Optimize Electrochemical Cell Cathode Reactions for Output
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cathode Technology Evolution and Objectives
Electrochemical cell technology has evolved significantly over the past century, with cathode development representing one of the most critical aspects of performance optimization. The journey began with simple metal oxide cathodes in the early 20th century and has progressed through multiple generations of increasingly sophisticated materials and designs. This evolution has been driven by the growing demand for higher energy density, improved cycle life, enhanced safety, and reduced environmental impact across various applications including energy storage, transportation, and portable electronics.
The fundamental challenge in cathode optimization lies in managing the complex electrochemical reactions that occur at the interface between the cathode material and the electrolyte. These reactions involve electron transfer, ion diffusion, and chemical transformations that directly impact the cell's output performance. Historical advancements have focused on improving reaction kinetics, reducing polarization losses, and enhancing structural stability during charge-discharge cycles.
Recent technological breakthroughs have centered on nanostructured cathode materials, which offer increased surface area for reactions and shorter diffusion paths for ions. Layered transition metal oxides, spinel structures, and olivine phosphates have emerged as promising cathode materials, each offering unique advantages in terms of energy density, power capability, and stability. The incorporation of dopants and surface coatings has further enhanced performance by mitigating degradation mechanisms and improving electronic conductivity.
The current technological trajectory is moving toward multi-component cathode systems that can simultaneously address multiple performance parameters. Advanced manufacturing techniques, including precision control of particle morphology and composition gradients, are enabling unprecedented control over cathode microstructure and, consequently, electrochemical behavior. Computational modeling and high-throughput screening approaches are accelerating the discovery and optimization of novel cathode materials with tailored properties.
The primary objectives for cathode optimization include achieving higher energy density (>300 Wh/kg at the cell level), faster charging capabilities (80% charge in under 15 minutes), extended cycle life (>1000 cycles with minimal capacity fade), and reduced reliance on critical raw materials. Additionally, there is growing emphasis on developing cathodes that operate efficiently across wider temperature ranges and that can be manufactured using environmentally sustainable processes.
Looking forward, the integration of artificial intelligence and machine learning approaches promises to revolutionize cathode development by enabling more efficient exploration of the vast materials design space. The ultimate goal is to develop cathode technologies that can deliver step-change improvements in electrochemical cell performance while meeting increasingly stringent requirements for sustainability, safety, and cost-effectiveness.
The fundamental challenge in cathode optimization lies in managing the complex electrochemical reactions that occur at the interface between the cathode material and the electrolyte. These reactions involve electron transfer, ion diffusion, and chemical transformations that directly impact the cell's output performance. Historical advancements have focused on improving reaction kinetics, reducing polarization losses, and enhancing structural stability during charge-discharge cycles.
Recent technological breakthroughs have centered on nanostructured cathode materials, which offer increased surface area for reactions and shorter diffusion paths for ions. Layered transition metal oxides, spinel structures, and olivine phosphates have emerged as promising cathode materials, each offering unique advantages in terms of energy density, power capability, and stability. The incorporation of dopants and surface coatings has further enhanced performance by mitigating degradation mechanisms and improving electronic conductivity.
The current technological trajectory is moving toward multi-component cathode systems that can simultaneously address multiple performance parameters. Advanced manufacturing techniques, including precision control of particle morphology and composition gradients, are enabling unprecedented control over cathode microstructure and, consequently, electrochemical behavior. Computational modeling and high-throughput screening approaches are accelerating the discovery and optimization of novel cathode materials with tailored properties.
The primary objectives for cathode optimization include achieving higher energy density (>300 Wh/kg at the cell level), faster charging capabilities (80% charge in under 15 minutes), extended cycle life (>1000 cycles with minimal capacity fade), and reduced reliance on critical raw materials. Additionally, there is growing emphasis on developing cathodes that operate efficiently across wider temperature ranges and that can be manufactured using environmentally sustainable processes.
Looking forward, the integration of artificial intelligence and machine learning approaches promises to revolutionize cathode development by enabling more efficient exploration of the vast materials design space. The ultimate goal is to develop cathode technologies that can deliver step-change improvements in electrochemical cell performance while meeting increasingly stringent requirements for sustainability, safety, and cost-effectiveness.
Market Analysis for High-Performance Battery Applications
The high-performance battery market is experiencing unprecedented growth, driven by the rapid expansion of electric vehicles (EVs), renewable energy storage systems, and portable electronics. The global high-performance battery market reached $41.8 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 18.7% through 2030, potentially reaching $178.5 billion. This growth trajectory is particularly significant for cathode optimization technologies, which directly impact battery performance metrics valued by end-users.
Electric vehicle applications represent the largest and fastest-growing segment for high-performance batteries, accounting for approximately 65% of the market. Major automotive manufacturers have committed over $300 billion to EV development in the next decade, with battery performance—particularly energy density and charging speed—cited as critical competitive factors. Cathode reaction optimization directly addresses these requirements, potentially increasing vehicle range by 25-40% and reducing charging times by up to 60%.
The grid-scale energy storage market presents another substantial opportunity, valued at $12.7 billion in 2022 with projected 24.3% annual growth. Utility companies and renewable energy providers are increasingly demanding batteries with improved cycle life and efficiency—both parameters that can be significantly enhanced through cathode reaction optimization. Recent installations in California and Australia demonstrate willingness to pay premium prices for batteries with superior performance characteristics.
Consumer electronics manufacturers represent a mature but innovation-driven market segment, where miniaturization and power density command premium pricing. Apple, Samsung, and other major manufacturers have indicated readiness to adopt next-generation battery technologies that offer 30%+ improvements in energy density, creating a potential $8.4 billion addressable market for optimized cathode technologies.
Regional analysis reveals Asia-Pacific dominates manufacturing capacity (71% of global production), while North America leads in research investment with $2.1 billion allocated to advanced battery research in 2022 alone. European markets show the highest premium pricing potential, with consumers willing to pay up to 40% more for products with superior battery performance.
Customer requirements analysis indicates three critical performance metrics driving market adoption: energy density (kWh/kg), charging speed (C-rate capability), and cycle life. Optimized cathode reactions can potentially deliver improvements across all three parameters simultaneously, creating significant competitive advantage and justifying premium pricing in multiple market segments.
Electric vehicle applications represent the largest and fastest-growing segment for high-performance batteries, accounting for approximately 65% of the market. Major automotive manufacturers have committed over $300 billion to EV development in the next decade, with battery performance—particularly energy density and charging speed—cited as critical competitive factors. Cathode reaction optimization directly addresses these requirements, potentially increasing vehicle range by 25-40% and reducing charging times by up to 60%.
The grid-scale energy storage market presents another substantial opportunity, valued at $12.7 billion in 2022 with projected 24.3% annual growth. Utility companies and renewable energy providers are increasingly demanding batteries with improved cycle life and efficiency—both parameters that can be significantly enhanced through cathode reaction optimization. Recent installations in California and Australia demonstrate willingness to pay premium prices for batteries with superior performance characteristics.
Consumer electronics manufacturers represent a mature but innovation-driven market segment, where miniaturization and power density command premium pricing. Apple, Samsung, and other major manufacturers have indicated readiness to adopt next-generation battery technologies that offer 30%+ improvements in energy density, creating a potential $8.4 billion addressable market for optimized cathode technologies.
Regional analysis reveals Asia-Pacific dominates manufacturing capacity (71% of global production), while North America leads in research investment with $2.1 billion allocated to advanced battery research in 2022 alone. European markets show the highest premium pricing potential, with consumers willing to pay up to 40% more for products with superior battery performance.
Customer requirements analysis indicates three critical performance metrics driving market adoption: energy density (kWh/kg), charging speed (C-rate capability), and cycle life. Optimized cathode reactions can potentially deliver improvements across all three parameters simultaneously, creating significant competitive advantage and justifying premium pricing in multiple market segments.
Current Cathode Materials Landscape and Technical Barriers
The current landscape of cathode materials for electrochemical cells is dominated by several key technologies, each with distinct advantages and limitations. Lithium-based cathodes, particularly lithium cobalt oxide (LCO), lithium nickel manganese cobalt oxide (NMC), and lithium iron phosphate (LFP), represent the mainstream commercial solutions. LCO offers high energy density but suffers from limited thermal stability and cobalt supply constraints. NMC provides a balanced performance profile with improved stability, while LFP delivers exceptional safety and cycle life at the cost of lower energy density.
Emerging materials include lithium-rich layered oxides and high-nickel content cathodes, which promise energy densities exceeding 250 Wh/kg. Sulfur-based cathodes have demonstrated theoretical capacities up to 1,675 mAh/g, significantly surpassing conventional materials, though they remain primarily in research stages due to stability issues.
Despite these advancements, several technical barriers impede optimization of cathode reactions. Foremost among these is the challenge of ion transport kinetics, where sluggish diffusion within cathode structures limits rate capability and power density. This becomes particularly problematic at high discharge rates or low temperatures, where reaction rates decrease exponentially.
Structural degradation presents another significant barrier. During cycling, cathode materials undergo volume changes and phase transitions that compromise long-term stability. This manifests as capacity fade, increased impedance, and reduced cycle life. Particularly in high-voltage operation (>4.5V vs. Li/Li+), structural collapse and transition metal dissolution accelerate degradation processes.
Interface stability between cathode materials and electrolytes constitutes a third major challenge. The formation of resistive surface layers (cathode-electrolyte interphase) impedes ion transport and increases cell impedance. This interface degradation is exacerbated at elevated temperatures and voltages, creating a significant barrier to high-energy density applications.
Resource constraints and manufacturing complexity further complicate cathode optimization. Critical materials like cobalt and nickel face supply chain vulnerabilities and price volatility. Additionally, advanced cathode materials often require precise synthesis conditions and complex processing steps, increasing production costs and limiting scalability.
Environmental considerations also present barriers, as conventional cathode manufacturing involves energy-intensive processes and potentially hazardous materials. The industry faces increasing pressure to develop more sustainable production methods while maintaining performance metrics.
Addressing these technical barriers requires interdisciplinary approaches combining materials science, electrochemistry, and manufacturing innovation to develop next-generation cathode materials capable of delivering enhanced electrochemical performance while overcoming current limitations.
Emerging materials include lithium-rich layered oxides and high-nickel content cathodes, which promise energy densities exceeding 250 Wh/kg. Sulfur-based cathodes have demonstrated theoretical capacities up to 1,675 mAh/g, significantly surpassing conventional materials, though they remain primarily in research stages due to stability issues.
Despite these advancements, several technical barriers impede optimization of cathode reactions. Foremost among these is the challenge of ion transport kinetics, where sluggish diffusion within cathode structures limits rate capability and power density. This becomes particularly problematic at high discharge rates or low temperatures, where reaction rates decrease exponentially.
Structural degradation presents another significant barrier. During cycling, cathode materials undergo volume changes and phase transitions that compromise long-term stability. This manifests as capacity fade, increased impedance, and reduced cycle life. Particularly in high-voltage operation (>4.5V vs. Li/Li+), structural collapse and transition metal dissolution accelerate degradation processes.
Interface stability between cathode materials and electrolytes constitutes a third major challenge. The formation of resistive surface layers (cathode-electrolyte interphase) impedes ion transport and increases cell impedance. This interface degradation is exacerbated at elevated temperatures and voltages, creating a significant barrier to high-energy density applications.
Resource constraints and manufacturing complexity further complicate cathode optimization. Critical materials like cobalt and nickel face supply chain vulnerabilities and price volatility. Additionally, advanced cathode materials often require precise synthesis conditions and complex processing steps, increasing production costs and limiting scalability.
Environmental considerations also present barriers, as conventional cathode manufacturing involves energy-intensive processes and potentially hazardous materials. The industry faces increasing pressure to develop more sustainable production methods while maintaining performance metrics.
Addressing these technical barriers requires interdisciplinary approaches combining materials science, electrochemistry, and manufacturing innovation to develop next-generation cathode materials capable of delivering enhanced electrochemical performance while overcoming current limitations.
Contemporary Cathode Optimization Methodologies
01 Cathode materials for enhanced electrochemical performance
Various materials can be used in electrochemical cell cathodes to enhance performance and output. These include specialized metal oxides, composite materials, and doped compounds that improve conductivity, stability, and energy density. The selection of appropriate cathode materials significantly impacts the overall efficiency and power output of electrochemical cells, with recent innovations focusing on nanomaterials and advanced composites to maximize electron transfer and electrochemical reactions.- Cathode materials for improved electrochemical performance: Various materials can be used as cathodes in electrochemical cells to enhance performance. These include metal oxides, sulfides, and composite materials that offer high energy density and improved cycling stability. The selection of cathode materials significantly impacts the cell's output voltage, capacity, and overall efficiency. Advanced cathode compositions can lead to higher power output and longer operational lifetimes in batteries and fuel cells.
- Cathode structure optimization for enhanced output: The physical structure and design of cathodes play a crucial role in determining electrochemical cell output. Optimized porosity, thickness, and surface area can improve ion transport and reaction kinetics. Structured cathodes with controlled morphology facilitate better electrolyte penetration and electron transfer, resulting in higher current densities and power output. Advanced manufacturing techniques enable precise control over cathode microstructure for maximized performance.
- Electrolyte interactions with cathode materials: The interface between cathode materials and electrolytes significantly affects electrochemical cell output. Optimizing this interface can reduce resistance and improve ion transfer rates. Selection of compatible electrolytes that form stable interfaces with cathode materials prevents degradation and maintains consistent output over time. Additives in the electrolyte can enhance cathode performance by forming protective layers or facilitating ion transport.
- Temperature effects on cathode output: Temperature significantly influences cathode performance in electrochemical cells. Operating temperature affects reaction kinetics, ion mobility, and internal resistance, all of which impact output. Cathode materials designed for specific temperature ranges can maintain stable performance under varying conditions. Thermal management systems can optimize cathode output by maintaining ideal operating temperatures and preventing degradation from thermal stress.
- Cathode dopants and additives for enhanced output: Incorporating dopants and additives into cathode materials can significantly enhance electrochemical cell output. These modifications can improve conductivity, structural stability, and electrochemical activity. Small amounts of metals, metal oxides, or carbon-based materials can create synergistic effects that boost cathode performance. Surface coatings and functional additives can protect cathode materials from degradation while maintaining high output over extended cycling.
02 Cathode structure optimization for improved output
The physical structure and design of cathodes play a crucial role in determining electrochemical cell output. Optimized cathode structures feature controlled porosity, specific surface area, and thickness that facilitate ion movement and reaction kinetics. Advanced manufacturing techniques allow for precise control of cathode architecture, including layered structures and gradient compositions that balance mechanical stability with electrochemical performance to maximize power output.Expand Specific Solutions03 Electrolyte interactions with cathode materials
The interface between cathode materials and electrolytes significantly affects electrochemical cell output. Specialized electrolyte formulations can enhance ion transport to and from the cathode, reduce interfacial resistance, and prevent degradation mechanisms. Research focuses on developing compatible electrolyte-cathode combinations that maintain stable performance over extended cycling, with innovations in solid-state electrolytes and electrolyte additives showing promise for improving cathode output efficiency.Expand Specific Solutions04 Temperature management for optimal cathode performance
Temperature significantly impacts cathode output in electrochemical cells. Effective thermal management systems help maintain optimal operating temperatures, preventing performance degradation at temperature extremes. Advanced cooling and heating mechanisms integrated into cell design ensure consistent cathode performance across varying environmental conditions, while thermally stable cathode materials resist degradation during temperature fluctuations, maintaining reliable output over the cell's operational lifetime.Expand Specific Solutions05 Cathode manufacturing processes for output enhancement
Manufacturing processes significantly influence cathode performance and output in electrochemical cells. Advanced fabrication techniques such as controlled deposition methods, precise mixing protocols, and specialized heat treatments optimize cathode microstructure and composition. Innovations in manufacturing include solvent-free processing, green manufacturing approaches, and scalable production methods that maintain consistent quality while reducing defects that could limit electrochemical output.Expand Specific Solutions
Leading Companies and Research Institutions in Cathode Technology
The electrochemical cell cathode optimization market is currently in a growth phase, with increasing demand driven by renewable energy storage needs and electric vehicle expansion. The market is projected to reach significant scale as battery technology becomes central to global energy transition strategies. Leading players represent diverse sectors: automotive manufacturers (Toyota, Honda, BMW, GM), specialized battery developers (Sion Power, LG Energy Solution), electronics giants (Toshiba, Hitachi), and research institutions (MIT, Caltech). Competition centers around improving energy density, durability, and cost-effectiveness, with companies like Energizer and Duracell focusing on consumer applications while automotive players prioritize high-performance cathodes for EVs. Technical maturity varies significantly across applications, with traditional lithium-ion technologies well-established but next-generation chemistries still evolving.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed several groundbreaking approaches to cathode optimization for electrochemical cells. Their work includes novel synthesis methods for high-nickel content cathode materials with controlled crystal facet exposure to enhance lithium-ion diffusion kinetics. MIT has pioneered the use of artificial intelligence and computational modeling to predict optimal cathode compositions and structures before physical synthesis, accelerating development cycles. Their research includes innovative single-atom catalysts anchored on conductive substrates that significantly reduce activation energy for oxygen reduction and evolution reactions in metal-air batteries and fuel cells. MIT has developed self-healing cathode materials incorporating dynamic chemical bonds that can repair structural damage during cycling, extending operational lifetime. Their work also encompasses advanced in-situ characterization techniques that provide atomic-level insights into degradation mechanisms at cathode surfaces during operation. Recent publications highlight their development of cathode architectures with precisely engineered porosity gradients that optimize both electronic and ionic transport pathways, resulting in superior rate capability and utilization of active materials.
Strengths: Cutting-edge fundamental understanding of electrochemical interfaces, access to advanced characterization facilities, and interdisciplinary approach combining materials science, chemistry, and computational modeling. Weaknesses: Potential challenges in scaling laboratory discoveries to commercial production, focus on fundamental research rather than manufacturing optimization, and longer timeline to market implementation.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered solid-state battery technology with optimized cathode-electrolyte interfaces to enhance electrochemical performance. Their approach focuses on sulfide-based solid electrolytes paired with high-voltage cathodes (>4.5V vs Li/Li+). Toyota's cathode optimization strategy involves precise control of crystal facet exposure and orientation to maximize active surface area while minimizing undesirable side reactions. They've developed proprietary surface modification techniques using nanoscale ceramic coatings that enhance lithium-ion transport at the cathode-electrolyte interface. Their research has yielded cathode materials with reduced cobalt content while maintaining structural stability during cycling. Toyota has also implemented advanced manufacturing processes that ensure uniform particle size distribution and optimal electrode microstructure, resulting in more efficient electrochemical reactions and improved power output. Recent patents reveal work on cathode materials that can operate efficiently at wider temperature ranges (-30°C to 60°C) without significant performance degradation.
Strengths: Superior thermal stability compared to conventional lithium-ion batteries, potential for higher energy density (400+ Wh/kg), and enhanced safety characteristics. Weaknesses: Higher manufacturing complexity, challenges in scaling production, and potentially higher initial costs compared to established technologies.
Critical Patents and Research in Electrochemical Cell Optimization
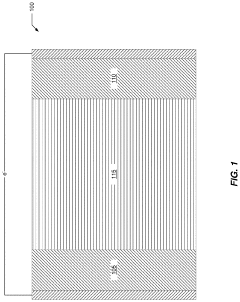
Optimization of electrochemical cell
PatentActiveUS20230084123A1
Innovation
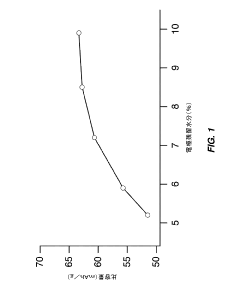
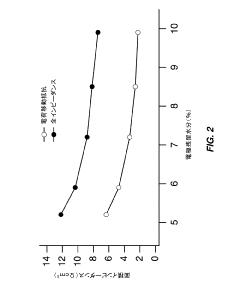
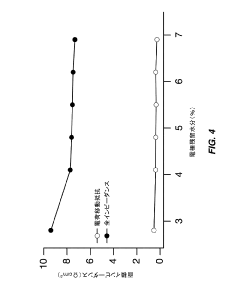
- Partial dehydration of transition metal cyanide coordination compound electrodes before assembly allows for a relaxed specification on water content in non-aqueous electrolytes, enabling the electrodes to absorb and remove water, thereby optimizing cell performance and reducing manufacturing costs by simplifying processing steps.
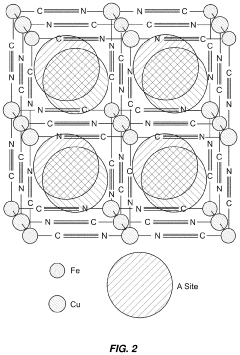
Electrochemical cell optimization
PatentPendingJP2023504210A
Innovation
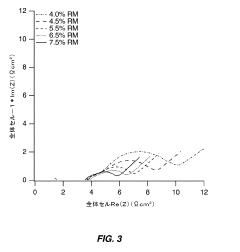
- Optimize the water content in TMCCC electrodes by maintaining a controlled residual moisture (RM) balance, allowing some lattice-bound water while minimizing non-coordinated water through a single dehydration/hydration process on a fully assembled cell stack, rather than individual electrodes.
Sustainability and Resource Considerations for Cathode Materials
The sustainability of cathode materials represents a critical dimension in the optimization of electrochemical cell performance. Current cathode technologies heavily rely on rare earth elements and transition metals such as cobalt, nickel, and lithium, which face significant supply chain vulnerabilities. Global reserves of these materials are concentrated in politically sensitive regions, with over 60% of cobalt sourced from the Democratic Republic of Congo and approximately 70% of lithium reserves located in the South American "Lithium Triangle."
Environmental impacts of cathode material extraction and processing present substantial challenges. Traditional mining operations generate approximately 10-15 tons of CO2 emissions per ton of cathode material produced, alongside significant water consumption and potential toxic waste generation. These environmental costs must be factored into the total lifecycle assessment of electrochemical cell technologies.
Recycling infrastructure for cathode materials remains underdeveloped, with current recovery rates averaging only 30-40% for most critical elements. This inefficiency creates a substantial gap between supply and demand projections, particularly as electrochemical applications expand beyond consumer electronics into transportation and grid storage sectors. Technological innovations in hydrometallurgical and direct recycling processes show promise for improving recovery rates to potentially 80-90%, but require further development.
Alternative material pathways are emerging to address these sustainability concerns. Sodium-ion cathodes offer a more abundant resource base, while manganese-rich formulations reduce dependence on cobalt. These alternatives currently deliver 15-20% lower energy density but demonstrate superior thermal stability and potentially longer cycle life. Research into organic cathode materials derived from sustainable carbon sources represents another promising direction, though challenges in conductivity and stability persist.
Manufacturing processes for cathode materials are evolving toward more resource-efficient approaches. Dry electrode processing techniques reduce solvent usage by up to 80% compared to traditional slurry-based methods, while co-precipitation synthesis routes enable more precise control of material morphology with lower energy inputs. These advancements simultaneously address sustainability concerns while potentially improving electrochemical performance.
Policy frameworks increasingly influence cathode material selection and supply chain development. The European Battery Directive and similar regulations in North America and Asia are establishing requirements for recycled content, carbon footprint limitations, and responsible sourcing documentation. These regulatory trends will likely accelerate the transition toward more sustainable cathode chemistries and manufacturing approaches in the coming decade.
Environmental impacts of cathode material extraction and processing present substantial challenges. Traditional mining operations generate approximately 10-15 tons of CO2 emissions per ton of cathode material produced, alongside significant water consumption and potential toxic waste generation. These environmental costs must be factored into the total lifecycle assessment of electrochemical cell technologies.
Recycling infrastructure for cathode materials remains underdeveloped, with current recovery rates averaging only 30-40% for most critical elements. This inefficiency creates a substantial gap between supply and demand projections, particularly as electrochemical applications expand beyond consumer electronics into transportation and grid storage sectors. Technological innovations in hydrometallurgical and direct recycling processes show promise for improving recovery rates to potentially 80-90%, but require further development.
Alternative material pathways are emerging to address these sustainability concerns. Sodium-ion cathodes offer a more abundant resource base, while manganese-rich formulations reduce dependence on cobalt. These alternatives currently deliver 15-20% lower energy density but demonstrate superior thermal stability and potentially longer cycle life. Research into organic cathode materials derived from sustainable carbon sources represents another promising direction, though challenges in conductivity and stability persist.
Manufacturing processes for cathode materials are evolving toward more resource-efficient approaches. Dry electrode processing techniques reduce solvent usage by up to 80% compared to traditional slurry-based methods, while co-precipitation synthesis routes enable more precise control of material morphology with lower energy inputs. These advancements simultaneously address sustainability concerns while potentially improving electrochemical performance.
Policy frameworks increasingly influence cathode material selection and supply chain development. The European Battery Directive and similar regulations in North America and Asia are establishing requirements for recycled content, carbon footprint limitations, and responsible sourcing documentation. These regulatory trends will likely accelerate the transition toward more sustainable cathode chemistries and manufacturing approaches in the coming decade.
Performance Metrics and Testing Standards for Cathode Evaluation
Standardized performance metrics and testing protocols are essential for the systematic evaluation and optimization of cathode materials in electrochemical cells. The industry has developed comprehensive frameworks that enable objective comparison across different cathode formulations and manufacturing processes.
Key performance indicators for cathode evaluation include specific capacity (mAh/g), which quantifies the charge storage capability per unit mass. This metric directly impacts the energy density of the final device and remains one of the primary benchmarks for cathode development. Cycling stability, measured through capacity retention over hundreds or thousands of cycles, provides critical insights into the long-term durability of cathode materials under operational conditions.
Rate capability testing, which evaluates performance across various charge-discharge rates (C-rates), has become increasingly important as applications demand both high power and energy density. Standard protocols typically include measurements at C/20, C/10, C/5, C/2, 1C, 2C, and 5C rates to generate comprehensive performance profiles.
Electrochemical impedance spectroscopy (EIS) serves as a powerful analytical technique for quantifying cathode reaction kinetics and interfacial properties. The standardization of EIS testing conditions and data interpretation methods has significantly improved the comparability of results across research institutions and manufacturing facilities.
Environmental testing standards have evolved to include performance evaluation under extreme temperature conditions (-20°C to 60°C), which is particularly relevant for automotive and aerospace applications. Accelerated aging protocols that simulate years of operation within weeks or months enable rapid screening of promising cathode formulations.
Safety testing standards for cathodes have become increasingly rigorous, incorporating thermal stability assessments, overcharge tolerance tests, and nail penetration evaluations. These protocols are essential for ensuring that performance optimization does not compromise the safety profile of the electrochemical system.
The emergence of high-throughput screening methodologies has revolutionized cathode evaluation, allowing researchers to assess hundreds of compositional variations simultaneously. These approaches typically employ miniaturized test cells and automated data collection systems, accelerating the optimization process while maintaining measurement accuracy.
International standardization bodies, including IEC, ISO, and ASTM, have established reference procedures that facilitate global collaboration and technology transfer. Adherence to these standards ensures that performance claims can be independently verified and that incremental improvements in cathode technology can be objectively quantified.
Key performance indicators for cathode evaluation include specific capacity (mAh/g), which quantifies the charge storage capability per unit mass. This metric directly impacts the energy density of the final device and remains one of the primary benchmarks for cathode development. Cycling stability, measured through capacity retention over hundreds or thousands of cycles, provides critical insights into the long-term durability of cathode materials under operational conditions.
Rate capability testing, which evaluates performance across various charge-discharge rates (C-rates), has become increasingly important as applications demand both high power and energy density. Standard protocols typically include measurements at C/20, C/10, C/5, C/2, 1C, 2C, and 5C rates to generate comprehensive performance profiles.
Electrochemical impedance spectroscopy (EIS) serves as a powerful analytical technique for quantifying cathode reaction kinetics and interfacial properties. The standardization of EIS testing conditions and data interpretation methods has significantly improved the comparability of results across research institutions and manufacturing facilities.
Environmental testing standards have evolved to include performance evaluation under extreme temperature conditions (-20°C to 60°C), which is particularly relevant for automotive and aerospace applications. Accelerated aging protocols that simulate years of operation within weeks or months enable rapid screening of promising cathode formulations.
Safety testing standards for cathodes have become increasingly rigorous, incorporating thermal stability assessments, overcharge tolerance tests, and nail penetration evaluations. These protocols are essential for ensuring that performance optimization does not compromise the safety profile of the electrochemical system.
The emergence of high-throughput screening methodologies has revolutionized cathode evaluation, allowing researchers to assess hundreds of compositional variations simultaneously. These approaches typically employ miniaturized test cells and automated data collection systems, accelerating the optimization process while maintaining measurement accuracy.
International standardization bodies, including IEC, ISO, and ASTM, have established reference procedures that facilitate global collaboration and technology transfer. Adherence to these standards ensures that performance claims can be independently verified and that incremental improvements in cathode technology can be objectively quantified.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!