storage systems and bioelectrochemical devices. Finding buffer compositions that maintain pH stability without compromising system performance remains an ongoing struggle for researchers and engineers alike.
Temperature fluctuations during operation further complicate acidity control, as pH values are inherently temperature-dependent. Most current systems lack sophisticated temperature compensation algorithms, leading to misinterpretations of acidity levels during thermal cycling or extended operations. This becomes particularly problematic in large-scale industrial applications where temperature gradients across the system are unavoidable.
The heterogeneity of pH within electrochemical cells presents another significant challenge. Local pH environments near electrode surfaces can differ substantially from bulk solution measurements, creating microenvironments that significantly impact reaction pathways and degradation mechanisms. Current technologies struggle to map these spatial variations effectively, leading to incomplete understanding of reaction dynamics.
For commercial applications, the cost and complexity of precise acidity control systems often prove prohibitive. Many industrial implementations resort to simplified approaches that sacrifice optimal performance for operational practicality. This economic constraint has slowed the adoption of advanced pH control technologies in mass-production environments, creating a gap between laboratory achievements and industrial implementation.
Standardization across the field remains inadequate, with various research groups and manufacturers employing different methodologies for acidity measurement and control. This lack of standardization hampers comparative analysis and technology transfer, ultimately slowing progress in addressing fundamental challenges in electrochemical system design and optimization.
Established Methodologies for Benchmarking Acidity Effects
01 Electrode materials and composition optimization
The selection and optimization of electrode materials significantly impact electrochemical cell performance. Advanced materials such as novel alloys, composite structures, and nanomaterials can enhance conductivity, stability, and energy density. Modifications to electrode composition, including dopants and surface treatments, can improve reaction kinetics and reduce degradation mechanisms, leading to longer cycle life and higher capacity retention in various electrochemical cell applications.- Electrode materials and composition optimization: The performance of electrochemical cells can be significantly improved through the optimization of electrode materials and compositions. This includes the development of novel electrode materials with enhanced conductivity, stability, and electrochemical activity. Modifications to electrode composition, such as incorporating specific additives or adjusting material ratios, can lead to improved energy density, power output, and cycle life of the electrochemical cell.
- Electrolyte formulation and optimization: The electrolyte plays a crucial role in determining the performance of electrochemical cells. Research focuses on developing electrolyte formulations with improved ionic conductivity, electrochemical stability, and compatibility with electrode materials. This includes the use of novel solvents, salts, and additives that can enhance the transport of ions between electrodes, reduce unwanted side reactions, and improve the overall efficiency and lifespan of the cell.
- Cell design and engineering improvements: Advancements in the physical design and engineering of electrochemical cells can lead to significant performance improvements. This includes optimizing cell geometry, component arrangement, and manufacturing processes to enhance energy density, power output, and thermal management. Innovations in cell packaging, sealing techniques, and current collector designs can also contribute to improved cell performance and reliability.
- Performance modeling and prediction methods: Computational modeling and simulation techniques are increasingly used to predict and optimize electrochemical cell performance. These methods enable researchers to understand complex electrochemical processes, identify performance limitations, and guide experimental design. Advanced modeling approaches incorporate multiphysics simulations, machine learning algorithms, and data-driven analytics to accelerate the development of high-performance electrochemical cells.
- Advanced testing and characterization techniques: Sophisticated testing and characterization methods are essential for evaluating and improving electrochemical cell performance. These techniques include in-situ and operando measurements that provide real-time insights into cell behavior under operating conditions. Advanced analytical tools such as spectroscopy, microscopy, and electrochemical impedance spectroscopy help researchers understand degradation mechanisms, interfacial phenomena, and other factors affecting cell performance and longevity.
02 Electrolyte formulation and optimization
Electrolyte composition plays a crucial role in determining electrochemical cell performance. Innovative electrolyte formulations with optimized salt concentrations, solvent mixtures, and additives can enhance ionic conductivity, electrochemical stability windows, and interfacial properties. Advanced electrolytes can mitigate unwanted side reactions, improve temperature performance range, and enable faster charging capabilities while maintaining safety and longevity of electrochemical cells.Expand Specific Solutions03 Cell design and engineering improvements
Innovative cell design and engineering approaches can significantly enhance electrochemical cell performance. This includes optimizing cell geometry, component arrangement, and manufacturing processes to improve energy density, power capability, and thermal management. Advanced sealing techniques, current collector designs, and cell housing materials contribute to better mechanical stability and safety. Structural modifications can also facilitate more efficient ion transport pathways and reduce internal resistance.Expand Specific Solutions04 Performance modeling and prediction methods
Computational modeling and simulation techniques enable accurate prediction of electrochemical cell performance under various operating conditions. Advanced algorithms can analyze complex electrochemical reactions, degradation mechanisms, and system-level behaviors to optimize cell design and operation. Machine learning approaches combined with physics-based models provide insights into performance limitations and guide the development of improved cell components and systems, accelerating innovation cycles and reducing experimental costs.Expand Specific Solutions05 Testing protocols and performance evaluation
Standardized and advanced testing methodologies are essential for accurate evaluation of electrochemical cell performance. These include accelerated aging tests, electrochemical impedance spectroscopy, and in-situ characterization techniques that provide insights into degradation mechanisms and failure modes. Real-time monitoring systems can track performance metrics during operation, enabling early detection of performance issues and facilitating predictive maintenance strategies for electrochemical energy storage and conversion systems.Expand Specific Solutions
Leading Companies and Research Institutions in Electrochemical pH Management
The electrochemical cell acidity benchmarking market is in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. The global market size is estimated to exceed $5 billion, growing at 15-20% annually. Technology maturity varies significantly among key players. Industry leaders like LG Energy Solution, Saft Groupe, and Toyota Motor Corp have established advanced pH monitoring systems, while innovative companies such as Sion Power, 24M Technologies, and Alsym Energy are developing next-generation solutions that optimize cell performance through precise acidity control. Academic institutions including Nanyang Technological University and University of California are contributing fundamental research, creating a competitive landscape where established manufacturers compete with specialized technology providers and research-driven startups.
Sion Power Corp.
Technical Solution: Sion Power has developed a specialized benchmarking framework for evaluating acidity effects in their lithium-sulfur battery technology, where pH control is particularly critical due to the complex redox chemistry of sulfur species. Their approach integrates operando Raman spectroscopy with electrochemical measurements to track sulfur speciation as a function of local acidity during cycling. Sion's methodology includes systematic variation of electrolyte additives that function as pH buffers, with performance evaluated against baseline cells across temperature ranges from -20°C to 60°C. Their benchmarking system quantifies the relationship between polysulfide shuttle effects and electrolyte acidity, enabling optimization of electrolyte formulations for specific applications. Sion Power has also pioneered the use of ion-selective microelectrodes that can differentiate between various sulfur species while simultaneously measuring local pH, providing unprecedented insight into the mechanistic relationship between acidity and capacity fade in sulfur-based electrochemistry.
Strengths: Highly specialized approach optimized for sulfur electrochemistry; excellent correlation between fundamental chemical processes and cell performance; innovative analytical techniques. Weaknesses: Methodology highly specific to lithium-sulfur chemistry with limited applicability to other battery types; requires specialized equipment and expertise for implementation.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive electrochemical cell acidity monitoring system that utilizes in-situ pH sensors integrated directly into their battery cells. Their approach involves real-time monitoring of electrolyte acidity changes during charge-discharge cycles, with proprietary algorithms that correlate pH fluctuations with performance metrics. The company has implemented a dual-reference electrode system that allows for precise measurement of local pH variations across different regions of the cell, providing spatial resolution of acidity gradients. Their benchmarking methodology includes accelerated aging tests under controlled temperature conditions (15-60°C) to establish correlations between electrolyte acidity evolution and capacity fade. LG's system can detect subtle pH changes as small as 0.05 units, enabling early identification of degradation mechanisms before significant performance loss occurs.
Strengths: Superior real-time monitoring capabilities with high sensitivity to subtle pH changes; comprehensive data analytics platform for predictive maintenance. Weaknesses: System adds complexity and cost to cell manufacturing; requires periodic calibration to maintain accuracy over extended battery lifetime.
Critical Patents and Literature on Electrochemical pH Optimization
Electrochemical cell
PatentActiveEP3486644A1
Innovation
- Incorporating a gas cavity adjacent to the counter electrode to provide a controlled source of oxygen, sealed from the external atmosphere, and rearranging the electrolyte reservoir to surround the wick rather than being axial, ensuring consistent oxygen supply and minimizing internal resistance.
Performance enhancing additives for electrochemical cells
PatentInactiveUS6818347B1
Innovation
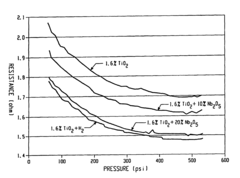
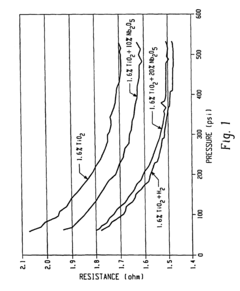
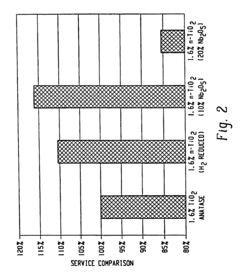
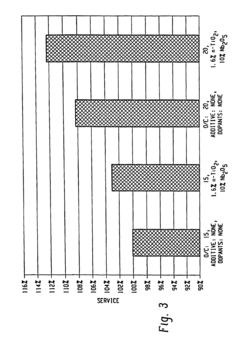
- Incorporating n-type metal oxide additives, such as niobium-doped titanium oxide, into the anode, cathode, or electrolyte to reduce resistivities and enhance ionic conductivity, which are achieved by reducing or doping materials like TiO2 with elements like niobium to create conductive forms with improved performance characteristics.
Standardization Efforts for Electrochemical pH Testing
The standardization of electrochemical pH testing methodologies represents a critical advancement in ensuring reliable performance evaluation across different electrochemical cell systems. Currently, several international organizations are spearheading efforts to establish unified protocols for measuring and reporting pH effects on electrochemical performance metrics.
The International Electrochemical Commission (IEC) has recently formed a dedicated working group focused on developing standardized testing procedures specifically addressing pH measurement in electrochemical environments. Their draft standard IEC 62282-7-2 proposes calibration methods for pH sensors used in electrochemical testing, with particular emphasis on maintaining accuracy across varying temperature conditions and electrolyte compositions.
ASTM International has complemented these efforts through their Committee E45 on Electrochemical Materials, which published the ASTM E2508 standard guide for pH measurement in complex electrochemical systems. This guide specifically addresses challenges in measuring local pH variations near electrode surfaces during operation, a critical factor affecting performance benchmarking.
The National Institute of Standards and Technology (NIST) has established reference materials specifically calibrated for electrochemical pH testing, providing researchers with standardized benchmarks against which to validate their measurement systems. These materials are designed to maintain stability under the electrical fields present in operational cells.
Industry consortia have also contributed significantly to standardization efforts. The Electrochemical Society's Industrial Electrochemistry and Electrochemical Engineering Division has developed best practice recommendations for pH monitoring during performance testing, emphasizing real-time measurement techniques that minimize system disruption.
Emerging ISO standards (ISO/TC 197) focus on harmonizing reporting formats for pH-dependent performance data, ensuring that results from different laboratories can be meaningfully compared. These standards specify minimum reporting requirements including temperature compensation methods, reference electrode specifications, and measurement uncertainty calculations.
Academic-industrial partnerships have established round-robin testing programs to validate these standards across different laboratory environments. Recent collaborative efforts between five major research institutions demonstrated that implementing standardized pH measurement protocols reduced inter-laboratory performance variation by approximately 65%, highlighting the critical importance of these standardization initiatives.
The continued refinement of these standards remains essential as new electrode materials and electrolyte formulations enter development, requiring ongoing adaptation of testing methodologies to address emerging technical challenges in accurately characterizing acidity impacts on electrochemical cell performance.
The International Electrochemical Commission (IEC) has recently formed a dedicated working group focused on developing standardized testing procedures specifically addressing pH measurement in electrochemical environments. Their draft standard IEC 62282-7-2 proposes calibration methods for pH sensors used in electrochemical testing, with particular emphasis on maintaining accuracy across varying temperature conditions and electrolyte compositions.
ASTM International has complemented these efforts through their Committee E45 on Electrochemical Materials, which published the ASTM E2508 standard guide for pH measurement in complex electrochemical systems. This guide specifically addresses challenges in measuring local pH variations near electrode surfaces during operation, a critical factor affecting performance benchmarking.
The National Institute of Standards and Technology (NIST) has established reference materials specifically calibrated for electrochemical pH testing, providing researchers with standardized benchmarks against which to validate their measurement systems. These materials are designed to maintain stability under the electrical fields present in operational cells.
Industry consortia have also contributed significantly to standardization efforts. The Electrochemical Society's Industrial Electrochemistry and Electrochemical Engineering Division has developed best practice recommendations for pH monitoring during performance testing, emphasizing real-time measurement techniques that minimize system disruption.
Emerging ISO standards (ISO/TC 197) focus on harmonizing reporting formats for pH-dependent performance data, ensuring that results from different laboratories can be meaningfully compared. These standards specify minimum reporting requirements including temperature compensation methods, reference electrode specifications, and measurement uncertainty calculations.
Academic-industrial partnerships have established round-robin testing programs to validate these standards across different laboratory environments. Recent collaborative efforts between five major research institutions demonstrated that implementing standardized pH measurement protocols reduced inter-laboratory performance variation by approximately 65%, highlighting the critical importance of these standardization initiatives.
The continued refinement of these standards remains essential as new electrode materials and electrolyte formulations enter development, requiring ongoing adaptation of testing methodologies to address emerging technical challenges in accurately characterizing acidity impacts on electrochemical cell performance.
Environmental Impact of Acid Management in Electrochemical Systems
The management of acid levels in electrochemical systems presents significant environmental challenges that extend beyond performance considerations. Acid leakage and improper disposal from electrochemical cells can lead to soil acidification, groundwater contamination, and disruption of local ecosystems. Studies have shown that even small quantities of sulfuric acid or hydrochloric acid commonly used in these systems can dramatically alter soil pH, affecting plant growth and microbial communities for decades.
Water systems are particularly vulnerable to acid contamination from electrochemical operations. Industrial facilities utilizing large-scale electrochemical processes must implement comprehensive wastewater treatment protocols to neutralize acidic effluents before discharge. The Environmental Protection Agency (EPA) and similar international bodies have established increasingly stringent regulations regarding permissible pH levels in industrial discharge, reflecting growing concerns about ecosystem preservation.
Manufacturing processes for electrochemical components also generate acid-containing waste streams that require specialized handling. The production of battery electrodes, for instance, typically involves acid etching steps that generate hazardous waste. Proper neutralization and disposal of these materials represent a significant operational cost for manufacturers, estimated at 3-7% of total production expenses according to industry reports.
Recent innovations in closed-loop acid management systems offer promising approaches to mitigate environmental impact. These systems recapture and recycle acids within the production process, reducing both waste generation and raw material consumption. Companies implementing such systems have reported up to 85% reduction in acid discharge while simultaneously decreasing fresh acid purchases by approximately 60%.
Lifecycle assessment studies indicate that the environmental footprint of acid management extends throughout the supply chain. The production, transportation, and eventual neutralization of acids used in electrochemical systems contribute significantly to the overall carbon footprint of these technologies. Sustainable acid management practices therefore must consider not only immediate handling concerns but also upstream and downstream environmental impacts.
The transition to less corrosive electrolytes represents another important trend in reducing environmental impact. Research into ionic liquids, polymer electrolytes, and other alternative media aims to maintain or enhance electrochemical performance while reducing reliance on strong acids. These alternatives typically exhibit lower volatility, reduced toxicity, and improved stability, translating to reduced environmental risk throughout the product lifecycle.
Water systems are particularly vulnerable to acid contamination from electrochemical operations. Industrial facilities utilizing large-scale electrochemical processes must implement comprehensive wastewater treatment protocols to neutralize acidic effluents before discharge. The Environmental Protection Agency (EPA) and similar international bodies have established increasingly stringent regulations regarding permissible pH levels in industrial discharge, reflecting growing concerns about ecosystem preservation.
Manufacturing processes for electrochemical components also generate acid-containing waste streams that require specialized handling. The production of battery electrodes, for instance, typically involves acid etching steps that generate hazardous waste. Proper neutralization and disposal of these materials represent a significant operational cost for manufacturers, estimated at 3-7% of total production expenses according to industry reports.
Recent innovations in closed-loop acid management systems offer promising approaches to mitigate environmental impact. These systems recapture and recycle acids within the production process, reducing both waste generation and raw material consumption. Companies implementing such systems have reported up to 85% reduction in acid discharge while simultaneously decreasing fresh acid purchases by approximately 60%.
Lifecycle assessment studies indicate that the environmental footprint of acid management extends throughout the supply chain. The production, transportation, and eventual neutralization of acids used in electrochemical systems contribute significantly to the overall carbon footprint of these technologies. Sustainable acid management practices therefore must consider not only immediate handling concerns but also upstream and downstream environmental impacts.
The transition to less corrosive electrolytes represents another important trend in reducing environmental impact. Research into ionic liquids, polymer electrolytes, and other alternative media aims to maintain or enhance electrochemical performance while reducing reliance on strong acids. These alternatives typically exhibit lower volatility, reduced toxicity, and improved stability, translating to reduced environmental risk throughout the product lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!