What Are the Biocompatibility Standards for Injectable Hydrogel
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Injectable Hydrogel Biocompatibility Background and Objectives
Injectable hydrogels have emerged as a revolutionary biomaterial platform in the field of regenerative medicine and drug delivery over the past three decades. These versatile materials transition from liquid to gel states upon injection into the body, offering minimally invasive administration while providing three-dimensional scaffolds for tissue engineering and controlled release systems for therapeutic agents. The evolution of injectable hydrogels began in the 1990s with simple polymer systems and has progressively advanced toward sophisticated, stimuli-responsive, and bioactive formulations that interact dynamically with biological environments.
The technological trajectory of injectable hydrogels has been characterized by significant milestones, including the development of in situ forming systems, integration of cell-instructive cues, and more recently, the incorporation of nanomaterials to enhance mechanical and biological properties. Current research trends are focusing on creating "smart" hydrogels that respond to specific biological triggers, mimic extracellular matrix functions, and facilitate precise spatiotemporal control over cellular processes and drug release kinetics.
Biocompatibility represents the cornerstone requirement for any injectable hydrogel system intended for clinical application. The concept extends beyond mere absence of toxicity to encompass appropriate host-material interactions that support intended therapeutic functions without eliciting adverse immune responses or compromising surrounding tissue integrity. Despite significant advances in hydrogel chemistry and engineering, standardized approaches to biocompatibility assessment remain fragmented and often inadequately address the unique challenges posed by these dynamic materials.
The primary objective of this technical research report is to comprehensively evaluate the current landscape of biocompatibility standards specifically applicable to injectable hydrogels. We aim to identify gaps in existing regulatory frameworks, analyze emerging methodologies for biocompatibility testing, and propose potential pathways toward more harmonized and clinically relevant assessment protocols. Additionally, this report seeks to establish correlations between hydrogel physicochemical properties and their biological performance to inform more predictive biocompatibility screening approaches.
Furthermore, this investigation intends to explore how evolving understanding of biomaterial-tissue interactions can inform next-generation biocompatibility standards that account for the dynamic nature of injectable hydrogels. By examining both traditional biocompatibility parameters and emerging considerations such as degradation profiles, mechanical property evolution in situ, and long-term tissue integration, we aim to contribute to the development of more comprehensive evaluation criteria that can accelerate clinical translation of promising injectable hydrogel technologies.
The technological trajectory of injectable hydrogels has been characterized by significant milestones, including the development of in situ forming systems, integration of cell-instructive cues, and more recently, the incorporation of nanomaterials to enhance mechanical and biological properties. Current research trends are focusing on creating "smart" hydrogels that respond to specific biological triggers, mimic extracellular matrix functions, and facilitate precise spatiotemporal control over cellular processes and drug release kinetics.
Biocompatibility represents the cornerstone requirement for any injectable hydrogel system intended for clinical application. The concept extends beyond mere absence of toxicity to encompass appropriate host-material interactions that support intended therapeutic functions without eliciting adverse immune responses or compromising surrounding tissue integrity. Despite significant advances in hydrogel chemistry and engineering, standardized approaches to biocompatibility assessment remain fragmented and often inadequately address the unique challenges posed by these dynamic materials.
The primary objective of this technical research report is to comprehensively evaluate the current landscape of biocompatibility standards specifically applicable to injectable hydrogels. We aim to identify gaps in existing regulatory frameworks, analyze emerging methodologies for biocompatibility testing, and propose potential pathways toward more harmonized and clinically relevant assessment protocols. Additionally, this report seeks to establish correlations between hydrogel physicochemical properties and their biological performance to inform more predictive biocompatibility screening approaches.
Furthermore, this investigation intends to explore how evolving understanding of biomaterial-tissue interactions can inform next-generation biocompatibility standards that account for the dynamic nature of injectable hydrogels. By examining both traditional biocompatibility parameters and emerging considerations such as degradation profiles, mechanical property evolution in situ, and long-term tissue integration, we aim to contribute to the development of more comprehensive evaluation criteria that can accelerate clinical translation of promising injectable hydrogel technologies.
Market Analysis for Biocompatible Injectable Hydrogels
The global market for biocompatible injectable hydrogels has experienced significant growth in recent years, driven by increasing applications in tissue engineering, drug delivery systems, and regenerative medicine. Current market valuations indicate that the injectable hydrogel sector reached approximately 16 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 9.8% through 2030.
Healthcare applications dominate the market landscape, accounting for over 65% of total revenue. Within this segment, wound healing and tissue regeneration represent the fastest-growing applications, particularly as minimally invasive surgical procedures gain popularity worldwide. The aging global population and rising prevalence of chronic wounds have further accelerated demand for advanced biomaterials like injectable hydrogels.
Regionally, North America holds the largest market share at 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate over the next decade, primarily due to increasing healthcare expenditure, expanding medical tourism, and growing research activities in countries like China, Japan, and South Korea.
From an end-user perspective, hospitals and specialized wound care centers constitute the primary consumers, though research institutions represent a significant market segment due to ongoing investigations into novel biocompatible formulations and applications. The cosmetic surgery sector has also emerged as a rapidly expanding market for injectable hydrogels, particularly for soft tissue augmentation procedures.
Key market drivers include technological advancements in polymer science, growing demand for minimally invasive procedures, and increasing research funding for regenerative medicine. The shift toward personalized medicine has further stimulated interest in customizable hydrogel systems that can be tailored to individual patient needs.
Market restraints primarily revolve around regulatory challenges, with stringent biocompatibility standards creating significant barriers to entry for new products. The high cost of development and clinical validation for novel injectable hydrogels also limits market penetration, particularly in developing economies where healthcare budgets remain constrained.
Consumer trends indicate growing preference for natural and synthetic-natural hybrid hydrogels over purely synthetic alternatives, driven by concerns regarding long-term biocompatibility and environmental sustainability. Additionally, there is increasing demand for multifunctional hydrogels that combine therapeutic delivery capabilities with structural support functions.
The competitive landscape features both established medical device manufacturers and specialized biomaterials companies, with recent years witnessing significant merger and acquisition activity as larger corporations seek to expand their regenerative medicine portfolios through strategic acquisitions of innovative startups with promising hydrogel technologies.
Healthcare applications dominate the market landscape, accounting for over 65% of total revenue. Within this segment, wound healing and tissue regeneration represent the fastest-growing applications, particularly as minimally invasive surgical procedures gain popularity worldwide. The aging global population and rising prevalence of chronic wounds have further accelerated demand for advanced biomaterials like injectable hydrogels.
Regionally, North America holds the largest market share at 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate over the next decade, primarily due to increasing healthcare expenditure, expanding medical tourism, and growing research activities in countries like China, Japan, and South Korea.
From an end-user perspective, hospitals and specialized wound care centers constitute the primary consumers, though research institutions represent a significant market segment due to ongoing investigations into novel biocompatible formulations and applications. The cosmetic surgery sector has also emerged as a rapidly expanding market for injectable hydrogels, particularly for soft tissue augmentation procedures.
Key market drivers include technological advancements in polymer science, growing demand for minimally invasive procedures, and increasing research funding for regenerative medicine. The shift toward personalized medicine has further stimulated interest in customizable hydrogel systems that can be tailored to individual patient needs.
Market restraints primarily revolve around regulatory challenges, with stringent biocompatibility standards creating significant barriers to entry for new products. The high cost of development and clinical validation for novel injectable hydrogels also limits market penetration, particularly in developing economies where healthcare budgets remain constrained.
Consumer trends indicate growing preference for natural and synthetic-natural hybrid hydrogels over purely synthetic alternatives, driven by concerns regarding long-term biocompatibility and environmental sustainability. Additionally, there is increasing demand for multifunctional hydrogels that combine therapeutic delivery capabilities with structural support functions.
The competitive landscape features both established medical device manufacturers and specialized biomaterials companies, with recent years witnessing significant merger and acquisition activity as larger corporations seek to expand their regenerative medicine portfolios through strategic acquisitions of innovative startups with promising hydrogel technologies.
Current Biocompatibility Standards and Technical Challenges
The current biocompatibility standards for injectable hydrogels are primarily governed by ISO 10993 series, particularly ISO 10993-1 which outlines the general principles for biological evaluation of medical devices. For injectable hydrogels specifically, standards focus on cytotoxicity (ISO 10993-5), sensitization (ISO 10993-10), irritation (ISO 10993-10), systemic toxicity (ISO 10993-11), and implantation tests (ISO 10993-6). These standards ensure that hydrogels do not cause adverse biological responses when introduced into the body.
The FDA guidance document "Use of International Standard ISO 10993-1" provides regulatory framework for evaluating biocompatibility of medical devices, including injectable hydrogels. Additionally, ASTM F2900 specifically addresses characterization of hydrogel-based products, providing standardized testing methodologies for physical, chemical, and biological properties.
Despite these established standards, significant technical challenges persist in the biocompatibility assessment of injectable hydrogels. The dynamic nature of hydrogels—transitioning from liquid to gel state in situ—creates unique testing difficulties not adequately addressed by current standards. Traditional biocompatibility tests were designed for solid materials with stable properties, whereas hydrogels undergo structural changes after injection.
Material degradation presents another major challenge. As hydrogels degrade, they release various compounds that may affect biocompatibility over time. Current standards lack comprehensive guidelines for evaluating the biocompatibility of degradation products and their potential long-term effects on surrounding tissues.
The variability in hydrogel composition further complicates standardization efforts. With numerous natural and synthetic polymers used in hydrogel formulations, each with unique biological interactions, developing universal testing protocols becomes extremely difficult. This diversity necessitates case-by-case evaluation approaches that are resource-intensive and time-consuming.
Sterilization compatibility represents another significant challenge. Many hydrogels are sensitive to conventional sterilization methods, which can alter their physical properties and biological performance. Current standards provide limited guidance on appropriate sterilization techniques that maintain hydrogel integrity while ensuring sterility.
Scaling issues also impact biocompatibility testing. Laboratory tests often fail to accurately predict in vivo performance due to differences in mechanical forces, biological environments, and degradation kinetics between test conditions and actual physiological environments. This translation gap between in vitro testing and clinical outcomes remains a major hurdle in hydrogel development.
Regulatory harmonization across different regions presents additional challenges. While ISO standards are widely recognized, regional variations in implementation and additional requirements create regulatory complexity for global development and commercialization of injectable hydrogel products.
The FDA guidance document "Use of International Standard ISO 10993-1" provides regulatory framework for evaluating biocompatibility of medical devices, including injectable hydrogels. Additionally, ASTM F2900 specifically addresses characterization of hydrogel-based products, providing standardized testing methodologies for physical, chemical, and biological properties.
Despite these established standards, significant technical challenges persist in the biocompatibility assessment of injectable hydrogels. The dynamic nature of hydrogels—transitioning from liquid to gel state in situ—creates unique testing difficulties not adequately addressed by current standards. Traditional biocompatibility tests were designed for solid materials with stable properties, whereas hydrogels undergo structural changes after injection.
Material degradation presents another major challenge. As hydrogels degrade, they release various compounds that may affect biocompatibility over time. Current standards lack comprehensive guidelines for evaluating the biocompatibility of degradation products and their potential long-term effects on surrounding tissues.
The variability in hydrogel composition further complicates standardization efforts. With numerous natural and synthetic polymers used in hydrogel formulations, each with unique biological interactions, developing universal testing protocols becomes extremely difficult. This diversity necessitates case-by-case evaluation approaches that are resource-intensive and time-consuming.
Sterilization compatibility represents another significant challenge. Many hydrogels are sensitive to conventional sterilization methods, which can alter their physical properties and biological performance. Current standards provide limited guidance on appropriate sterilization techniques that maintain hydrogel integrity while ensuring sterility.
Scaling issues also impact biocompatibility testing. Laboratory tests often fail to accurately predict in vivo performance due to differences in mechanical forces, biological environments, and degradation kinetics between test conditions and actual physiological environments. This translation gap between in vitro testing and clinical outcomes remains a major hurdle in hydrogel development.
Regulatory harmonization across different regions presents additional challenges. While ISO standards are widely recognized, regional variations in implementation and additional requirements create regulatory complexity for global development and commercialization of injectable hydrogel products.
Current Biocompatibility Assessment Methodologies
01 Natural polymer-based injectable hydrogels
Injectable hydrogels derived from natural polymers such as collagen, hyaluronic acid, and alginate demonstrate excellent biocompatibility due to their similarity to the extracellular matrix. These materials provide a supportive environment for cell growth and tissue regeneration while minimizing immune responses. Their biodegradable nature allows for gradual replacement by native tissue, making them particularly suitable for tissue engineering and drug delivery applications.- Natural polymer-based injectable hydrogels: Injectable hydrogels derived from natural polymers such as collagen, hyaluronic acid, and alginate demonstrate excellent biocompatibility due to their similarity to the extracellular matrix. These materials provide a favorable microenvironment for cell growth and tissue regeneration while minimizing immune responses. Their biodegradable nature allows for gradual replacement by native tissue, making them ideal for various biomedical applications including drug delivery, tissue engineering, and wound healing.
- Synthetic polymer hydrogels with enhanced biocompatibility: Synthetic polymer-based injectable hydrogels, including polyethylene glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), and polyacrylamide derivatives, can be engineered with specific biocompatible properties. These materials offer advantages such as controlled degradation rates, tunable mechanical properties, and reduced batch-to-batch variation compared to natural polymers. Modifications such as incorporating cell-adhesion motifs or combining with bioactive molecules can significantly improve their biocompatibility for clinical applications.
- Hybrid and composite hydrogel systems: Hybrid injectable hydrogels combining natural and synthetic polymers leverage the advantages of both material types to enhance biocompatibility. These composite systems often exhibit improved mechanical properties while maintaining excellent biological performance. Additionally, incorporating nanoparticles, bioactive glass, or ceramic materials into hydrogels can enhance their functionality for specific applications such as bone regeneration or controlled drug release, while maintaining good biocompatibility with host tissues.
- In vivo biocompatibility assessment methods: Various techniques for evaluating the biocompatibility of injectable hydrogels in vivo include subcutaneous implantation, histological analysis, immunohistochemistry, and functional recovery assessment. These methods help determine inflammatory responses, foreign body reactions, tissue integration, and long-term safety. Advanced imaging techniques such as MRI and fluorescence imaging allow for real-time monitoring of hydrogel degradation and tissue regeneration, providing comprehensive data on biocompatibility performance under physiological conditions.
- Bioactive and stimuli-responsive injectable hydrogels: Injectable hydrogels incorporating bioactive molecules such as growth factors, peptides, and therapeutic drugs can enhance biocompatibility while providing additional therapeutic benefits. Stimuli-responsive hydrogels that react to changes in temperature, pH, or enzymatic activity offer controlled gelation and drug release properties. These smart materials can adapt to the physiological environment, improving integration with host tissues and reducing adverse reactions, making them particularly valuable for targeted drug delivery and regenerative medicine applications.
02 Synthetic polymer hydrogels with enhanced biocompatibility
Synthetic polymer-based injectable hydrogels, including polyethylene glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), and polyvinyl alcohol (PVA) derivatives, can be engineered with specific biocompatible properties. These materials offer tunable mechanical strength, degradation rates, and functional groups that can be modified to improve cell adhesion and reduce foreign body responses. Their synthetic nature allows for consistent production and customization for specific biomedical applications.Expand Specific Solutions03 Composite hydrogels for improved biocompatibility
Composite injectable hydrogels combining multiple materials (such as natural/synthetic polymer blends or polymer-nanoparticle combinations) demonstrate enhanced biocompatibility profiles. These hybrid systems leverage the advantages of each component while mitigating their individual limitations. The incorporation of bioactive components like growth factors, peptides, or minerals can further improve cell attachment, proliferation, and tissue integration, making these composites particularly effective for complex tissue engineering applications.Expand Specific Solutions04 In-situ forming hydrogels with minimized inflammatory response
In-situ forming injectable hydrogels that solidify after administration demonstrate improved biocompatibility by minimizing surgical trauma and conforming perfectly to tissue defects. These systems utilize various crosslinking mechanisms including temperature-sensitive, pH-responsive, or photo-crosslinking approaches. Their ability to form under physiological conditions without harmful solvents or initiators reduces inflammatory responses and promotes better integration with surrounding tissues, making them ideal for minimally invasive therapeutic applications.Expand Specific Solutions05 Biocompatibility assessment methods for injectable hydrogels
Advanced methods for evaluating the biocompatibility of injectable hydrogels include in vitro cytotoxicity assays, protein adsorption studies, inflammatory marker analysis, and in vivo implantation models. These comprehensive assessment approaches help identify potential adverse reactions and optimize formulations before clinical application. Long-term biocompatibility monitoring techniques, including non-invasive imaging and biomarker detection, provide crucial information about the integration and performance of hydrogels in biological environments over extended periods.Expand Specific Solutions
Key Industry Players in Biocompatible Hydrogel Development
The injectable hydrogel biocompatibility standards market is currently in a growth phase, with increasing adoption across medical applications. Market size is expanding rapidly due to rising demand in drug delivery, tissue engineering, and regenerative medicine sectors. From a technical maturity perspective, the field shows varied development levels among key players. Academic institutions like Northwestern University, Sichuan University, and Fudan University are driving fundamental research, while companies such as Laboratoires Vivacy, Contraline, and Ocular Therapeutix are commercializing specific applications. Johnson & Johnson Vision Care and Alkermes represent established pharmaceutical players integrating hydrogel technologies into their portfolios. SentryX and Rochal Technologies exemplify emerging companies with specialized hydrogel innovations. The regulatory landscape remains complex, with standards still evolving as the technology advances from research to clinical implementation.
Northwestern University
Technical Solution: Northwestern University has pioneered innovative injectable hydrogel technologies through its Biomedical Engineering department. Their research team has developed a self-assembling peptide amphiphile (PA) hydrogel system that forms nanofibrous structures mimicking natural extracellular matrix. These hydrogels incorporate bioactive epitopes that promote cell adhesion, proliferation, and differentiation. Northwestern's approach includes rigorous biocompatibility assessment protocols that evaluate both short-term inflammatory responses and long-term tissue integration. Their injectable hydrogels have demonstrated particular promise in regenerative medicine applications, including spinal cord repair and cardiac tissue regeneration. The university has established comprehensive testing standards that include in vitro cytotoxicity assays using multiple cell lines, protein adsorption studies, and in vivo biocompatibility assessments in various animal models. Their research has advanced the understanding of hydrogel-tissue interactions, particularly regarding the importance of mechanical properties and degradation kinetics in determining biocompatibility outcomes.
Strengths: Cutting-edge research in self-assembling peptide hydrogels; strong focus on biomimetic approaches; extensive academic publication record establishing scientific credibility. Weaknesses: As an academic institution, faces challenges in scaling up manufacturing and commercialization; research-focused approach may require industrial partnerships for clinical translation.
Johnson & Johnson Vision Care, Inc.
Technical Solution: Johnson & Johnson Vision Care has developed advanced biocompatible hydrogel technologies for ophthalmic applications, particularly for contact lenses and injectable ocular treatments. Their ACUVUE® OASYS with Transitions™ utilizes a proprietary senofilcon A hydrogel material that incorporates photochromic molecules. For injectable applications, they've developed crosslinked hyaluronic acid-based hydrogels with enhanced viscoelastic properties for intraocular procedures. The company implements comprehensive biocompatibility testing protocols that exceed ISO 10993 requirements, including specialized ocular biocompatibility assessments such as corneal epithelial barrier function tests and tear film interaction studies. Their hydrogel formulations undergo rigorous evaluation for protein adsorption, inflammatory response, and long-term tissue compatibility. J&J has established proprietary manufacturing processes that ensure consistent hydrogel properties with minimal batch-to-batch variation, critical for meeting stringent medical device regulations.
Strengths: Extensive R&D resources and manufacturing infrastructure; comprehensive biocompatibility testing capabilities; strong regulatory expertise and established quality management systems. Weaknesses: Primary focus on ophthalmic applications may limit broader medical applications; complex corporate structure may slow innovation compared to smaller, more agile competitors.
Critical Patents and Research in Hydrogel Biocompatibility
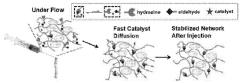
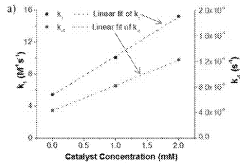
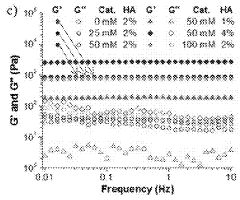
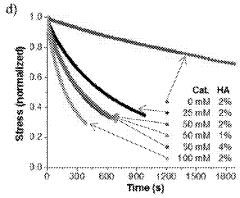
Injectable and stable hydrogels with dynamic properties modulated by biocompatible catalysts
PatentActiveUS20180104348A1
Innovation
- A biocompatible organic catalyst is used to modulate the dynamic properties of hyaluronic acid-based hydrogels through dynamic covalent hydrazone crosslinking, accelerating crosslink exchange during injection and slowing it down post-injection to achieve both high injectability and stability.
Biocompatible hydrogel comprising hyaluronic acid and polyethylene glycol
PatentPendingUS20220280684A1
Innovation
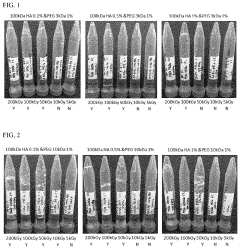
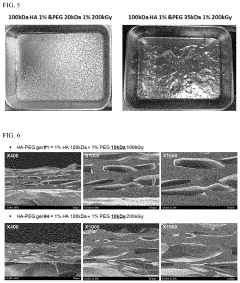
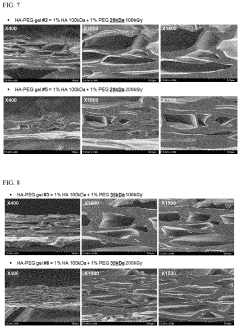
- A biocompatible hydrogel is created through inter-molecular and/or intra-molecular cross-linking of hyaluronic acid and polyethylene glycol using radiation without external cross-linking agents, achieved by preparing a solution of hyaluronic acid and polyethylene glycol in water and inducing cross-linking with radiation.
Regulatory Framework for Injectable Biomaterials
The regulatory landscape for injectable biomaterials is governed by a complex framework of international standards, national regulations, and industry guidelines. The FDA in the United States classifies most injectable hydrogels as Class II or III medical devices, requiring either 510(k) clearance or Premarket Approval (PMA) depending on their intended use and risk profile. The regulatory pathway is significantly influenced by the hydrogel's composition, degradation profile, and therapeutic claims.
In the European Union, injectable biomaterials fall under the Medical Device Regulation (MDR) or the newer In Vitro Diagnostic Regulation (IVDR), with classification based on risk categories. The CE marking process requires manufacturers to demonstrate compliance with Essential Requirements, including biocompatibility testing according to ISO 10993 standards. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implements a similar risk-based approach but with distinct requirements for foreign manufacturers.
Biocompatibility assessment for injectable hydrogels typically follows ISO 10993-1 guidelines, which outline a systematic approach to biological evaluation. This includes cytotoxicity (ISO 10993-5), sensitization (ISO 10993-10), and genotoxicity testing (ISO 10993-3). For hydrogels intended for long-term implantation, additional tests for carcinogenicity and reproductive toxicity may be required.
The ASTM F2900 standard specifically addresses the characterization of hydrogels used in medical applications, providing guidance on physical, chemical, and biological property testing. This standard complements the broader ISO framework by addressing the unique properties of hydrogels, such as swelling behavior, mechanical properties, and degradation kinetics.
Regulatory bodies increasingly emphasize a risk-based approach to biocompatibility testing, allowing manufacturers to justify the omission of certain tests based on material characterization data and existing scientific literature. This approach, outlined in FDA's guidance document "Use of International Standard ISO 10993-1," aims to reduce unnecessary animal testing while maintaining safety standards.
Post-market surveillance requirements for injectable biomaterials have become more stringent in recent years, with regulatory bodies requiring manufacturers to implement comprehensive vigilance systems. The EU MDR, for instance, mandates more robust post-market clinical follow-up and periodic safety update reports for higher-risk devices, including many injectable hydrogels.
Harmonization efforts between major regulatory bodies through the International Medical Device Regulators Forum (IMDRF) have led to more consistent approaches to biocompatibility assessment globally, though significant regional differences remain. These differences can present challenges for manufacturers seeking multi-market approval for novel injectable hydrogel technologies.
In the European Union, injectable biomaterials fall under the Medical Device Regulation (MDR) or the newer In Vitro Diagnostic Regulation (IVDR), with classification based on risk categories. The CE marking process requires manufacturers to demonstrate compliance with Essential Requirements, including biocompatibility testing according to ISO 10993 standards. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implements a similar risk-based approach but with distinct requirements for foreign manufacturers.
Biocompatibility assessment for injectable hydrogels typically follows ISO 10993-1 guidelines, which outline a systematic approach to biological evaluation. This includes cytotoxicity (ISO 10993-5), sensitization (ISO 10993-10), and genotoxicity testing (ISO 10993-3). For hydrogels intended for long-term implantation, additional tests for carcinogenicity and reproductive toxicity may be required.
The ASTM F2900 standard specifically addresses the characterization of hydrogels used in medical applications, providing guidance on physical, chemical, and biological property testing. This standard complements the broader ISO framework by addressing the unique properties of hydrogels, such as swelling behavior, mechanical properties, and degradation kinetics.
Regulatory bodies increasingly emphasize a risk-based approach to biocompatibility testing, allowing manufacturers to justify the omission of certain tests based on material characterization data and existing scientific literature. This approach, outlined in FDA's guidance document "Use of International Standard ISO 10993-1," aims to reduce unnecessary animal testing while maintaining safety standards.
Post-market surveillance requirements for injectable biomaterials have become more stringent in recent years, with regulatory bodies requiring manufacturers to implement comprehensive vigilance systems. The EU MDR, for instance, mandates more robust post-market clinical follow-up and periodic safety update reports for higher-risk devices, including many injectable hydrogels.
Harmonization efforts between major regulatory bodies through the International Medical Device Regulators Forum (IMDRF) have led to more consistent approaches to biocompatibility assessment globally, though significant regional differences remain. These differences can present challenges for manufacturers seeking multi-market approval for novel injectable hydrogel technologies.
Long-term Safety Monitoring Protocols
Long-term safety monitoring of injectable hydrogels represents a critical component in establishing comprehensive biocompatibility standards. Current protocols typically extend monitoring periods from 6 months to 5 years post-implantation, depending on the hydrogel's degradation profile and intended clinical application. These monitoring frameworks must account for both local tissue responses and potential systemic effects that may manifest only after extended periods.
Standard monitoring protocols incorporate regular imaging assessments using MRI, CT, or ultrasound to evaluate hydrogel integrity, degradation patterns, and host tissue integration. These imaging techniques allow for non-invasive longitudinal tracking of material performance without necessitating additional surgical interventions. Advanced imaging protocols now include contrast-enhanced techniques specifically optimized for hydrogel visualization within biological environments.
Biochemical monitoring constitutes another essential element, involving periodic blood and urine analyses to detect potential degradation products, inflammatory markers, or immune responses. Established protocols recommend baseline measurements prior to implantation, followed by assessments at 1, 3, 6, 12 months, and annually thereafter. Parameters typically monitored include complete blood count, liver and kidney function tests, and specific inflammatory biomarkers such as IL-6, TNF-α, and C-reactive protein.
Histopathological evaluation remains the gold standard for assessing long-term tissue compatibility. This requires standardized protocols for tissue sampling, processing, and evaluation using both conventional histology and immunohistochemistry techniques. The FDA and ISO guidelines specify evaluation criteria focusing on chronic inflammation, fibrotic encapsulation, neovascularization patterns, and cell phenotype characterization at the implant interface.
Patient-reported outcomes have gained increasing prominence in safety monitoring protocols, with validated assessment tools now incorporated into standard follow-up procedures. These instruments capture subjective experiences related to pain, functional limitations, and quality of life metrics that may indicate subtle adverse effects not detectable through conventional clinical assessments.
Emerging technologies in safety monitoring include implantable biosensors capable of real-time monitoring of local tissue environment parameters such as pH, oxygen tension, and specific biomarkers. These advanced monitoring approaches promise to revolutionize long-term safety assessment by providing continuous data streams rather than periodic snapshots, potentially enabling earlier detection of adverse responses before clinical manifestation.
Regulatory frameworks increasingly emphasize the importance of centralized adverse event reporting systems specific to biomaterials, facilitating the aggregation of long-term safety data across multiple clinical sites and applications. This systematic approach to data collection supports continuous refinement of safety standards and monitoring protocols based on real-world evidence.
Standard monitoring protocols incorporate regular imaging assessments using MRI, CT, or ultrasound to evaluate hydrogel integrity, degradation patterns, and host tissue integration. These imaging techniques allow for non-invasive longitudinal tracking of material performance without necessitating additional surgical interventions. Advanced imaging protocols now include contrast-enhanced techniques specifically optimized for hydrogel visualization within biological environments.
Biochemical monitoring constitutes another essential element, involving periodic blood and urine analyses to detect potential degradation products, inflammatory markers, or immune responses. Established protocols recommend baseline measurements prior to implantation, followed by assessments at 1, 3, 6, 12 months, and annually thereafter. Parameters typically monitored include complete blood count, liver and kidney function tests, and specific inflammatory biomarkers such as IL-6, TNF-α, and C-reactive protein.
Histopathological evaluation remains the gold standard for assessing long-term tissue compatibility. This requires standardized protocols for tissue sampling, processing, and evaluation using both conventional histology and immunohistochemistry techniques. The FDA and ISO guidelines specify evaluation criteria focusing on chronic inflammation, fibrotic encapsulation, neovascularization patterns, and cell phenotype characterization at the implant interface.
Patient-reported outcomes have gained increasing prominence in safety monitoring protocols, with validated assessment tools now incorporated into standard follow-up procedures. These instruments capture subjective experiences related to pain, functional limitations, and quality of life metrics that may indicate subtle adverse effects not detectable through conventional clinical assessments.
Emerging technologies in safety monitoring include implantable biosensors capable of real-time monitoring of local tissue environment parameters such as pH, oxygen tension, and specific biomarkers. These advanced monitoring approaches promise to revolutionize long-term safety assessment by providing continuous data streams rather than periodic snapshots, potentially enabling earlier detection of adverse responses before clinical manifestation.
Regulatory frameworks increasingly emphasize the importance of centralized adverse event reporting systems specific to biomaterials, facilitating the aggregation of long-term safety data across multiple clinical sites and applications. This systematic approach to data collection supports continuous refinement of safety standards and monitoring protocols based on real-world evidence.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!