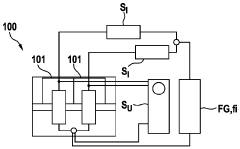
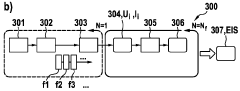
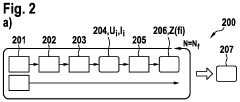
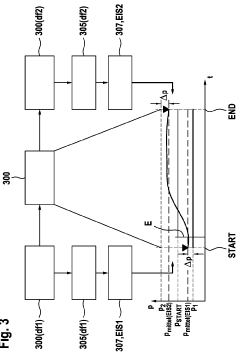
Electrochemical Cell Response to Frequency Modulation
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Frequency Modulation Background and Objectives
Electrochemical cells have been fundamental components in energy storage and conversion systems since their inception in the late 18th century. The evolution of these systems has been marked by continuous improvements in materials, design, and operational parameters. Among these parameters, frequency modulation has emerged as a critical factor influencing cell performance, efficiency, and longevity. This technical exploration aims to comprehensively examine how electrochemical cells respond to various frequency modulation techniques and to establish clear objectives for advancing this technology.
The historical trajectory of electrochemical frequency modulation research began with rudimentary DC applications, evolving through basic AC implementations, and now encompasses sophisticated variable frequency techniques. Early research in the 1950s and 1960s primarily focused on understanding impedance characteristics, while the 1970s and 1980s saw the development of electrochemical impedance spectroscopy (EIS) as a powerful analytical tool. Recent decades have witnessed exponential growth in this field, driven by the increasing demands of renewable energy storage, electric vehicles, and portable electronics.
Current technological trends indicate a shift toward higher frequency applications, with particular emphasis on understanding the nanoscale dynamics at electrode-electrolyte interfaces. The miniaturization of electrochemical systems and the integration of advanced materials such as graphene, carbon nanotubes, and novel electrolytes have opened new avenues for frequency-dependent applications. Additionally, the emergence of machine learning algorithms for real-time frequency optimization represents a promising frontier in this domain.
The primary objectives of this technical investigation are multifaceted. First, we aim to characterize the fundamental mechanisms governing electrochemical cell response to frequency modulation across different cell chemistries and architectures. Second, we seek to identify optimal frequency ranges and modulation patterns for specific applications, particularly focusing on energy density, power density, and cycle life improvements. Third, we intend to develop predictive models that can accurately forecast cell behavior under various frequency conditions.
Furthermore, this research aims to explore the potential for frequency modulation as a diagnostic tool for cell health monitoring and failure prediction. By analyzing frequency response signatures, it may be possible to detect early signs of degradation mechanisms such as lithium plating, dendrite formation, or electrode delamination before they lead to catastrophic failure.
The ultimate goal is to establish a comprehensive framework for implementing frequency modulation strategies in next-generation electrochemical energy systems, potentially revolutionizing how we design, operate, and maintain these critical technologies in an increasingly electrified world.
The historical trajectory of electrochemical frequency modulation research began with rudimentary DC applications, evolving through basic AC implementations, and now encompasses sophisticated variable frequency techniques. Early research in the 1950s and 1960s primarily focused on understanding impedance characteristics, while the 1970s and 1980s saw the development of electrochemical impedance spectroscopy (EIS) as a powerful analytical tool. Recent decades have witnessed exponential growth in this field, driven by the increasing demands of renewable energy storage, electric vehicles, and portable electronics.
Current technological trends indicate a shift toward higher frequency applications, with particular emphasis on understanding the nanoscale dynamics at electrode-electrolyte interfaces. The miniaturization of electrochemical systems and the integration of advanced materials such as graphene, carbon nanotubes, and novel electrolytes have opened new avenues for frequency-dependent applications. Additionally, the emergence of machine learning algorithms for real-time frequency optimization represents a promising frontier in this domain.
The primary objectives of this technical investigation are multifaceted. First, we aim to characterize the fundamental mechanisms governing electrochemical cell response to frequency modulation across different cell chemistries and architectures. Second, we seek to identify optimal frequency ranges and modulation patterns for specific applications, particularly focusing on energy density, power density, and cycle life improvements. Third, we intend to develop predictive models that can accurately forecast cell behavior under various frequency conditions.
Furthermore, this research aims to explore the potential for frequency modulation as a diagnostic tool for cell health monitoring and failure prediction. By analyzing frequency response signatures, it may be possible to detect early signs of degradation mechanisms such as lithium plating, dendrite formation, or electrode delamination before they lead to catastrophic failure.
The ultimate goal is to establish a comprehensive framework for implementing frequency modulation strategies in next-generation electrochemical energy systems, potentially revolutionizing how we design, operate, and maintain these critical technologies in an increasingly electrified world.
Market Applications and Demand Analysis for Frequency-Modulated Electrochemical Systems
The frequency-modulated electrochemical systems market is experiencing significant growth driven by advancements in energy storage, sensing technologies, and analytical applications. Current market analysis indicates that the global electrochemical sensor market is expanding at a compound annual growth rate of approximately 9.5% through 2028, with frequency-modulated systems representing an emerging high-value segment within this space.
Energy storage applications constitute the largest market segment for frequency-modulated electrochemical systems. Battery manufacturers are increasingly adopting these technologies to enhance charge-discharge efficiency, extend battery lifespan, and improve safety profiles. The electric vehicle industry particularly values these advancements as they address critical consumer concerns regarding range anxiety and charging times.
Healthcare and biomedical applications represent another rapidly growing market segment. Frequency-modulated electrochemical biosensors enable real-time monitoring of biological markers with unprecedented sensitivity and specificity. The continuous glucose monitoring market alone is projected to reach substantial market value, with frequency modulation techniques offering improved accuracy and reduced interference from biological matrices.
Environmental monitoring applications are gaining traction as regulatory requirements for pollution control become more stringent globally. Frequency-modulated electrochemical sensors provide advantages in detecting trace contaminants in air and water with higher sensitivity than traditional methods. Municipal water treatment facilities and industrial compliance monitoring represent significant market opportunities in this sector.
Industrial process control represents a mature but evolving market for these technologies. The chemical manufacturing industry increasingly relies on frequency-modulated electrochemical systems for real-time quality control and process optimization. These applications deliver substantial return on investment through reduced waste, improved product consistency, and enhanced operational efficiency.
Consumer electronics manufacturers are exploring frequency modulation techniques to develop next-generation power management systems. This emerging application area focuses on extending device battery life and enabling faster charging capabilities, addressing key consumer pain points in portable electronics.
Regional market analysis reveals that North America currently leads in adoption of frequency-modulated electrochemical technologies, followed closely by Europe and East Asia. However, the fastest growth is anticipated in emerging economies where rapid industrialization and infrastructure development create substantial demand for advanced sensing and energy storage solutions.
Market barriers include high initial implementation costs, technical complexity requiring specialized expertise, and competition from established alternative technologies. Despite these challenges, the unique performance advantages of frequency-modulated electrochemical systems continue to drive market expansion across multiple sectors.
Energy storage applications constitute the largest market segment for frequency-modulated electrochemical systems. Battery manufacturers are increasingly adopting these technologies to enhance charge-discharge efficiency, extend battery lifespan, and improve safety profiles. The electric vehicle industry particularly values these advancements as they address critical consumer concerns regarding range anxiety and charging times.
Healthcare and biomedical applications represent another rapidly growing market segment. Frequency-modulated electrochemical biosensors enable real-time monitoring of biological markers with unprecedented sensitivity and specificity. The continuous glucose monitoring market alone is projected to reach substantial market value, with frequency modulation techniques offering improved accuracy and reduced interference from biological matrices.
Environmental monitoring applications are gaining traction as regulatory requirements for pollution control become more stringent globally. Frequency-modulated electrochemical sensors provide advantages in detecting trace contaminants in air and water with higher sensitivity than traditional methods. Municipal water treatment facilities and industrial compliance monitoring represent significant market opportunities in this sector.
Industrial process control represents a mature but evolving market for these technologies. The chemical manufacturing industry increasingly relies on frequency-modulated electrochemical systems for real-time quality control and process optimization. These applications deliver substantial return on investment through reduced waste, improved product consistency, and enhanced operational efficiency.
Consumer electronics manufacturers are exploring frequency modulation techniques to develop next-generation power management systems. This emerging application area focuses on extending device battery life and enabling faster charging capabilities, addressing key consumer pain points in portable electronics.
Regional market analysis reveals that North America currently leads in adoption of frequency-modulated electrochemical technologies, followed closely by Europe and East Asia. However, the fastest growth is anticipated in emerging economies where rapid industrialization and infrastructure development create substantial demand for advanced sensing and energy storage solutions.
Market barriers include high initial implementation costs, technical complexity requiring specialized expertise, and competition from established alternative technologies. Despite these challenges, the unique performance advantages of frequency-modulated electrochemical systems continue to drive market expansion across multiple sectors.
Current Technical Limitations and Challenges in Electrochemical Frequency Response
Despite significant advancements in electrochemical frequency response analysis, several technical limitations and challenges persist that hinder the full exploitation of this technique. One fundamental challenge lies in the signal-to-noise ratio, particularly when working with low-amplitude signals. As frequency modulation techniques require precise measurement of small perturbations, environmental electromagnetic interference and instrument-based noise can significantly compromise data quality, especially at high frequencies above 100 kHz.
The non-linear behavior of electrochemical systems presents another major obstacle. While most analytical models assume linearity for mathematical simplicity, real electrochemical cells often exhibit non-linear responses when subjected to frequency modulation. This discrepancy becomes particularly problematic when applying traditional equivalent circuit models to interpret impedance data, leading to potential misinterpretations of underlying physicochemical processes.
Time-variant characteristics of electrochemical systems further complicate frequency response analysis. Many electrochemical cells undergo continuous changes during measurement due to processes such as electrode fouling, solution depletion, or surface modification. These temporal variations introduce artifacts in frequency response data, making it difficult to distinguish between genuine electrochemical phenomena and measurement artifacts.
Electrode surface heterogeneity represents another significant challenge. Most theoretical models assume uniform electrode surfaces, whereas real electrodes often possess microscopic irregularities that create localized variations in reaction rates and diffusion patterns. These heterogeneities can produce distributed time constants that broaden frequency response peaks and valleys, complicating data interpretation.
Instrumentation limitations also constrain the practical application of frequency modulation techniques. Current potentiostats typically offer frequency ranges from 10 μHz to 1 MHz, which may be insufficient for capturing ultrafast electrochemical processes or very slow phenomena. Additionally, the phase accuracy of many commercial instruments deteriorates at frequency extremes, introducing systematic errors in impedance measurements.
Data analysis and interpretation remain challenging aspects of electrochemical frequency response studies. The inverse problem of extracting physical parameters from impedance spectra is often ill-posed, meaning multiple physical models can fit the same experimental data equally well. This ambiguity complicates the determination of unique mechanistic insights from frequency response measurements.
Temperature and pressure dependencies introduce additional complexities, as most electrochemical processes are highly sensitive to these parameters. Maintaining stable conditions throughout frequency sweep measurements, which can span hours for comprehensive low-frequency analysis, presents significant experimental challenges that affect measurement reproducibility and reliability.
The non-linear behavior of electrochemical systems presents another major obstacle. While most analytical models assume linearity for mathematical simplicity, real electrochemical cells often exhibit non-linear responses when subjected to frequency modulation. This discrepancy becomes particularly problematic when applying traditional equivalent circuit models to interpret impedance data, leading to potential misinterpretations of underlying physicochemical processes.
Time-variant characteristics of electrochemical systems further complicate frequency response analysis. Many electrochemical cells undergo continuous changes during measurement due to processes such as electrode fouling, solution depletion, or surface modification. These temporal variations introduce artifacts in frequency response data, making it difficult to distinguish between genuine electrochemical phenomena and measurement artifacts.
Electrode surface heterogeneity represents another significant challenge. Most theoretical models assume uniform electrode surfaces, whereas real electrodes often possess microscopic irregularities that create localized variations in reaction rates and diffusion patterns. These heterogeneities can produce distributed time constants that broaden frequency response peaks and valleys, complicating data interpretation.
Instrumentation limitations also constrain the practical application of frequency modulation techniques. Current potentiostats typically offer frequency ranges from 10 μHz to 1 MHz, which may be insufficient for capturing ultrafast electrochemical processes or very slow phenomena. Additionally, the phase accuracy of many commercial instruments deteriorates at frequency extremes, introducing systematic errors in impedance measurements.
Data analysis and interpretation remain challenging aspects of electrochemical frequency response studies. The inverse problem of extracting physical parameters from impedance spectra is often ill-posed, meaning multiple physical models can fit the same experimental data equally well. This ambiguity complicates the determination of unique mechanistic insights from frequency response measurements.
Temperature and pressure dependencies introduce additional complexities, as most electrochemical processes are highly sensitive to these parameters. Maintaining stable conditions throughout frequency sweep measurements, which can span hours for comprehensive low-frequency analysis, presents significant experimental challenges that affect measurement reproducibility and reliability.
State-of-the-Art Frequency Modulation Methods for Electrochemical Cells
01 Electrochemical cell response monitoring and control systems
Advanced monitoring and control systems are essential for optimizing electrochemical cell performance. These systems typically include sensors that measure various parameters such as voltage, current, temperature, and chemical composition in real-time. The data collected is processed through algorithms that can detect anomalies, predict failures, and automatically adjust operating conditions to maintain optimal performance. These control systems enhance efficiency, extend cell lifespan, and improve safety by preventing dangerous operating conditions.- Electrochemical cell response monitoring and control systems: Advanced monitoring and control systems are designed to optimize electrochemical cell performance by continuously analyzing cell response parameters. These systems employ sensors to measure voltage, current, temperature, and other critical variables in real-time. The collected data is processed through algorithms that can detect anomalies, predict potential failures, and automatically adjust operating conditions to maintain optimal performance. Such systems enhance cell efficiency, extend operational lifespan, and improve safety by preventing dangerous conditions.
- Electrode materials and configurations for improved cell response: Innovative electrode materials and configurations significantly impact electrochemical cell response characteristics. Advanced materials including novel alloys, composites, and nanostructured surfaces enhance electron transfer rates and catalytic activity. Specialized electrode geometries optimize surface area and reaction kinetics, while multi-layered electrode designs incorporate functional layers for specific purposes such as protection, conductivity enhancement, or selective ion transport. These developments result in cells with faster response times, higher sensitivity, and improved stability under various operating conditions.
- Electrolyte formulations for enhanced cell performance: Specialized electrolyte formulations play a crucial role in determining electrochemical cell response characteristics. Advanced electrolytes incorporate additives that improve ionic conductivity, enhance stability, and prevent unwanted side reactions. Some formulations include compounds that form protective interfaces at electrode surfaces, reducing degradation and extending cell lifespan. Temperature-responsive electrolytes maintain optimal performance across varying environmental conditions, while others are designed to minimize resistance and maximize power output. These innovations result in cells with more consistent response, improved efficiency, and greater durability.
- Sensing and diagnostic techniques for electrochemical cells: Advanced sensing and diagnostic techniques enable comprehensive analysis of electrochemical cell response. Impedance spectroscopy methods characterize cell behavior across frequency ranges, revealing information about reaction kinetics and transport processes. Voltammetric techniques provide insights into redox reactions and electrode processes. Non-invasive monitoring approaches use external signals to assess internal cell conditions without disrupting operation. Machine learning algorithms analyze complex response patterns to identify subtle changes indicating potential issues. These techniques allow for early detection of performance degradation, more accurate state estimation, and targeted maintenance strategies.
- Environmental and operational factors affecting cell response: Environmental and operational factors significantly influence electrochemical cell response characteristics. Temperature fluctuations affect reaction kinetics, transport properties, and overall cell efficiency. Pressure variations impact gas-involving reactions and can alter electrode-electrolyte interfaces. Humidity levels affect water management in certain cell types. Operational factors such as charge/discharge rates, duty cycles, and rest periods determine stress levels and degradation patterns. Understanding these influences enables the development of adaptive control strategies, protective measures, and optimized operating protocols that maintain consistent cell response under varying conditions.
02 Electrode materials and configurations for improved cell response
The selection and configuration of electrode materials significantly impact electrochemical cell response characteristics. Advanced electrode designs incorporate nanomaterials, composite structures, and specialized coatings to enhance conductivity, catalytic activity, and stability. These innovations lead to faster response times, higher current densities, and improved resistance to degradation mechanisms such as corrosion and fouling. Optimized electrode geometries also contribute to more uniform current distribution and better overall cell performance.Expand Specific Solutions03 Electrolyte composition effects on cell performance
The composition of electrolytes plays a crucial role in determining electrochemical cell response. Researchers have developed specialized electrolyte formulations that enhance ionic conductivity, stability, and compatibility with electrode materials. Additives are incorporated to suppress unwanted side reactions, prevent dendrite formation, and extend operational temperature ranges. Advanced electrolytes also feature self-healing properties and improved safety characteristics, reducing the risk of thermal runaway and other failure modes.Expand Specific Solutions04 Temperature management for optimized cell response
Effective temperature management is critical for maintaining optimal electrochemical cell response. Thermal control systems regulate operating temperatures to prevent performance degradation, extend cell lifespan, and ensure safety. Advanced cooling and heating mechanisms include phase-change materials, liquid cooling circuits, and intelligent thermal management algorithms. These systems respond dynamically to changing load conditions and environmental factors, maintaining cells within their ideal temperature range to maximize efficiency and prevent thermal runaway incidents.Expand Specific Solutions05 Diagnostic and predictive analytics for cell response
Modern electrochemical cells benefit from sophisticated diagnostic and predictive analytics that evaluate cell response characteristics. These systems employ machine learning algorithms and statistical models to analyze performance data, identify degradation patterns, and predict remaining useful life. By detecting subtle changes in cell response before visible failure occurs, these analytics enable proactive maintenance, optimize charging protocols, and prevent catastrophic failures. Implementation of these technologies significantly improves reliability and operational efficiency in various electrochemical applications.Expand Specific Solutions
Leading Research Institutions and Companies in Electrochemical Cell Technology
The electrochemical cell response to frequency modulation market is currently in a growth phase, with increasing applications in energy storage, fuel cells, and sensing technologies. The global market size is expanding rapidly, driven by renewable energy integration and electrification trends. Technologically, the field shows varying maturity levels across applications. Leading players include Robert Bosch GmbH and Boeing, focusing on automotive and aerospace applications respectively, while Contemporary Amperex Technology and FuelCell Energy are advancing battery and fuel cell technologies. Academic institutions like California Institute of Technology and research organizations like Centre National de la Recherche Scientifique are driving fundamental innovations, while companies like Kyocera and 3M are developing commercial applications in materials and components for electrochemical systems responding to frequency modulation.
Robert Bosch GmbH
Technical Solution: Robert Bosch GmbH has pioneered frequency domain analysis techniques for electrochemical cells with applications in fuel cells, batteries, and sensors. Their approach utilizes multi-sine excitation signals to simultaneously probe cell response across multiple frequencies, significantly reducing characterization time compared to traditional sequential frequency sweeps. Bosch's technology incorporates adaptive frequency selection algorithms that dynamically focus on frequency ranges most relevant to specific electrochemical processes of interest. Their system employs digital signal processing techniques to extract meaningful parameters from frequency response data, including charge transfer resistance, double-layer capacitance, and mass transport limitations. Bosch has implemented this technology in their automotive battery management systems to enable predictive maintenance and optimize battery performance throughout vehicle lifecycles. The company has also developed specialized hardware with high precision current control and voltage measurement capabilities to ensure accurate frequency response measurements even in noisy environments.
Strengths: Highly integrated with automotive systems; robust performance in field conditions; fast measurement capabilities through parallel frequency testing. Weaknesses: Proprietary system with limited compatibility with third-party equipment; requires significant computational resources for real-time analysis; primarily optimized for automotive applications rather than general research purposes.
Morimura SOFC Technology Co Ltd
Technical Solution: Morimura SOFC Technology has developed specialized electrochemical frequency response analysis techniques for solid oxide fuel cells operating at temperatures between 600-900°C. Their approach focuses on correlating electrochemical impedance spectroscopy (EIS) measurements with microstructural characteristics of their proprietary ceramic materials. The company employs a multi-scale frequency analysis approach that spans from 0.001 Hz to 1 MHz to capture both fast electrode kinetics and slow mass transport phenomena. Their technology incorporates reference electrodes with precise geometric control to isolate anode, cathode, and electrolyte contributions to the overall cell impedance. Morimura has developed temperature-controlled measurement chambers that maintain thermal stability within ±0.5°C during frequency sweep measurements, eliminating thermal artifacts from the impedance data. The company utilizes equivalent circuit modeling with physically meaningful components to translate frequency response data into actionable insights about cell performance and degradation mechanisms. Their system can detect subtle changes in electrode microstructure, electrolyte conductivity, and interfacial resistance long before these manifest as observable performance losses.
Strengths: Deep specialization in high-temperature SOFC systems; excellent correlation between frequency response and material microstructure; highly sensitive detection of early degradation indicators. Weaknesses: Extremely narrow focus on SOFC technology limits broader application; requires sophisticated high-temperature testing equipment; complex data interpretation requiring specialized expertise in ceramic electrochemistry.
Critical Patents and Literature on Electrochemical Frequency Response
Method for examining an electrochemical cell by electrochemical impedance spectroscopy
PatentPendingDE102022202521A1
Innovation
- A method using Rapid and Local Electrochemical Impedance Spectroscopy (RaLo EIS) that measures impedance spectra with both temporal and spatial resolution by impressing all frequencies within a specific range before evaluation, allowing for quick and reliable determination of parameters like membrane resistance during transient conditions.
Electrochemical cell systems and associated power systems, components, and methods
PatentWO2025160055A1
Innovation
- Employing pulse width modulation (PWM) controllers to intermittently interrupt power supply to electrochemical cells, coupled with high-speed monitoring tools to measure voltage changes and calculate cell resistance in real-time, allowing for in situ monitoring and efficient load distribution.
Materials Science Considerations for Enhanced Frequency Response
The selection of materials for electrochemical cells significantly impacts their frequency response characteristics. Traditional electrode materials such as carbon, platinum, and various metal oxides exhibit distinct electrochemical behaviors when subjected to frequency modulation. Recent advances in nanomaterials have revolutionized this field, with graphene, carbon nanotubes, and metal-organic frameworks demonstrating superior conductivity and surface area properties that enhance frequency response.
Material composition directly influences the double-layer capacitance at the electrode-electrolyte interface, a critical factor in determining frequency response limitations. Nanostructured materials with optimized pore sizes and distributions can significantly reduce ion diffusion limitations, allowing for more efficient charge transfer at higher frequencies. Research indicates that hierarchical porous structures combining macro, meso, and micropores provide optimal pathways for ion transport across multiple frequency ranges.
Surface modification techniques have emerged as powerful tools for tailoring frequency response characteristics. Functionalization with specific chemical groups can alter the surface charge distribution and wettability, thereby influencing the electrode-electrolyte interactions. Plasma treatment, chemical vapor deposition, and atomic layer deposition have proven effective in creating precisely engineered surfaces with enhanced frequency response properties.
Composite materials represent another frontier in materials science for electrochemical frequency response applications. By combining materials with complementary properties, researchers have developed electrodes that maintain high capacitance across broader frequency ranges. For instance, metal oxide/carbon composites leverage the high capacitance of metal oxides while mitigating their poor conductivity through carbon integration.
The crystalline structure of electrode materials plays a crucial role in frequency response behavior. Materials with well-defined crystalline structures often exhibit more predictable frequency-dependent characteristics compared to amorphous counterparts. Defect engineering has emerged as a strategic approach to manipulate frequency response, with controlled introduction of vacancies, dislocations, and grain boundaries serving to create additional active sites for electrochemical reactions.
Temperature stability represents another critical consideration, as thermal fluctuations can significantly alter material properties and consequently frequency response characteristics. Advanced ceramic materials and temperature-resistant polymers have been developed to maintain consistent electrochemical performance across varying thermal conditions, essential for applications requiring stable frequency response under fluctuating environmental conditions.
Recent developments in flexible and stretchable materials have opened new possibilities for electrochemical devices with consistent frequency response under mechanical deformation. Conductive polymers, elastomeric composites, and liquid metal alloys maintain electrical properties during bending, stretching, and compression, enabling next-generation wearable and conformable electrochemical systems with reliable frequency-modulated performance.
Material composition directly influences the double-layer capacitance at the electrode-electrolyte interface, a critical factor in determining frequency response limitations. Nanostructured materials with optimized pore sizes and distributions can significantly reduce ion diffusion limitations, allowing for more efficient charge transfer at higher frequencies. Research indicates that hierarchical porous structures combining macro, meso, and micropores provide optimal pathways for ion transport across multiple frequency ranges.
Surface modification techniques have emerged as powerful tools for tailoring frequency response characteristics. Functionalization with specific chemical groups can alter the surface charge distribution and wettability, thereby influencing the electrode-electrolyte interactions. Plasma treatment, chemical vapor deposition, and atomic layer deposition have proven effective in creating precisely engineered surfaces with enhanced frequency response properties.
Composite materials represent another frontier in materials science for electrochemical frequency response applications. By combining materials with complementary properties, researchers have developed electrodes that maintain high capacitance across broader frequency ranges. For instance, metal oxide/carbon composites leverage the high capacitance of metal oxides while mitigating their poor conductivity through carbon integration.
The crystalline structure of electrode materials plays a crucial role in frequency response behavior. Materials with well-defined crystalline structures often exhibit more predictable frequency-dependent characteristics compared to amorphous counterparts. Defect engineering has emerged as a strategic approach to manipulate frequency response, with controlled introduction of vacancies, dislocations, and grain boundaries serving to create additional active sites for electrochemical reactions.
Temperature stability represents another critical consideration, as thermal fluctuations can significantly alter material properties and consequently frequency response characteristics. Advanced ceramic materials and temperature-resistant polymers have been developed to maintain consistent electrochemical performance across varying thermal conditions, essential for applications requiring stable frequency response under fluctuating environmental conditions.
Recent developments in flexible and stretchable materials have opened new possibilities for electrochemical devices with consistent frequency response under mechanical deformation. Conductive polymers, elastomeric composites, and liquid metal alloys maintain electrical properties during bending, stretching, and compression, enabling next-generation wearable and conformable electrochemical systems with reliable frequency-modulated performance.
Standardization and Testing Protocols for Electrochemical Cell Performance
The standardization of testing protocols for electrochemical cell performance in response to frequency modulation represents a critical area requiring industry-wide consensus. Current testing methodologies vary significantly across research institutions and manufacturers, creating challenges in comparing results and establishing reliable performance benchmarks. This inconsistency hampers technological advancement and market growth in applications ranging from energy storage to sensing technologies.
Internationally recognized bodies such as ASTM International, IEC, and ISO have begun developing standardized protocols, though these efforts remain fragmented across different electrochemical cell types and applications. The IEEE Standards Association has recently formed a working group specifically addressing frequency response characterization methodologies, aiming to unify testing approaches by 2025.
Key parameters requiring standardization include frequency range specifications (typically 1 mHz to 1 MHz), amplitude modulation protocols, temperature control parameters (±0.5°C precision), and reference electrode configurations. The establishment of standard equivalent circuit models for data interpretation represents another crucial aspect, as current analytical approaches vary widely, leading to inconsistent performance evaluations.
Testing equipment calibration presents a significant challenge, with inter-laboratory studies revealing measurement variations exceeding 15% for identical cells under nominally identical conditions. This highlights the urgent need for certified reference materials and calibration standards specific to frequency modulation response measurements.
Data reporting formats also require standardization, as the current landscape features proprietary formats that impede cross-platform analysis. The emergence of open-source data formats such as the Electrochemical Markup Language (EChemML) offers promising solutions, though adoption remains limited in commercial settings.
Validation methodologies constitute another critical component, with round-robin testing programs emerging as the gold standard for protocol verification. Recent initiatives by the Battery Innovation Center and similar organizations have demonstrated that standardized protocols can reduce inter-laboratory variation to below 5% when properly implemented.
The economic impact of standardization extends beyond technical considerations, potentially reducing R&D costs by 20-30% through elimination of redundant testing and enabling more accurate performance comparisons across different manufacturers' products. This would accelerate market adoption of advanced electrochemical technologies while providing consumers with more reliable performance metrics.
Internationally recognized bodies such as ASTM International, IEC, and ISO have begun developing standardized protocols, though these efforts remain fragmented across different electrochemical cell types and applications. The IEEE Standards Association has recently formed a working group specifically addressing frequency response characterization methodologies, aiming to unify testing approaches by 2025.
Key parameters requiring standardization include frequency range specifications (typically 1 mHz to 1 MHz), amplitude modulation protocols, temperature control parameters (±0.5°C precision), and reference electrode configurations. The establishment of standard equivalent circuit models for data interpretation represents another crucial aspect, as current analytical approaches vary widely, leading to inconsistent performance evaluations.
Testing equipment calibration presents a significant challenge, with inter-laboratory studies revealing measurement variations exceeding 15% for identical cells under nominally identical conditions. This highlights the urgent need for certified reference materials and calibration standards specific to frequency modulation response measurements.
Data reporting formats also require standardization, as the current landscape features proprietary formats that impede cross-platform analysis. The emergence of open-source data formats such as the Electrochemical Markup Language (EChemML) offers promising solutions, though adoption remains limited in commercial settings.
Validation methodologies constitute another critical component, with round-robin testing programs emerging as the gold standard for protocol verification. Recent initiatives by the Battery Innovation Center and similar organizations have demonstrated that standardized protocols can reduce inter-laboratory variation to below 5% when properly implemented.
The economic impact of standardization extends beyond technical considerations, potentially reducing R&D costs by 20-30% through elimination of redundant testing and enabling more accurate performance comparisons across different manufacturers' products. This would accelerate market adoption of advanced electrochemical technologies while providing consumers with more reliable performance metrics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!