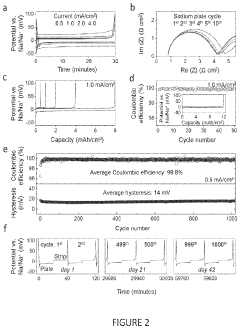
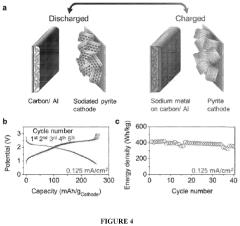
Electrochemical Cell Vs Chemical Cell: Energy Density Benchmark
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical vs Chemical Cell Technology Background
Energy storage technologies have evolved significantly over the past century, with electrochemical and chemical cells representing two fundamental approaches to storing and converting energy. Electrochemical cells, which include batteries and fuel cells, convert chemical energy directly into electrical energy through redox reactions at electrodes separated by an electrolyte. This technology dates back to Alessandro Volta's pioneering work in 1800, but has seen revolutionary advancements in recent decades.
Chemical cells, by contrast, store energy in chemical bonds and release it through combustion or other chemical reactions. These include traditional fossil fuels, hydrogen storage systems, and various synthetic fuels. The fundamental difference lies in the energy conversion pathway: electrochemical cells convert chemical energy directly to electrical energy, while chemical cells typically require an intermediate thermal energy conversion step.
The energy density benchmark between these technologies has been a critical factor driving research and development efforts. Historically, chemical cells have demonstrated superior gravimetric energy density, with petroleum-based fuels offering 12,000-13,000 Wh/kg compared to early lead-acid batteries at merely 30-40 Wh/kg. This substantial gap has positioned chemical cells as the dominant energy source for transportation and many industrial applications throughout the 20th century.
However, the technological trajectory has shifted dramatically in recent years. Modern lithium-ion batteries have achieved commercial energy densities of 250-300 Wh/kg, with laboratory prototypes approaching 400-500 Wh/kg. Next-generation technologies like lithium-sulfur and solid-state batteries promise to push these boundaries further, potentially reaching 500-700 Wh/kg in practical applications.
The evolution of electrochemical cells has been characterized by incremental improvements in electrode materials, electrolyte compositions, and cell architectures. Key milestones include the commercialization of lithium-ion technology in the 1990s, the development of high-capacity cathode materials in the 2000s, and recent breakthroughs in solid-state electrolytes. Each advancement has narrowed the energy density gap with chemical cells.
Environmental considerations have become increasingly important in this technological comparison. While chemical cells typically offer higher energy density, their utilization efficiency and environmental impact present significant drawbacks. Electrochemical cells offer higher round-trip efficiency, reduced emissions, and increasingly competitive energy storage capabilities, driving their adoption across multiple sectors.
The convergence of these technologies is evident in emerging hybrid systems, such as flow batteries and metal-air batteries, which combine aspects of both electrochemical and chemical energy storage principles to maximize performance characteristics and address specific application requirements.
Chemical cells, by contrast, store energy in chemical bonds and release it through combustion or other chemical reactions. These include traditional fossil fuels, hydrogen storage systems, and various synthetic fuels. The fundamental difference lies in the energy conversion pathway: electrochemical cells convert chemical energy directly to electrical energy, while chemical cells typically require an intermediate thermal energy conversion step.
The energy density benchmark between these technologies has been a critical factor driving research and development efforts. Historically, chemical cells have demonstrated superior gravimetric energy density, with petroleum-based fuels offering 12,000-13,000 Wh/kg compared to early lead-acid batteries at merely 30-40 Wh/kg. This substantial gap has positioned chemical cells as the dominant energy source for transportation and many industrial applications throughout the 20th century.
However, the technological trajectory has shifted dramatically in recent years. Modern lithium-ion batteries have achieved commercial energy densities of 250-300 Wh/kg, with laboratory prototypes approaching 400-500 Wh/kg. Next-generation technologies like lithium-sulfur and solid-state batteries promise to push these boundaries further, potentially reaching 500-700 Wh/kg in practical applications.
The evolution of electrochemical cells has been characterized by incremental improvements in electrode materials, electrolyte compositions, and cell architectures. Key milestones include the commercialization of lithium-ion technology in the 1990s, the development of high-capacity cathode materials in the 2000s, and recent breakthroughs in solid-state electrolytes. Each advancement has narrowed the energy density gap with chemical cells.
Environmental considerations have become increasingly important in this technological comparison. While chemical cells typically offer higher energy density, their utilization efficiency and environmental impact present significant drawbacks. Electrochemical cells offer higher round-trip efficiency, reduced emissions, and increasingly competitive energy storage capabilities, driving their adoption across multiple sectors.
The convergence of these technologies is evident in emerging hybrid systems, such as flow batteries and metal-air batteries, which combine aspects of both electrochemical and chemical energy storage principles to maximize performance characteristics and address specific application requirements.
Market Demand Analysis for High Energy Density Cells
The global market for high energy density cells is experiencing unprecedented growth, driven primarily by the expanding electric vehicle (EV) sector, consumer electronics, and renewable energy storage systems. Current projections indicate the market will reach $127 billion by 2025, with a compound annual growth rate of 16.4% between 2020-2025. This acceleration reflects the intensifying demand for energy storage solutions that deliver superior performance in increasingly compact form factors.
Electric vehicle manufacturers represent the largest demand segment, seeking cells that maximize range while minimizing weight and volume. Tesla, BYD, and Volkswagen have publicly stated targets to increase energy density by at least 30% in their next-generation vehicles. The automotive sector's requirements are particularly stringent, demanding cells that not only offer high energy density but also maintain safety, longevity, and fast-charging capabilities.
Consumer electronics manufacturers constitute another significant market driver, with Apple, Samsung, and other major players continuously pushing for batteries that enable slimmer devices with longer operating times. The wearable technology segment specifically requires energy densities exceeding 700 Wh/L to support advanced functionality in space-constrained designs.
Geographically, Asia-Pacific dominates both production and consumption of high-energy-density cells, with China, Japan, and South Korea collectively accounting for 67% of global manufacturing capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian suppliers, with the European Battery Alliance committing €20 billion toward developing regional production capabilities.
Market research indicates a clear preference shift toward electrochemical cells over traditional chemical cells, particularly lithium-based technologies that demonstrate superior energy density metrics. Solid-state batteries represent the fastest-growing segment, with venture capital investments exceeding $3.6 billion in 2021 alone, despite remaining largely pre-commercial.
Price sensitivity varies significantly by application sector. While consumer electronics manufacturers demonstrate willingness to pay premium prices for incremental density improvements, grid storage applications remain highly cost-sensitive, prioritizing levelized cost of storage over absolute energy density metrics.
Regulatory frameworks increasingly influence market dynamics, with several jurisdictions implementing energy density requirements and safety standards. The European Union's proposed Battery Directive revision includes minimum energy density thresholds for various applications, potentially creating market access barriers for lower-performing technologies.
Electric vehicle manufacturers represent the largest demand segment, seeking cells that maximize range while minimizing weight and volume. Tesla, BYD, and Volkswagen have publicly stated targets to increase energy density by at least 30% in their next-generation vehicles. The automotive sector's requirements are particularly stringent, demanding cells that not only offer high energy density but also maintain safety, longevity, and fast-charging capabilities.
Consumer electronics manufacturers constitute another significant market driver, with Apple, Samsung, and other major players continuously pushing for batteries that enable slimmer devices with longer operating times. The wearable technology segment specifically requires energy densities exceeding 700 Wh/L to support advanced functionality in space-constrained designs.
Geographically, Asia-Pacific dominates both production and consumption of high-energy-density cells, with China, Japan, and South Korea collectively accounting for 67% of global manufacturing capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian suppliers, with the European Battery Alliance committing €20 billion toward developing regional production capabilities.
Market research indicates a clear preference shift toward electrochemical cells over traditional chemical cells, particularly lithium-based technologies that demonstrate superior energy density metrics. Solid-state batteries represent the fastest-growing segment, with venture capital investments exceeding $3.6 billion in 2021 alone, despite remaining largely pre-commercial.
Price sensitivity varies significantly by application sector. While consumer electronics manufacturers demonstrate willingness to pay premium prices for incremental density improvements, grid storage applications remain highly cost-sensitive, prioritizing levelized cost of storage over absolute energy density metrics.
Regulatory frameworks increasingly influence market dynamics, with several jurisdictions implementing energy density requirements and safety standards. The European Union's proposed Battery Directive revision includes minimum energy density thresholds for various applications, potentially creating market access barriers for lower-performing technologies.
Current Technical Challenges in Energy Storage Systems
Energy storage systems face significant technical challenges that impede their widespread adoption and optimal performance. The comparison between electrochemical cells (like lithium-ion batteries) and chemical cells (such as fuel cells) reveals several critical limitations in current technologies.
Energy density remains a fundamental challenge, with even advanced lithium-ion batteries achieving only 250-300 Wh/kg, far below theoretical limits and insufficient for many applications requiring extended operation. Chemical cells like hydrogen fuel cells offer higher theoretical energy densities but struggle with practical implementation due to storage and conversion inefficiencies.
Cycle life degradation presents another major obstacle. Electrochemical cells typically experience capacity fade of 20% after 500-1000 cycles, limiting their long-term economic viability. Chemical cells face different but equally challenging durability issues, including catalyst poisoning and membrane degradation that reduce operational lifetimes.
Safety concerns persist across both technologies. Electrochemical cells, particularly lithium-based systems, remain vulnerable to thermal runaway events, while chemical cells often involve highly reactive or pressurized substances that require complex safety systems, adding weight and cost to overall systems.
Temperature sensitivity significantly impacts performance, with most electrochemical cells losing up to 50% capacity at low temperatures (-20°C) and experiencing accelerated degradation at high temperatures. Chemical cells similarly struggle with temperature extremes, requiring sophisticated thermal management systems that reduce overall system efficiency.
Material supply constraints represent a growing challenge, particularly for electrochemical cells relying on cobalt, nickel, and lithium. Chemical cells face similar resource limitations regarding platinum group metals used as catalysts, driving research toward alternative materials with often compromised performance.
Charging infrastructure compatibility remains problematic, with electrochemical cells requiring standardized charging protocols and chemical cells needing specialized refueling systems. This fragmentation hinders market adoption and increases implementation costs across various applications.
Cost barriers continue to limit widespread deployment, with high-performance electrochemical cells still costing $150-300/kWh at pack level. Chemical cell systems often have lower cell costs but higher system-level expenses due to balance-of-plant requirements, making total cost of ownership calculations complex and application-specific.
These multifaceted challenges necessitate continued research and development across multiple fronts, from fundamental materials science to systems engineering, to achieve the performance metrics required for next-generation energy storage applications.
Energy density remains a fundamental challenge, with even advanced lithium-ion batteries achieving only 250-300 Wh/kg, far below theoretical limits and insufficient for many applications requiring extended operation. Chemical cells like hydrogen fuel cells offer higher theoretical energy densities but struggle with practical implementation due to storage and conversion inefficiencies.
Cycle life degradation presents another major obstacle. Electrochemical cells typically experience capacity fade of 20% after 500-1000 cycles, limiting their long-term economic viability. Chemical cells face different but equally challenging durability issues, including catalyst poisoning and membrane degradation that reduce operational lifetimes.
Safety concerns persist across both technologies. Electrochemical cells, particularly lithium-based systems, remain vulnerable to thermal runaway events, while chemical cells often involve highly reactive or pressurized substances that require complex safety systems, adding weight and cost to overall systems.
Temperature sensitivity significantly impacts performance, with most electrochemical cells losing up to 50% capacity at low temperatures (-20°C) and experiencing accelerated degradation at high temperatures. Chemical cells similarly struggle with temperature extremes, requiring sophisticated thermal management systems that reduce overall system efficiency.
Material supply constraints represent a growing challenge, particularly for electrochemical cells relying on cobalt, nickel, and lithium. Chemical cells face similar resource limitations regarding platinum group metals used as catalysts, driving research toward alternative materials with often compromised performance.
Charging infrastructure compatibility remains problematic, with electrochemical cells requiring standardized charging protocols and chemical cells needing specialized refueling systems. This fragmentation hinders market adoption and increases implementation costs across various applications.
Cost barriers continue to limit widespread deployment, with high-performance electrochemical cells still costing $150-300/kWh at pack level. Chemical cell systems often have lower cell costs but higher system-level expenses due to balance-of-plant requirements, making total cost of ownership calculations complex and application-specific.
These multifaceted challenges necessitate continued research and development across multiple fronts, from fundamental materials science to systems engineering, to achieve the performance metrics required for next-generation energy storage applications.
Benchmark Methodology for Energy Density Comparison
01 Lithium-ion battery energy density improvements
Lithium-ion batteries represent a significant advancement in electrochemical cell technology with high energy density. Various approaches to improve their energy density include advanced electrode materials, novel electrolyte compositions, and optimized cell designs. These improvements allow for greater energy storage capacity in smaller volumes, making them ideal for portable electronics and electric vehicles.- Lithium-ion battery energy density improvements: Lithium-ion batteries represent a significant advancement in electrochemical cell technology with high energy density. Various approaches to improve their energy density include advanced electrode materials, novel electrolyte compositions, and optimized cell designs. These improvements allow for greater energy storage capacity in smaller volumes, making them suitable for applications ranging from portable electronics to electric vehicles.
- Flow battery systems for large-scale energy storage: Flow batteries offer unique advantages for large-scale energy storage applications due to their scalable design where energy capacity and power output can be independently sized. These electrochemical cells store energy in liquid electrolyte solutions that flow through the system, allowing for extended cycle life and flexible operation. Innovations in membrane technology and electrolyte chemistry have significantly increased their energy density potential.
- Solid-state battery technology: Solid-state batteries represent a breakthrough in electrochemical cell design by replacing liquid electrolytes with solid ion-conducting materials. This configuration offers potential for higher energy density, improved safety, and longer lifespan compared to conventional battery technologies. Research focuses on developing new solid electrolyte materials with high ionic conductivity and stable interfaces with electrode materials.
- Supercapacitor and hybrid energy storage systems: Supercapacitors and hybrid energy storage systems combine features of both batteries and capacitors to achieve optimal performance characteristics. While traditional batteries offer high energy density and capacitors provide high power density, hybrid systems aim to bridge this gap. These electrochemical cells utilize specialized electrode materials and electrolyte formulations to store energy through both faradaic and non-faradaic processes.
- Metal-air battery technology: Metal-air batteries represent a class of electrochemical cells with exceptionally high theoretical energy density due to their use of atmospheric oxygen as the cathode reactant. These systems, particularly those based on lithium, zinc, or aluminum, can achieve energy densities several times higher than conventional lithium-ion batteries. Research focuses on addressing challenges related to rechargeability, air electrode efficiency, and electrolyte stability to realize their full potential.
02 Flow battery systems for grid-scale energy storage
Flow batteries offer unique advantages for large-scale energy storage applications with their scalable energy density. These electrochemical systems store energy in liquid electrolytes contained in external tanks, allowing independent scaling of power and energy capacity. This technology is particularly valuable for grid-scale energy storage, renewable energy integration, and load-leveling applications.Expand Specific Solutions03 Solid-state battery technology
Solid-state batteries represent a next-generation approach to increasing energy density by replacing liquid electrolytes with solid ion-conducting materials. This technology offers potential advantages including higher energy density, improved safety, and longer cycle life compared to conventional lithium-ion batteries with liquid electrolytes. The solid electrolyte prevents dendrite formation and allows for the use of high-capacity electrode materials.Expand Specific Solutions04 Supercapacitor and hybrid energy storage systems
Supercapacitors and hybrid energy storage systems combine features of both batteries and capacitors to optimize energy and power density. These systems utilize electrochemical double-layer capacitance and pseudocapacitance mechanisms to store energy. Hybrid approaches integrate high energy density chemical storage with high power density electrochemical storage to create versatile energy solutions for applications requiring both characteristics.Expand Specific Solutions05 Metal-air battery technology
Metal-air batteries offer exceptionally high theoretical energy density by utilizing oxygen from the ambient air as the cathode reactant. These chemical cells, particularly those based on lithium, zinc, or aluminum, can achieve energy densities several times higher than conventional lithium-ion batteries. The open-air cathode design reduces weight while the metal anode provides high energy capacity, though challenges remain in cycle life and practical implementation.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The electrochemical vs chemical cell energy density landscape is currently in a growth phase, with the market expanding rapidly due to increasing demand for high-energy storage solutions in electric vehicles and renewable energy sectors. The global market size is projected to reach significant scale as companies invest heavily in research and development. Technologically, established players like Sony, Panasonic, and LG Energy Solution lead in conventional lithium-ion technology, while emerging companies such as 24M Technologies, Sion Power, and Alsym Energy are developing next-generation solutions with higher energy densities. Research institutions including MIT and CNRS are advancing fundamental science in this field. Automotive manufacturers (GM, Bosch) and specialized battery producers (CATL, A123 Systems) are competing to develop cells with optimal energy-to-weight ratios for various applications, driving continuous innovation in this competitive sector.
Sony Group Corp.
Technical Solution: Sony Group has pioneered significant advancements in electrochemical cell technology since commercializing the first lithium-ion battery in 1991. Their current lithium-ion cells achieve energy densities of 250-280 Wh/kg, substantially outperforming traditional chemical cells which typically deliver only 40-60 Wh/kg for primary cells. Sony's proprietary Nexelion hybrid lithium-ion technology incorporates tin-based amorphous anodes that offer higher capacity than conventional graphite anodes. Their sulfur-based cathode research has demonstrated potential energy densities exceeding 500 Wh/kg in laboratory settings. Sony has also developed olivine-structured lithium iron phosphate (LFP) cathodes with enhanced thermal stability and cycle life, though at lower energy densities (140-160 Wh/kg) than their NMC variants. Their advanced manufacturing processes enable thinner electrodes and separators, maximizing the volumetric energy density to approximately 600-650 Wh/L, compared to 200-300 Wh/L for typical chemical cells.
Strengths: Pioneering expertise in lithium-ion technology; excellent quality control and safety record; diverse product portfolio spanning consumer electronics to industrial applications; proprietary manufacturing techniques for high-density cells. Weaknesses: Higher cost structure compared to newer Asian manufacturers; energy density improvements have been incremental rather than revolutionary in recent years; limited public information on next-generation battery research compared to competitors.
Ningde Amperex Technology Ltd.
Technical Solution: CATL (Ningde Amperex Technology) has developed cutting-edge electrochemical cell technology that significantly outperforms traditional chemical cells in energy density benchmarks. Their CTP (Cell-to-Pack) technology eliminates conventional module structures, increasing energy density by 15-20% at the pack level. CATL's latest generation NMC (Nickel Manganese Cobalt) batteries achieve energy densities of approximately 300 Wh/kg at the cell level, while their LFP (Lithium Iron Phosphate) cells deliver around 180-200 Wh/kg with superior safety profiles. The company has pioneered sodium-ion battery technology as an alternative to lithium-ion, achieving energy densities of 160 Wh/kg with significantly lower costs and better low-temperature performance. CATL's Qilin battery, utilizing their third-generation CTP technology, achieves a record volume utilization efficiency of 72% and an energy density of up to 255 Wh/kg, enabling electric vehicles to achieve ranges exceeding 1,000 km on a single charge.
Strengths: Industry-leading energy density in mass production cells; excellent thermal management systems; diverse chemistry portfolio allowing application-specific optimization; cost-effective manufacturing at scale. Weaknesses: Energy density still lower than theoretical limits; high dependence on critical raw materials; trade-off between energy density and cycle life in some formulations; higher initial cost compared to traditional chemical cells.
Core Patents and Research in Cell Energy Density Enhancement
Electrochemical cell
PatentWO2025110834A1
Innovation
- An electrochemical cell design featuring a solid electrolyte with a first ion conductor, a complex hydride, and a second ion conductor, a sulfur compound, positioned between the lithium metal cathode and anode, effectively suppressing side reactions and enhancing interfacial stability.
Electrochemical cells and methods of making and using thereof
PatentInactiveUS20200058922A1
Innovation
- An 'anode-free' design using a carbon nucleation layer on an aluminum current collector for efficient plating and stripping of sodium metal, which reduces nucleation overpotential and facilitates high-rate capabilities, achieving energy densities exceeding 400 Wh/kg while using naturally abundant materials and cost-effective aqueous processing.
Environmental Impact and Sustainability Considerations
The environmental footprint of energy storage technologies has become increasingly critical in the global transition towards sustainable energy systems. When comparing electrochemical cells (such as lithium-ion batteries) with chemical cells (like hydrogen fuel cells), their environmental impacts differ significantly across their life cycles.
Electrochemical cells, particularly lithium-ion batteries, face sustainability challenges related to raw material extraction. Mining of lithium, cobalt, and nickel creates substantial ecological disruption, water pollution, and carbon emissions. These materials are often sourced from regions with limited environmental regulations, exacerbating their impact. However, recent advancements in battery recycling technologies have shown promise in recovering up to 95% of these critical materials, potentially creating a more circular economy for battery components.
Chemical cells, especially hydrogen fuel cells, present a different environmental profile. While they produce only water as a direct emission during operation, the upstream environmental impact varies dramatically based on hydrogen production methods. Grey hydrogen derived from natural gas reforming generates significant carbon emissions, whereas green hydrogen produced via electrolysis powered by renewable energy offers a substantially lower carbon footprint. The platinum catalysts used in fuel cells also present sustainability concerns due to their scarcity and energy-intensive mining processes.
Manufacturing energy requirements represent another key environmental consideration. The production of lithium-ion batteries is energy-intensive, consuming approximately 50-150 kWh of energy per kWh of battery capacity produced. Fuel cell manufacturing generally requires less energy but involves more specialized materials and processes that may have concentrated environmental impacts.
End-of-life management presents distinct challenges for both technologies. While lithium-ion batteries contain valuable materials that incentivize recycling, current global recycling rates remain below 5%. Fuel cell systems have longer operational lifespans but contain precious metals that require specialized recovery processes.
Water usage patterns also differ significantly between these technologies. Battery production requires substantial water for processing and cooling, while hydrogen production via electrolysis consumes approximately 9 kg of water per kg of hydrogen produced. This water footprint becomes particularly relevant in water-stressed regions.
Overall, the environmental superiority between electrochemical and chemical cells depends heavily on specific implementation contexts, including energy sources, manufacturing practices, and end-of-life management. A comprehensive life cycle assessment approach is essential when evaluating these technologies for specific applications and regional deployment.
Electrochemical cells, particularly lithium-ion batteries, face sustainability challenges related to raw material extraction. Mining of lithium, cobalt, and nickel creates substantial ecological disruption, water pollution, and carbon emissions. These materials are often sourced from regions with limited environmental regulations, exacerbating their impact. However, recent advancements in battery recycling technologies have shown promise in recovering up to 95% of these critical materials, potentially creating a more circular economy for battery components.
Chemical cells, especially hydrogen fuel cells, present a different environmental profile. While they produce only water as a direct emission during operation, the upstream environmental impact varies dramatically based on hydrogen production methods. Grey hydrogen derived from natural gas reforming generates significant carbon emissions, whereas green hydrogen produced via electrolysis powered by renewable energy offers a substantially lower carbon footprint. The platinum catalysts used in fuel cells also present sustainability concerns due to their scarcity and energy-intensive mining processes.
Manufacturing energy requirements represent another key environmental consideration. The production of lithium-ion batteries is energy-intensive, consuming approximately 50-150 kWh of energy per kWh of battery capacity produced. Fuel cell manufacturing generally requires less energy but involves more specialized materials and processes that may have concentrated environmental impacts.
End-of-life management presents distinct challenges for both technologies. While lithium-ion batteries contain valuable materials that incentivize recycling, current global recycling rates remain below 5%. Fuel cell systems have longer operational lifespans but contain precious metals that require specialized recovery processes.
Water usage patterns also differ significantly between these technologies. Battery production requires substantial water for processing and cooling, while hydrogen production via electrolysis consumes approximately 9 kg of water per kg of hydrogen produced. This water footprint becomes particularly relevant in water-stressed regions.
Overall, the environmental superiority between electrochemical and chemical cells depends heavily on specific implementation contexts, including energy sources, manufacturing practices, and end-of-life management. A comprehensive life cycle assessment approach is essential when evaluating these technologies for specific applications and regional deployment.
Regulatory Framework for Energy Storage Technologies
The regulatory landscape for energy storage technologies has evolved significantly in response to the growing importance of these systems in modern energy infrastructure. When comparing electrochemical cells (such as lithium-ion batteries) with chemical cells (like hydrogen fuel cells), regulatory frameworks play a crucial role in determining market adoption, safety standards, and performance benchmarks.
International organizations including the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) have established comprehensive standards for energy density measurement and reporting. These standards ensure consistent comparison methodologies when evaluating the energy density of different cell technologies, which is particularly important when benchmarking electrochemical against chemical cells.
Safety regulations represent a significant component of the regulatory framework, with electrochemical cells facing stringent requirements due to thermal runaway risks. The UN Transportation Testing standards (UN 38.3) and IEC 62133 specifically address lithium-ion battery safety, while hydrogen storage in chemical cells must comply with high-pressure vessel regulations such as those from the American Society of Mechanical Engineers (ASME).
Environmental regulations increasingly influence energy storage technology development, with the EU's Battery Directive and similar legislation in other regions mandating recycling requirements and restricting hazardous materials. These regulations typically impose stricter controls on electrochemical cells containing heavy metals compared to some chemical cell technologies.
Grid integration regulations vary significantly by region, affecting how different cell technologies can participate in energy markets. FERC Order 841 in the United States has opened electricity markets to energy storage resources, while the EU's Clean Energy Package includes provisions for storage participation in capacity markets. These frameworks often do not distinguish between storage technologies but focus on performance characteristics.
Performance certification systems like UL 1973 for batteries and similar standards for fuel cells establish minimum requirements for energy density, cycle life, and efficiency. These certifications serve as important benchmarks when comparing electrochemical and chemical cell technologies in commercial applications.
Emerging regulatory trends indicate a move toward technology-neutral frameworks that focus on performance metrics rather than specific technologies. This approach may benefit innovative solutions in both electrochemical and chemical cell categories, particularly as regulators seek to encourage higher energy density solutions for transportation and grid applications.
International organizations including the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) have established comprehensive standards for energy density measurement and reporting. These standards ensure consistent comparison methodologies when evaluating the energy density of different cell technologies, which is particularly important when benchmarking electrochemical against chemical cells.
Safety regulations represent a significant component of the regulatory framework, with electrochemical cells facing stringent requirements due to thermal runaway risks. The UN Transportation Testing standards (UN 38.3) and IEC 62133 specifically address lithium-ion battery safety, while hydrogen storage in chemical cells must comply with high-pressure vessel regulations such as those from the American Society of Mechanical Engineers (ASME).
Environmental regulations increasingly influence energy storage technology development, with the EU's Battery Directive and similar legislation in other regions mandating recycling requirements and restricting hazardous materials. These regulations typically impose stricter controls on electrochemical cells containing heavy metals compared to some chemical cell technologies.
Grid integration regulations vary significantly by region, affecting how different cell technologies can participate in energy markets. FERC Order 841 in the United States has opened electricity markets to energy storage resources, while the EU's Clean Energy Package includes provisions for storage participation in capacity markets. These frameworks often do not distinguish between storage technologies but focus on performance characteristics.
Performance certification systems like UL 1973 for batteries and similar standards for fuel cells establish minimum requirements for energy density, cycle life, and efficiency. These certifications serve as important benchmarks when comparing electrochemical and chemical cell technologies in commercial applications.
Emerging regulatory trends indicate a move toward technology-neutral frameworks that focus on performance metrics rather than specific technologies. This approach may benefit innovative solutions in both electrochemical and chemical cell categories, particularly as regulators seek to encourage higher energy density solutions for transportation and grid applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!