Measure Electrochemical Cell Electrolyte Ion Concentration
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Electrolyte Ion Sensing Background and Objectives
Electrochemical cell systems have evolved significantly over the past several decades, transforming from simple laboratory devices to sophisticated components integral to numerous technological applications. The measurement and monitoring of electrolyte ion concentration represent a critical aspect of electrochemical cell performance and safety management. This technological domain has witnessed accelerated development due to the expanding applications of batteries, fuel cells, and electrochemical sensors across industries including automotive, consumer electronics, medical devices, and renewable energy systems.
The evolution of ion concentration measurement techniques has progressed from basic conductivity measurements to advanced spectroscopic and electrochemical methods. Early approaches relied primarily on offline sampling and laboratory analysis, which provided limited real-time monitoring capabilities. The technological trajectory has since moved toward in-situ and operando measurement techniques that enable continuous monitoring of electrolyte conditions during cell operation.
Recent advancements in miniaturized sensors, wireless communication technologies, and data analytics have further propelled this field forward. The integration of these technologies has enabled more precise, reliable, and cost-effective monitoring solutions that can be deployed in various operational environments. The development of novel electrode materials, reference systems, and signal processing algorithms has significantly enhanced measurement accuracy and stability under challenging conditions.
The global push toward electrification and renewable energy integration has intensified the need for advanced electrolyte monitoring systems. As battery technologies become increasingly central to energy storage solutions and electric mobility, the demand for sophisticated ion concentration measurement techniques continues to grow. This trend is further reinforced by stringent safety requirements and performance optimization needs across industries.
The primary objectives of current research and development efforts in this domain include enhancing measurement accuracy across wider concentration ranges, improving sensor durability in harsh chemical environments, reducing system costs, and enabling real-time data integration with battery management systems. Additionally, there is significant focus on developing non-invasive measurement techniques that minimize interference with cell operation while providing comprehensive insights into electrolyte composition and dynamics.
Future technological trajectories are likely to emphasize miniaturization, multi-parameter sensing capabilities, and integration with artificial intelligence for predictive analytics. The convergence of electrochemical sensing with advanced materials science and digital technologies presents promising opportunities for breakthrough innovations in this field. These developments aim to address the growing complexity of electrochemical systems while meeting the increasing demands for reliability, efficiency, and safety in various applications.
The evolution of ion concentration measurement techniques has progressed from basic conductivity measurements to advanced spectroscopic and electrochemical methods. Early approaches relied primarily on offline sampling and laboratory analysis, which provided limited real-time monitoring capabilities. The technological trajectory has since moved toward in-situ and operando measurement techniques that enable continuous monitoring of electrolyte conditions during cell operation.
Recent advancements in miniaturized sensors, wireless communication technologies, and data analytics have further propelled this field forward. The integration of these technologies has enabled more precise, reliable, and cost-effective monitoring solutions that can be deployed in various operational environments. The development of novel electrode materials, reference systems, and signal processing algorithms has significantly enhanced measurement accuracy and stability under challenging conditions.
The global push toward electrification and renewable energy integration has intensified the need for advanced electrolyte monitoring systems. As battery technologies become increasingly central to energy storage solutions and electric mobility, the demand for sophisticated ion concentration measurement techniques continues to grow. This trend is further reinforced by stringent safety requirements and performance optimization needs across industries.
The primary objectives of current research and development efforts in this domain include enhancing measurement accuracy across wider concentration ranges, improving sensor durability in harsh chemical environments, reducing system costs, and enabling real-time data integration with battery management systems. Additionally, there is significant focus on developing non-invasive measurement techniques that minimize interference with cell operation while providing comprehensive insights into electrolyte composition and dynamics.
Future technological trajectories are likely to emphasize miniaturization, multi-parameter sensing capabilities, and integration with artificial intelligence for predictive analytics. The convergence of electrochemical sensing with advanced materials science and digital technologies presents promising opportunities for breakthrough innovations in this field. These developments aim to address the growing complexity of electrochemical systems while meeting the increasing demands for reliability, efficiency, and safety in various applications.
Market Analysis for Ion Concentration Measurement Technologies
The global market for ion concentration measurement technologies has experienced significant growth in recent years, driven primarily by increasing applications in battery research, environmental monitoring, and healthcare diagnostics. The market size for electrochemical analysis equipment reached approximately $2.3 billion in 2022, with ion concentration measurement devices constituting about 18% of this segment. Industry analysts project a compound annual growth rate (CAGR) of 5.7% through 2028, potentially expanding the market to $3.4 billion.
Battery manufacturing represents the fastest-growing application sector, with demand for precise electrolyte ion concentration measurement increasing by nearly 24% annually since 2020. This surge correlates directly with the expansion of electric vehicle production and stationary energy storage systems. Major automotive manufacturers have increased their investment in battery testing equipment by an average of 32% year-over-year, highlighting the critical importance of electrolyte monitoring technologies.
The healthcare and pharmaceutical sectors collectively account for approximately 27% of the current market share. Hospitals and clinical laboratories increasingly rely on ion-selective electrodes and similar technologies for blood electrolyte analysis, creating a stable demand base with moderate growth of 4-6% annually. Point-of-care testing devices that incorporate ion concentration measurement capabilities have seen particularly strong adoption, with unit sales increasing by 15% in 2022 alone.
Environmental monitoring applications, including water quality assessment and soil analysis, represent another significant market segment at 22% of total demand. Government regulations regarding water quality standards in North America, Europe, and increasingly in Asia have created sustained demand for advanced ion measurement technologies. The industrial sector, particularly semiconductor manufacturing and chemical processing, contributes an additional 19% to market demand.
Geographically, North America leads with 38% market share, followed by Europe (29%) and Asia-Pacific (26%). However, the Asia-Pacific region demonstrates the highest growth rate at 8.3% annually, driven by China's expanding manufacturing base and increasing environmental regulations. Latin America and Africa remain relatively small markets but show promising growth potential as water quality monitoring infrastructure develops.
Customer preferences increasingly favor portable, digital devices with wireless connectivity and cloud data integration capabilities. The premium segment of the market has shifted toward multi-parameter instruments that can simultaneously measure various ion concentrations, offering comprehensive analysis in a single device. Price sensitivity varies significantly by application, with research institutions willing to pay premium prices for high-accuracy instruments, while industrial users often prioritize durability and cost-effectiveness.
Battery manufacturing represents the fastest-growing application sector, with demand for precise electrolyte ion concentration measurement increasing by nearly 24% annually since 2020. This surge correlates directly with the expansion of electric vehicle production and stationary energy storage systems. Major automotive manufacturers have increased their investment in battery testing equipment by an average of 32% year-over-year, highlighting the critical importance of electrolyte monitoring technologies.
The healthcare and pharmaceutical sectors collectively account for approximately 27% of the current market share. Hospitals and clinical laboratories increasingly rely on ion-selective electrodes and similar technologies for blood electrolyte analysis, creating a stable demand base with moderate growth of 4-6% annually. Point-of-care testing devices that incorporate ion concentration measurement capabilities have seen particularly strong adoption, with unit sales increasing by 15% in 2022 alone.
Environmental monitoring applications, including water quality assessment and soil analysis, represent another significant market segment at 22% of total demand. Government regulations regarding water quality standards in North America, Europe, and increasingly in Asia have created sustained demand for advanced ion measurement technologies. The industrial sector, particularly semiconductor manufacturing and chemical processing, contributes an additional 19% to market demand.
Geographically, North America leads with 38% market share, followed by Europe (29%) and Asia-Pacific (26%). However, the Asia-Pacific region demonstrates the highest growth rate at 8.3% annually, driven by China's expanding manufacturing base and increasing environmental regulations. Latin America and Africa remain relatively small markets but show promising growth potential as water quality monitoring infrastructure develops.
Customer preferences increasingly favor portable, digital devices with wireless connectivity and cloud data integration capabilities. The premium segment of the market has shifted toward multi-parameter instruments that can simultaneously measure various ion concentrations, offering comprehensive analysis in a single device. Price sensitivity varies significantly by application, with research institutions willing to pay premium prices for high-accuracy instruments, while industrial users often prioritize durability and cost-effectiveness.
Current Challenges in Electrolyte Ion Concentration Detection
Despite significant advancements in electrochemical sensing technologies, measuring electrolyte ion concentration in electrochemical cells continues to present several formidable challenges. The dynamic nature of electrochemical environments creates inherent difficulties in obtaining accurate, real-time measurements of ion concentrations, particularly in complex electrolyte solutions with multiple ionic species.
One of the primary challenges lies in achieving sufficient selectivity for specific ions in mixed electrolyte environments. Current sensors often struggle to distinguish between similar ionic species, especially in the presence of interfering ions with comparable charge or size characteristics. This selectivity issue becomes particularly problematic in applications such as lithium-ion batteries, where accurately differentiating between lithium ions and other alkali metal ions is crucial for performance monitoring.
Sensitivity limitations represent another significant hurdle. Many existing detection methods fail to provide adequate resolution at the concentration ranges relevant to specific electrochemical applications. This is especially evident in dilute electrolyte systems or when monitoring trace-level ionic species that play critical roles in electrochemical processes. The detection limits of conventional techniques often fall short of the requirements for advanced electrochemical systems.
Temporal resolution presents a substantial challenge for real-time monitoring applications. The rapid dynamics of ion transport during electrochemical reactions demand measurement techniques capable of capturing concentration changes on millisecond or even microsecond timescales. Current technologies frequently exhibit response times that are inadequate for tracking fast electrochemical processes, resulting in measurement lag that compromises data interpretation.
Spatial resolution constraints further complicate accurate ion concentration measurements. Concentration gradients within electrochemical cells can vary significantly across microscopic distances, particularly near electrode surfaces where electrochemical reactions occur. Existing measurement techniques typically provide bulk concentration values rather than spatially resolved data, obscuring critical localized phenomena that influence overall cell performance.
Stability and durability of sensing elements in harsh electrochemical environments pose persistent challenges. Exposure to extreme pH conditions, high ionic strengths, organic solvents, and elevated temperatures can rapidly degrade sensor performance. This degradation leads to measurement drift, reduced sensitivity, and shortened operational lifetimes, necessitating frequent recalibration or replacement of sensing components.
Miniaturization requirements for integration into modern electrochemical devices create additional complications. As electrochemical cells become increasingly compact, conventional ion concentration measurement techniques often prove too bulky or intrusive for practical implementation without disrupting normal cell operation or introducing measurement artifacts.
One of the primary challenges lies in achieving sufficient selectivity for specific ions in mixed electrolyte environments. Current sensors often struggle to distinguish between similar ionic species, especially in the presence of interfering ions with comparable charge or size characteristics. This selectivity issue becomes particularly problematic in applications such as lithium-ion batteries, where accurately differentiating between lithium ions and other alkali metal ions is crucial for performance monitoring.
Sensitivity limitations represent another significant hurdle. Many existing detection methods fail to provide adequate resolution at the concentration ranges relevant to specific electrochemical applications. This is especially evident in dilute electrolyte systems or when monitoring trace-level ionic species that play critical roles in electrochemical processes. The detection limits of conventional techniques often fall short of the requirements for advanced electrochemical systems.
Temporal resolution presents a substantial challenge for real-time monitoring applications. The rapid dynamics of ion transport during electrochemical reactions demand measurement techniques capable of capturing concentration changes on millisecond or even microsecond timescales. Current technologies frequently exhibit response times that are inadequate for tracking fast electrochemical processes, resulting in measurement lag that compromises data interpretation.
Spatial resolution constraints further complicate accurate ion concentration measurements. Concentration gradients within electrochemical cells can vary significantly across microscopic distances, particularly near electrode surfaces where electrochemical reactions occur. Existing measurement techniques typically provide bulk concentration values rather than spatially resolved data, obscuring critical localized phenomena that influence overall cell performance.
Stability and durability of sensing elements in harsh electrochemical environments pose persistent challenges. Exposure to extreme pH conditions, high ionic strengths, organic solvents, and elevated temperatures can rapidly degrade sensor performance. This degradation leads to measurement drift, reduced sensitivity, and shortened operational lifetimes, necessitating frequent recalibration or replacement of sensing components.
Miniaturization requirements for integration into modern electrochemical devices create additional complications. As electrochemical cells become increasingly compact, conventional ion concentration measurement techniques often prove too bulky or intrusive for practical implementation without disrupting normal cell operation or introducing measurement artifacts.
State-of-the-Art Ion Concentration Measurement Solutions
01 Optimization of electrolyte ion concentration for battery performance
The concentration of ions in electrolytes significantly impacts battery performance metrics such as energy density, power output, and cycle life. Optimizing the ion concentration can enhance ionic conductivity while maintaining electrochemical stability. Research shows that carefully controlled ion concentrations can reduce internal resistance, improve charge transfer at electrode interfaces, and prevent unwanted side reactions that lead to capacity fade. These optimizations are particularly important in lithium-ion and next-generation battery technologies.- Optimizing ion concentration for improved electrochemical performance: The concentration of ions in electrolytes significantly impacts the performance of electrochemical cells. Optimizing ion concentration can enhance conductivity, energy density, and overall cell efficiency. Research shows that carefully controlled ion concentrations can reduce internal resistance, improve charge transfer rates, and extend the operational life of batteries and other electrochemical devices. Various methods for measuring and maintaining optimal ion concentrations have been developed to maximize electrochemical performance.
- Novel electrolyte compositions with specific ion concentrations: Innovative electrolyte formulations with precisely controlled ion concentrations have been developed to address specific challenges in electrochemical cells. These compositions may include unique combinations of salts, solvents, and additives that work synergistically to enhance performance. Some formulations focus on increasing specific ion mobility, while others aim to suppress unwanted side reactions or improve stability at extreme temperatures. These novel electrolyte compositions represent significant advancements in tailoring ion concentrations for specialized applications.
- Temperature effects on electrolyte ion concentration: Temperature significantly influences ion concentration and behavior in electrochemical cell electrolytes. As temperature changes, ion mobility, solubility, and interaction dynamics are affected, impacting overall cell performance. Research has focused on developing temperature-stable electrolytes that maintain optimal ion concentrations across wide operating ranges. Some approaches include using temperature-responsive additives, eutectic mixtures, or specially engineered solvents that compensate for temperature-induced concentration changes, ensuring consistent electrochemical performance in varying environmental conditions.
- Ion concentration monitoring and control systems: Advanced systems for real-time monitoring and control of electrolyte ion concentration have been developed to optimize electrochemical cell performance. These systems employ various sensing technologies, including electrochemical impedance spectroscopy, optical methods, and miniaturized sensors integrated directly into cells. Feedback control mechanisms can automatically adjust ion concentrations in response to changing operating conditions or performance requirements. Such monitoring and control systems are particularly valuable in large-scale energy storage applications, where maintaining optimal ion concentration is critical for efficiency and longevity.
- Solid-state electrolytes with controlled ion concentration: Solid-state electrolytes with precisely engineered ion concentrations offer advantages in safety, stability, and performance for next-generation electrochemical cells. These materials feature carefully controlled ion distribution within their crystal structures or polymer matrices, enabling selective ion transport while blocking dendrite formation. Research has focused on optimizing ion doping levels, distribution patterns, and migration pathways to achieve high ionic conductivity comparable to liquid electrolytes. Solid-state systems with tailored ion concentrations show promise for high-energy density applications where conventional liquid electrolytes present safety or stability limitations.
02 Novel electrolyte compositions with controlled ion concentrations
Advanced electrolyte formulations incorporate specific ion concentrations to address challenges in electrochemical cells. These compositions may include mixed salt systems, ionic liquids, or polymer electrolytes with precisely controlled ion ratios. By manipulating the concentration of different ionic species, researchers have developed electrolytes with enhanced thermal stability, wider electrochemical windows, and improved safety characteristics. These novel compositions often enable operation under extreme conditions or with high-voltage electrode materials that conventional electrolytes cannot support.Expand Specific Solutions03 Ion concentration gradient engineering in electrochemical systems
Creating controlled ion concentration gradients within electrochemical cells can enhance performance and functionality. This approach involves strategically varying ion concentrations across different regions of the electrolyte or at electrode-electrolyte interfaces. Such gradient engineering can facilitate directional ion transport, mitigate dendrite formation, and improve rate capability. Advanced techniques for establishing and maintaining these gradients include structured electrolytes, concentration polarization management, and dynamic control systems that respond to operating conditions.Expand Specific Solutions04 Measurement and monitoring of electrolyte ion concentration
Accurate measurement and real-time monitoring of ion concentrations in electrochemical cell electrolytes are crucial for performance optimization and safety management. Various analytical techniques and sensors have been developed to track ion concentrations during cell operation, including spectroscopic methods, electrochemical impedance spectroscopy, and specialized ion-selective electrodes. These monitoring systems can detect concentration changes that might indicate degradation processes, allowing for preventive maintenance or adaptive control strategies to extend cell lifetime.Expand Specific Solutions05 Impact of temperature and pressure on electrolyte ion concentration
Temperature and pressure conditions significantly affect ion concentration behavior in electrochemical cell electrolytes. Research has explored how these parameters influence ion solvation, mobility, and distribution within the electrolyte. Understanding these relationships enables the development of electrochemical systems that maintain optimal ion concentrations across varying environmental conditions. Advanced electrolyte formulations incorporate additives or stabilizers that help maintain consistent ion concentration profiles despite temperature fluctuations or pressure changes, improving the reliability and performance consistency of electrochemical devices.Expand Specific Solutions
Leading Companies and Research Institutions in Electrochemical Sensing
The electrochemical cell electrolyte ion concentration measurement market is in a growth phase, driven by increasing demand for battery technologies and energy storage solutions. The market size is expanding rapidly, with projections indicating significant growth as electric vehicle adoption accelerates globally. Technologically, the field shows varying maturity levels, with established players like Hitachi High-Tech, Agilent Technologies, and Robert Bosch offering sophisticated measurement solutions, while Chinese companies such as Tianjin Lishen Battery, Beijing WeLion, and LG Energy Solution are advancing rapidly in battery-specific applications. Academic institutions like Tsinghua University and Swiss Federal Institute of Technology contribute fundamental research, creating a competitive landscape where collaboration between industry and academia is driving innovation in more precise, reliable, and cost-effective measurement technologies for next-generation battery systems.
Robert Bosch GmbH
Technical Solution: Bosch has engineered sophisticated sensor systems for monitoring electrolyte ion concentrations in automotive and industrial battery applications. Their technology combines impedance-based measurements with advanced signal processing to determine ion mobility and concentration in operating cells. Bosch's sensors utilize microelectrode arrays fabricated using semiconductor manufacturing techniques, enabling miniaturization and cost-effective mass production. Their systems incorporate temperature compensation algorithms that maintain measurement accuracy across the wide temperature ranges experienced in automotive applications (-40°C to +85°C). Bosch has developed specialized reference electrodes with extended lifespans in aggressive electrolyte environments, addressing a common failure point in long-term monitoring systems. Their technology can detect early indicators of electrolyte degradation by measuring changes in specific ion ratios, providing predictive maintenance capabilities for critical battery systems.
Strengths: Robust designs suitable for harsh automotive environments; excellent temperature stability; integration capabilities with vehicle diagnostic systems. Weaknesses: Primarily focused on automotive applications; may have limited sensitivity for research-grade measurements; proprietary data formats can complicate integration with third-party analysis tools.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed advanced ion chromatography systems specifically designed for electrochemical cell electrolyte analysis. Their ICP-MS (Inductively Coupled Plasma Mass Spectrometry) technology enables precise measurement of electrolyte ion concentrations at parts-per-billion levels. The company's 5800 ICP-OES system incorporates vertical torch design and advanced optical systems for superior detection limits when analyzing lithium-ion battery electrolytes. Agilent's integrated workflow solutions combine sample preparation automation with sophisticated software algorithms that compensate for matrix effects common in complex electrolyte solutions. Their systems can simultaneously measure multiple ion species (Li+, Na+, K+, etc.) with minimal cross-interference, providing comprehensive electrolyte composition profiles essential for battery performance optimization.
Strengths: Industry-leading detection limits and measurement precision; comprehensive multi-element analysis capabilities; robust systems designed for high-throughput industrial environments. Weaknesses: Higher initial investment costs compared to simpler analytical methods; requires specialized operator training; some systems have larger footprints requiring dedicated laboratory space.
Key Patents and Scientific Advances in Electrochemical Sensing
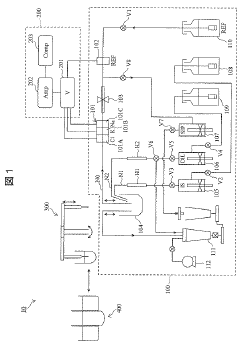
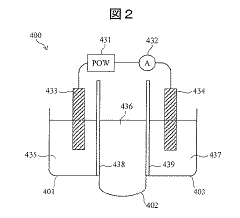
Electrolyte concentration measuring device
PatentInactiveJP2020034449A
Innovation
- An electrolyte concentration measuring device equipped with an ion-selective electrode, reference electrode, comparison electrode, and an electrolyte standard solution generator that can generate and adjust the concentration of electrolyte standard solutions automatically, allowing for self-calibration without user intervention.
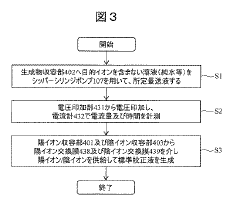
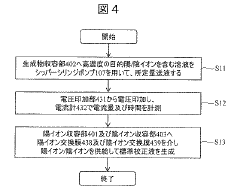
Electrolyte concentration measuring device and method for obtaining selectivity coefficient
PatentWO2023157421A1
Innovation
- An electrolyte concentration measuring device that includes an ion-selective electrode, a comparison electrode, and a control unit to calculate the selection coefficient of interfering ions without a dedicated sample, using machine learning algorithms to process potential differences and determine the interfering ion concentration based on measured potentials from solutions containing target and interfering ions.
Calibration and Standardization Methodologies
Accurate calibration and standardization methodologies are fundamental to ensuring reliable measurements of electrolyte ion concentration in electrochemical cells. The primary calibration approach involves the preparation of standard solutions with precisely known ion concentrations, typically covering the expected measurement range. These standards must be prepared using analytical-grade reagents and ultrapure water to minimize contamination that could affect calibration accuracy.
Multi-point calibration techniques are generally preferred over single-point methods, as they account for non-linear responses across different concentration ranges. For most ion-selective electrode applications, a minimum of three calibration points is recommended, with logarithmically spaced concentration intervals to optimize accuracy across the measurement range. The calibration curve typically follows the Nernst equation, relating electrode potential to ion activity logarithmically.
Standard addition methods represent another valuable calibration approach, particularly useful when matrix effects are significant. This technique involves adding known quantities of the target ion to the sample and measuring the corresponding change in signal. By extrapolating the resulting curve, the original ion concentration can be determined with minimal matrix interference.
Regular recalibration schedules must be established based on electrode stability, measurement frequency, and required accuracy. In high-precision applications, calibration may be necessary before each measurement series, while less critical applications might require daily or weekly calibration. Environmental factors such as temperature fluctuations necessitate more frequent calibration, as they can significantly affect electrode response.
Traceability to recognized standards is essential for ensuring measurement validity. Reference materials certified by national metrology institutes like NIST (USA) or PTB (Germany) should be used whenever possible. For specialized applications where certified reference materials are unavailable, laboratory-prepared standards must be validated through interlaboratory comparison studies or alternative analytical methods.
Quality control measures should include regular verification using check standards, drift monitoring, and statistical process control techniques. Implementing blind samples and duplicate analyses helps identify systematic errors in the calibration process. Documentation of all calibration procedures, including reagent sources, preparation methods, and environmental conditions, is crucial for maintaining measurement traceability and facilitating troubleshooting when deviations occur.
Multi-point calibration techniques are generally preferred over single-point methods, as they account for non-linear responses across different concentration ranges. For most ion-selective electrode applications, a minimum of three calibration points is recommended, with logarithmically spaced concentration intervals to optimize accuracy across the measurement range. The calibration curve typically follows the Nernst equation, relating electrode potential to ion activity logarithmically.
Standard addition methods represent another valuable calibration approach, particularly useful when matrix effects are significant. This technique involves adding known quantities of the target ion to the sample and measuring the corresponding change in signal. By extrapolating the resulting curve, the original ion concentration can be determined with minimal matrix interference.
Regular recalibration schedules must be established based on electrode stability, measurement frequency, and required accuracy. In high-precision applications, calibration may be necessary before each measurement series, while less critical applications might require daily or weekly calibration. Environmental factors such as temperature fluctuations necessitate more frequent calibration, as they can significantly affect electrode response.
Traceability to recognized standards is essential for ensuring measurement validity. Reference materials certified by national metrology institutes like NIST (USA) or PTB (Germany) should be used whenever possible. For specialized applications where certified reference materials are unavailable, laboratory-prepared standards must be validated through interlaboratory comparison studies or alternative analytical methods.
Quality control measures should include regular verification using check standards, drift monitoring, and statistical process control techniques. Implementing blind samples and duplicate analyses helps identify systematic errors in the calibration process. Documentation of all calibration procedures, including reagent sources, preparation methods, and environmental conditions, is crucial for maintaining measurement traceability and facilitating troubleshooting when deviations occur.
Environmental Impact and Sustainability Considerations
The measurement of electrochemical cell electrolyte ion concentration carries significant environmental implications that must be considered in both research and industrial applications. Traditional measurement techniques often involve chemicals that can be harmful to ecosystems when improperly disposed of, including heavy metals and organic solvents used in reference electrodes and calibration solutions.
Sustainable approaches to ion concentration measurement are increasingly prioritized, with emphasis on developing sensors that utilize environmentally benign materials. Recent advancements include the replacement of mercury-based reference electrodes with alternatives using non-toxic ionic liquids and the development of biodegradable sensor components that minimize electronic waste.
Energy consumption represents another critical environmental consideration. Conventional ion concentration measurement systems may require substantial power, particularly in continuous monitoring applications. The trend toward miniaturized, low-power sensors utilizing energy-efficient microelectronics and energy harvesting technologies significantly reduces the carbon footprint associated with these measurements.
Life cycle assessment (LCA) studies of electrochemical sensors reveal that the manufacturing phase often accounts for the largest environmental impact. Researchers are addressing this by exploring green synthesis methods and sustainable material sourcing. For instance, carbon-based electrodes derived from renewable biomass rather than petroleum-based precursors demonstrate comparable performance with substantially reduced environmental impact.
Water usage in sensor production and calibration presents additional sustainability challenges. Advanced manufacturing techniques that minimize water consumption and closed-loop calibration systems that recycle calibration solutions are being implemented to address these concerns. These approaches not only reduce environmental impact but often result in cost savings for industrial applications.
The environmental benefits of accurate ion concentration measurement must also be acknowledged. In wastewater treatment, precise monitoring enables optimal chemical dosing, reducing excess chemical usage and environmental discharge. Similarly, in natural water monitoring, these technologies enable early detection of contamination events, potentially preventing widespread ecological damage.
Regulatory frameworks increasingly incorporate sustainability requirements for analytical instrumentation. The European Union's Restriction of Hazardous Substances (RoHS) directive and similar regulations worldwide have accelerated the transition to greener measurement technologies. Companies developing new ion concentration measurement solutions must now consider environmental compliance from the earliest design stages.
Sustainable approaches to ion concentration measurement are increasingly prioritized, with emphasis on developing sensors that utilize environmentally benign materials. Recent advancements include the replacement of mercury-based reference electrodes with alternatives using non-toxic ionic liquids and the development of biodegradable sensor components that minimize electronic waste.
Energy consumption represents another critical environmental consideration. Conventional ion concentration measurement systems may require substantial power, particularly in continuous monitoring applications. The trend toward miniaturized, low-power sensors utilizing energy-efficient microelectronics and energy harvesting technologies significantly reduces the carbon footprint associated with these measurements.
Life cycle assessment (LCA) studies of electrochemical sensors reveal that the manufacturing phase often accounts for the largest environmental impact. Researchers are addressing this by exploring green synthesis methods and sustainable material sourcing. For instance, carbon-based electrodes derived from renewable biomass rather than petroleum-based precursors demonstrate comparable performance with substantially reduced environmental impact.
Water usage in sensor production and calibration presents additional sustainability challenges. Advanced manufacturing techniques that minimize water consumption and closed-loop calibration systems that recycle calibration solutions are being implemented to address these concerns. These approaches not only reduce environmental impact but often result in cost savings for industrial applications.
The environmental benefits of accurate ion concentration measurement must also be acknowledged. In wastewater treatment, precise monitoring enables optimal chemical dosing, reducing excess chemical usage and environmental discharge. Similarly, in natural water monitoring, these technologies enable early detection of contamination events, potentially preventing widespread ecological damage.
Regulatory frameworks increasingly incorporate sustainability requirements for analytical instrumentation. The European Union's Restriction of Hazardous Substances (RoHS) directive and similar regulations worldwide have accelerated the transition to greener measurement technologies. Companies developing new ion concentration measurement solutions must now consider environmental compliance from the earliest design stages.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!