The Effectiveness of Injectable Hydrogel in Hemostatic Agents
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Injectable Hydrogel Hemostatic Technology Background and Objectives
Injectable hydrogel hemostatic agents represent a significant advancement in the field of wound management and surgical interventions. The development of these materials can be traced back to the early 2000s when researchers began exploring biocompatible polymers capable of rapid gelation upon contact with blood or tissue fluids. This technology evolved from traditional hemostatic approaches such as mechanical compression, thermal cautery, and topical agents, which often presented limitations in efficacy and application versatility.
The evolution of injectable hydrogels has been driven by increasing surgical complexity and the need for minimally invasive procedures. Early formulations focused primarily on natural polymers like chitosan, alginate, and fibrin, while recent advancements have incorporated synthetic materials and hybrid compositions to enhance performance characteristics. The integration of nanotechnology and biomimetic approaches has further refined these systems over the past decade.
Current injectable hydrogel hemostatic technologies aim to address several critical challenges in modern medicine. These include achieving rapid hemostasis in complex surgical scenarios, managing bleeding in patients with coagulopathies, providing effective solutions for battlefield trauma, and developing hemostatic agents suitable for laparoscopic and robotic surgical applications. The ideal injectable hydrogel must balance immediate efficacy with biocompatibility and eventual biodegradation.
The technical objectives for advanced injectable hydrogel hemostatic agents encompass multiple performance parameters. These include rapid gelation time (typically under 30 seconds), strong adhesion to wet tissue surfaces, effective sealing of irregular wound geometries, sufficient mechanical strength to withstand physiological pressures, and controlled biodegradation profiles. Additionally, these materials must demonstrate minimal inflammatory response, absence of cytotoxicity, and compatibility with the wound healing process.
Recent technological trends indicate a shift toward multifunctional injectable hydrogels that combine hemostatic properties with antimicrobial activity, drug delivery capabilities, and tissue regeneration promotion. The incorporation of bioactive components such as growth factors, stem cells, and therapeutic agents represents an emerging frontier in this field, potentially transforming these materials from passive hemostatic agents to active participants in the wound healing cascade.
The global research landscape shows accelerating interest in injectable hydrogel hemostatics, with significant contributions from academic institutions and medical device companies in North America, Europe, and East Asia. Publication trends indicate a compound annual growth rate of approximately 15% in research output over the past five years, highlighting the increasing recognition of this technology's potential to address unmet clinical needs in hemostasis management.
The evolution of injectable hydrogels has been driven by increasing surgical complexity and the need for minimally invasive procedures. Early formulations focused primarily on natural polymers like chitosan, alginate, and fibrin, while recent advancements have incorporated synthetic materials and hybrid compositions to enhance performance characteristics. The integration of nanotechnology and biomimetic approaches has further refined these systems over the past decade.
Current injectable hydrogel hemostatic technologies aim to address several critical challenges in modern medicine. These include achieving rapid hemostasis in complex surgical scenarios, managing bleeding in patients with coagulopathies, providing effective solutions for battlefield trauma, and developing hemostatic agents suitable for laparoscopic and robotic surgical applications. The ideal injectable hydrogel must balance immediate efficacy with biocompatibility and eventual biodegradation.
The technical objectives for advanced injectable hydrogel hemostatic agents encompass multiple performance parameters. These include rapid gelation time (typically under 30 seconds), strong adhesion to wet tissue surfaces, effective sealing of irregular wound geometries, sufficient mechanical strength to withstand physiological pressures, and controlled biodegradation profiles. Additionally, these materials must demonstrate minimal inflammatory response, absence of cytotoxicity, and compatibility with the wound healing process.
Recent technological trends indicate a shift toward multifunctional injectable hydrogels that combine hemostatic properties with antimicrobial activity, drug delivery capabilities, and tissue regeneration promotion. The incorporation of bioactive components such as growth factors, stem cells, and therapeutic agents represents an emerging frontier in this field, potentially transforming these materials from passive hemostatic agents to active participants in the wound healing cascade.
The global research landscape shows accelerating interest in injectable hydrogel hemostatics, with significant contributions from academic institutions and medical device companies in North America, Europe, and East Asia. Publication trends indicate a compound annual growth rate of approximately 15% in research output over the past five years, highlighting the increasing recognition of this technology's potential to address unmet clinical needs in hemostasis management.
Market Analysis for Advanced Hemostatic Solutions
The global market for advanced hemostatic solutions has experienced significant growth in recent years, driven by increasing surgical procedures, trauma cases, and the rising prevalence of bleeding disorders. The injectable hydrogel segment within this market has emerged as a particularly promising area, with a compound annual growth rate exceeding traditional hemostatic agents. This growth trajectory is expected to continue as healthcare systems worldwide seek more effective and efficient bleeding control solutions.
Surgical applications represent the largest market segment for injectable hydrogel hemostatic agents, particularly in cardiovascular, orthopedic, and general surgeries where controlling bleeding quickly and effectively is critical. The trauma management sector follows closely, with emergency departments and military medical units showing increased adoption of advanced hemostatic technologies including injectable hydrogels.
Geographically, North America dominates the market due to its advanced healthcare infrastructure, high healthcare expenditure, and early adoption of innovative medical technologies. Europe represents the second-largest market, with countries like Germany, France, and the UK leading in adoption rates. The Asia-Pacific region is witnessing the fastest growth, attributed to improving healthcare infrastructure, increasing surgical procedures, and rising awareness about advanced hemostatic solutions.
Key market drivers include the growing geriatric population requiring surgical interventions, increasing prevalence of chronic diseases necessitating surgical procedures, and rising incidence of traumatic injuries. Additionally, the shift toward minimally invasive surgeries has created demand for specialized hemostatic agents that can be delivered through small incisions or endoscopically, where injectable hydrogels offer significant advantages.
Market restraints include the high cost of advanced hemostatic agents compared to conventional options, stringent regulatory approval processes, and limited awareness in developing regions. Reimbursement challenges also pose significant barriers to market penetration in certain regions.
Customer segmentation reveals that large hospitals and academic medical centers are the primary adopters of injectable hydrogel hemostatic agents, while smaller healthcare facilities tend to rely on traditional options due to cost considerations. Military medical units represent another significant customer segment, particularly for combat-ready hemostatic solutions.
The competitive landscape features both established medical device companies and innovative startups. Major players have been actively pursuing strategic acquisitions and partnerships to strengthen their product portfolios and expand market reach. This consolidation trend is expected to continue as the market matures and competition intensifies.
Surgical applications represent the largest market segment for injectable hydrogel hemostatic agents, particularly in cardiovascular, orthopedic, and general surgeries where controlling bleeding quickly and effectively is critical. The trauma management sector follows closely, with emergency departments and military medical units showing increased adoption of advanced hemostatic technologies including injectable hydrogels.
Geographically, North America dominates the market due to its advanced healthcare infrastructure, high healthcare expenditure, and early adoption of innovative medical technologies. Europe represents the second-largest market, with countries like Germany, France, and the UK leading in adoption rates. The Asia-Pacific region is witnessing the fastest growth, attributed to improving healthcare infrastructure, increasing surgical procedures, and rising awareness about advanced hemostatic solutions.
Key market drivers include the growing geriatric population requiring surgical interventions, increasing prevalence of chronic diseases necessitating surgical procedures, and rising incidence of traumatic injuries. Additionally, the shift toward minimally invasive surgeries has created demand for specialized hemostatic agents that can be delivered through small incisions or endoscopically, where injectable hydrogels offer significant advantages.
Market restraints include the high cost of advanced hemostatic agents compared to conventional options, stringent regulatory approval processes, and limited awareness in developing regions. Reimbursement challenges also pose significant barriers to market penetration in certain regions.
Customer segmentation reveals that large hospitals and academic medical centers are the primary adopters of injectable hydrogel hemostatic agents, while smaller healthcare facilities tend to rely on traditional options due to cost considerations. Military medical units represent another significant customer segment, particularly for combat-ready hemostatic solutions.
The competitive landscape features both established medical device companies and innovative startups. Major players have been actively pursuing strategic acquisitions and partnerships to strengthen their product portfolios and expand market reach. This consolidation trend is expected to continue as the market matures and competition intensifies.
Current Status and Challenges in Hemostatic Technology
Hemostatic technology has evolved significantly over the past decade, with injectable hydrogels emerging as promising candidates for effective bleeding control. Currently, the global market for hemostatic agents is dominated by traditional methods such as mechanical compression, topical agents, and surgical interventions. However, these conventional approaches often fall short in addressing uncontrolled hemorrhage in complex trauma scenarios, particularly in battlefield injuries and deep tissue wounds.
Injectable hydrogel-based hemostatic agents represent a cutting-edge solution that has gained considerable attention in recent years. These materials offer unique advantages including conformability to irregular wound geometries, minimally invasive application, and the ability to form in situ barriers against blood loss. Despite these promising attributes, several technical challenges persist in their widespread clinical adoption.
A significant limitation of current injectable hydrogels is their mechanical stability under high-pressure bleeding conditions. Many formulations exhibit inadequate adhesion to wet tissue surfaces, resulting in displacement during active hemorrhage. This challenge is particularly pronounced in arterial bleeding scenarios where blood pressure can easily dislodge the applied material before effective hemostasis is achieved.
Another critical challenge lies in the biocompatibility and biodegradation profiles of these materials. While rapid gelation is essential for effective hemostasis, the crosslinking mechanisms employed must not induce cytotoxicity or inflammatory responses. Balancing the competing requirements of fast gelation kinetics and biocompatibility remains a significant technical hurdle.
The scalability of production and shelf stability present additional obstacles. Many advanced hydrogel formulations require complex synthesis procedures and specialized storage conditions, limiting their practical deployment in emergency settings. Furthermore, regulatory approval pathways for these novel biomaterials are often lengthy and complex, slowing their transition from laboratory to clinical practice.
Geographically, research in injectable hydrogel hemostatic agents is concentrated primarily in North America, Europe, and East Asia, with the United States, China, and Japan leading patent filings in this domain. Academic-industry partnerships have accelerated in recent years, particularly in developing multifunctional hydrogels that combine hemostatic properties with antimicrobial or wound-healing capabilities.
The integration of nanotechnology with injectable hydrogels represents another frontier, with nanocomposite hydrogels demonstrating enhanced mechanical properties and bioactivity. However, concerns regarding the long-term safety of nanomaterials in vivo have tempered enthusiasm for their immediate clinical translation.
Cost-effectiveness remains a persistent challenge, as advanced hydrogel formulations typically involve expensive precursors and processing techniques. This economic barrier has limited their adoption in resource-constrained healthcare settings, creating a significant gap between technological capability and global accessibility.
Injectable hydrogel-based hemostatic agents represent a cutting-edge solution that has gained considerable attention in recent years. These materials offer unique advantages including conformability to irregular wound geometries, minimally invasive application, and the ability to form in situ barriers against blood loss. Despite these promising attributes, several technical challenges persist in their widespread clinical adoption.
A significant limitation of current injectable hydrogels is their mechanical stability under high-pressure bleeding conditions. Many formulations exhibit inadequate adhesion to wet tissue surfaces, resulting in displacement during active hemorrhage. This challenge is particularly pronounced in arterial bleeding scenarios where blood pressure can easily dislodge the applied material before effective hemostasis is achieved.
Another critical challenge lies in the biocompatibility and biodegradation profiles of these materials. While rapid gelation is essential for effective hemostasis, the crosslinking mechanisms employed must not induce cytotoxicity or inflammatory responses. Balancing the competing requirements of fast gelation kinetics and biocompatibility remains a significant technical hurdle.
The scalability of production and shelf stability present additional obstacles. Many advanced hydrogel formulations require complex synthesis procedures and specialized storage conditions, limiting their practical deployment in emergency settings. Furthermore, regulatory approval pathways for these novel biomaterials are often lengthy and complex, slowing their transition from laboratory to clinical practice.
Geographically, research in injectable hydrogel hemostatic agents is concentrated primarily in North America, Europe, and East Asia, with the United States, China, and Japan leading patent filings in this domain. Academic-industry partnerships have accelerated in recent years, particularly in developing multifunctional hydrogels that combine hemostatic properties with antimicrobial or wound-healing capabilities.
The integration of nanotechnology with injectable hydrogels represents another frontier, with nanocomposite hydrogels demonstrating enhanced mechanical properties and bioactivity. However, concerns regarding the long-term safety of nanomaterials in vivo have tempered enthusiasm for their immediate clinical translation.
Cost-effectiveness remains a persistent challenge, as advanced hydrogel formulations typically involve expensive precursors and processing techniques. This economic barrier has limited their adoption in resource-constrained healthcare settings, creating a significant gap between technological capability and global accessibility.
Current Injectable Hydrogel Hemostatic Solutions
01 Composition and formulation of injectable hydrogels
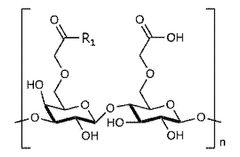
Injectable hydrogels can be formulated with various polymers and cross-linking agents to achieve desired mechanical properties and biocompatibility. These formulations often include natural polymers like hyaluronic acid, collagen, or synthetic polymers that can form a gel-like structure upon injection. The composition can be tailored to control degradation rates, swelling behavior, and mechanical strength, which directly impacts the effectiveness of the hydrogel for specific applications.- Composition and formulation of injectable hydrogels: Injectable hydrogels can be formulated with various polymers and cross-linking agents to achieve desired mechanical properties and biocompatibility. These formulations often include natural polymers like hyaluronic acid, collagen, or synthetic polymers that can form a gel matrix upon injection. The composition can be tailored to control degradation rate, swelling behavior, and drug release kinetics, which directly impacts the effectiveness of the hydrogel for specific medical applications.
- Drug delivery applications of injectable hydrogels: Injectable hydrogels serve as effective drug delivery systems due to their ability to encapsulate therapeutic agents and provide controlled release. These hydrogels can be designed to respond to specific stimuli such as pH, temperature, or enzymatic activity, allowing for targeted and sustained release of pharmaceuticals. The effectiveness of drug delivery is enhanced by the hydrogel's ability to protect drugs from degradation and maintain therapeutic concentrations at the target site over extended periods.
- Tissue engineering and regenerative medicine applications: Injectable hydrogels provide an effective scaffold for tissue engineering and regenerative medicine applications. They can be loaded with cells, growth factors, and other bioactive molecules to promote tissue regeneration. The three-dimensional network of hydrogels mimics the extracellular matrix, supporting cell adhesion, proliferation, and differentiation. These properties make injectable hydrogels particularly effective for wound healing, cartilage repair, and other tissue regeneration applications.
- Mechanical properties and stability of injectable hydrogels: The effectiveness of injectable hydrogels is significantly influenced by their mechanical properties and stability. Factors such as stiffness, elasticity, and shear-thinning behavior determine how well the hydrogel maintains its structure after injection. Advanced formulations incorporate reinforcement strategies like nanoparticle inclusion or dual-network systems to enhance mechanical strength while maintaining injectability. The stability of hydrogels in physiological environments affects their longevity and functional performance in medical applications.
- Biocompatibility and biodegradation of injectable hydrogels: The biocompatibility and biodegradation profile of injectable hydrogels are crucial for their clinical effectiveness. Hydrogels must not elicit adverse immune responses when introduced into the body and should degrade at rates appropriate for their intended application. The degradation products must be non-toxic and easily cleared from the body. Innovations in this area include hydrogels with tunable degradation rates and those incorporating anti-inflammatory or immunomodulatory components to enhance biocompatibility and overall therapeutic effectiveness.
02 Drug delivery applications of injectable hydrogels
Injectable hydrogels serve as effective drug delivery systems due to their ability to encapsulate therapeutic agents and provide controlled release. These hydrogels can be designed to respond to specific stimuli such as pH, temperature, or enzymatic activity, allowing for targeted and sustained release of drugs. The effectiveness of drug delivery is enhanced by the hydrogel's ability to protect the therapeutic agents from degradation and maintain their bioactivity until reaching the target site.Expand Specific Solutions03 Tissue engineering and regenerative medicine applications
Injectable hydrogels provide a three-dimensional scaffold for cell growth and tissue regeneration. They can be loaded with cells, growth factors, and other bioactive molecules to promote tissue repair and regeneration. The effectiveness of these hydrogels in tissue engineering depends on their ability to mimic the extracellular matrix, support cell adhesion and proliferation, and integrate with the surrounding tissue. These properties make them particularly useful for cartilage repair, wound healing, and other regenerative applications.Expand Specific Solutions04 Biocompatibility and biodegradability characteristics
The effectiveness of injectable hydrogels is significantly influenced by their biocompatibility and biodegradability. Hydrogels that elicit minimal immune response and integrate well with host tissues show better therapeutic outcomes. The degradation rate can be engineered to match the rate of tissue regeneration or drug release requirements. Materials that break down into non-toxic byproducts and are eventually eliminated from the body are preferred for clinical applications, ensuring both safety and efficacy.Expand Specific Solutions05 Smart and responsive injectable hydrogel systems
Advanced injectable hydrogels incorporate responsive elements that can adapt to physiological conditions or external stimuli. These smart systems can change their properties in response to temperature, pH, light, or specific biomolecules, enhancing their therapeutic effectiveness. Some hydrogels can self-heal after injection, improving their mechanical stability and longevity. Others can form in situ upon injection through various cross-linking mechanisms, allowing for minimally invasive delivery while providing robust mechanical support once formed.Expand Specific Solutions
Key Industry Players in Hemostatic Materials
The injectable hydrogel hemostatic agent market is currently in a growth phase, with increasing adoption across surgical and emergency medicine applications. The global market size for hemostatic agents is projected to reach approximately $5 billion by 2027, with injectable hydrogels representing a rapidly expanding segment due to their superior efficacy and ease of application in difficult-to-access bleeding sites. Technologically, the field is advancing from early-stage development toward clinical maturity, with companies like Baxter International, Ferrosan Medical Devices, and Genzyme leading commercial applications. Research institutions including Shenzhen Institutes of Advanced Technology, Tianjin University, and Seoul National University Hospital are driving innovation through novel formulations with enhanced biocompatibility and hemostatic properties. Regional players such as Theracion Biomedical and Shenzhen Huanuo Biotechnology are emerging with specialized applications, indicating the technology's global expansion and diversification.
Ferrosan Medical Devices A/S
Technical Solution: Ferrosan Medical Devices has pioneered the Surgiflo® Hemostatic Matrix, an advanced injectable hydrogel system utilizing porcine gelatin particles that form a matrix at the bleeding site. The technology incorporates a patented thrombin-delivery mechanism that activates upon contact with blood, accelerating the natural clotting cascade. Their proprietary manufacturing process ensures consistent particle size distribution (20-50 μm), optimizing surface area for platelet activation while maintaining injectability through fine-gauge needles. Recent innovations include temperature-stable formulations that eliminate cold-chain requirements and antimicrobial-infused variants that reduce infection risk. Clinical evaluations demonstrate hemostasis achievement in 95% of cases within 3 minutes of application, with the matrix being completely absorbed within 4-6 weeks without fibrotic tissue formation.
Strengths: Excellent flowability allowing access to difficult-to-reach bleeding sites, compatibility with minimally invasive procedures, and complete bioabsorption without foreign body reaction. Weaknesses: Animal-derived components may raise concerns in certain patient populations, and the product requires reconstitution which adds preparation time in emergency situations.
The Shenzhen Institutes of Advanced Technology
Technical Solution: The Shenzhen Institutes of Advanced Technology has developed a multifunctional injectable hydrogel hemostatic platform based on oxidized alginate and chitosan derivatives. Their technology employs a rapid in-situ crosslinking mechanism triggered by Schiff base formation between aldehyde groups on oxidized alginate and amino groups on chitosan, achieving gelation within 5-10 seconds upon mixing. The hydrogel exhibits excellent adhesion to tissue surfaces (adhesion strength >15 kPa) even in the presence of blood, preventing displacement during active bleeding. A key innovation is the incorporation of calcium phosphate nanoparticles that serve dual functions: reinforcing the hydrogel network while simultaneously releasing calcium ions that activate the coagulation cascade. Preclinical studies demonstrate that this formulation reduces clotting time by approximately 40% compared to conventional methods and maintains hemostatic efficacy even in heparinized models. The material undergoes controlled degradation over 2-4 weeks, with degradation products that stimulate tissue regeneration through upregulation of growth factors including VEGF and TGF-β.
Strengths: Extremely rapid gelation suitable for emergency applications, strong tissue adhesion properties, and additional wound healing promotion effects beyond hemostasis. Weaknesses: Complex preparation process that may limit point-of-care applications, and potential challenges in scaling up production while maintaining consistent crosslinking kinetics.
Critical Patents and Research in Hemostatic Hydrogels
Marine-derived gelatin-based injectable hydrogel hemostatic agent, and use thereof and application method therefor
PatentWO2021237543A1
Innovation
- An injectable hydrogel hemostatic agent based on marine gelatin is used, which contains chemically modified marine gelatin, photo-cross-linked gel factor, tissue adhesion factor and photoinitiator. It achieves rapid curing through ultraviolet irradiation and has strong tissue adhesion. Adhesion and mechanical properties, and has degradable properties.
Hydrogel including phenol derivative-modified cellulose and use thereof
PatentWO2022216134A1
Innovation
- A hydrogel is created by modifying cellulose with phenol derivatives, such as dopamine or 5-hydroxydopamine, through substitution and cross-linking, which can be used for hemostasis, tissue adhesion, cell culture, and drug delivery, offering improved biocompatibility and biodegradability.
Biocompatibility and Safety Considerations
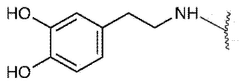
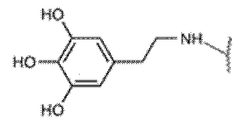
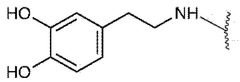
Biocompatibility represents a critical consideration in the development and application of injectable hydrogel hemostatic agents. These materials must interact with biological systems without eliciting adverse reactions, particularly when applied to open wounds or surgical sites. Current research indicates that hydrogels composed of natural polymers such as chitosan, alginate, and hyaluronic acid demonstrate superior biocompatibility profiles compared to synthetic alternatives. These natural polymers closely mimic the extracellular matrix, facilitating integration with host tissues and minimizing foreign body responses.
Safety evaluations for injectable hydrogels encompass multiple dimensions, including cytotoxicity, immunogenicity, and long-term tissue effects. Comprehensive in vitro and in vivo testing protocols have been established to assess these parameters. Recent studies have shown that hydrogels with controlled degradation rates significantly reduce the risk of chronic inflammation and granuloma formation, which were common complications in earlier formulations. The degradation byproducts must be non-toxic and easily metabolized or excreted from the body.
Immunological considerations present another crucial aspect of hydrogel safety profiles. Some synthetic polymers and cross-linking agents can trigger immune responses, leading to delayed healing or rejection. Advanced immunocompatibility testing now includes evaluation of complement activation, macrophage polarization, and T-cell responses to better predict clinical outcomes. Notably, PEG-based hydrogels have demonstrated reduced immunogenicity through their "stealth" properties that minimize protein adsorption and subsequent immune recognition.
Regulatory frameworks for hemostatic hydrogels have evolved substantially, with the FDA and EMA establishing specific guidelines for biocompatibility assessment. These include ISO 10993 standards for biological evaluation of medical devices, with particular emphasis on hemocompatibility for blood-contacting applications. Manufacturers must now provide comprehensive safety data packages that address both immediate and long-term biocompatibility concerns.
The potential for systemic effects represents an emerging area of safety research. While injectable hydrogels are typically designed for localized action, components may enter the circulation, particularly in highly vascularized wound beds. Recent pharmacokinetic studies have focused on tracking hydrogel components and degradation products to ensure they do not accumulate in vital organs or disrupt normal physiological functions. Advanced imaging techniques such as fluorescent labeling and MRI contrast enhancement have facilitated this monitoring process.
Sterilization compatibility presents another critical consideration, as many hydrogel formulations contain temperature-sensitive components that may degrade during conventional sterilization processes. Radiation sterilization methods have shown promise for preserving hydrogel integrity while ensuring microbial safety, though careful validation is required to prevent formation of toxic byproducts or alterations in mechanical properties that could compromise hemostatic efficacy.
Safety evaluations for injectable hydrogels encompass multiple dimensions, including cytotoxicity, immunogenicity, and long-term tissue effects. Comprehensive in vitro and in vivo testing protocols have been established to assess these parameters. Recent studies have shown that hydrogels with controlled degradation rates significantly reduce the risk of chronic inflammation and granuloma formation, which were common complications in earlier formulations. The degradation byproducts must be non-toxic and easily metabolized or excreted from the body.
Immunological considerations present another crucial aspect of hydrogel safety profiles. Some synthetic polymers and cross-linking agents can trigger immune responses, leading to delayed healing or rejection. Advanced immunocompatibility testing now includes evaluation of complement activation, macrophage polarization, and T-cell responses to better predict clinical outcomes. Notably, PEG-based hydrogels have demonstrated reduced immunogenicity through their "stealth" properties that minimize protein adsorption and subsequent immune recognition.
Regulatory frameworks for hemostatic hydrogels have evolved substantially, with the FDA and EMA establishing specific guidelines for biocompatibility assessment. These include ISO 10993 standards for biological evaluation of medical devices, with particular emphasis on hemocompatibility for blood-contacting applications. Manufacturers must now provide comprehensive safety data packages that address both immediate and long-term biocompatibility concerns.
The potential for systemic effects represents an emerging area of safety research. While injectable hydrogels are typically designed for localized action, components may enter the circulation, particularly in highly vascularized wound beds. Recent pharmacokinetic studies have focused on tracking hydrogel components and degradation products to ensure they do not accumulate in vital organs or disrupt normal physiological functions. Advanced imaging techniques such as fluorescent labeling and MRI contrast enhancement have facilitated this monitoring process.
Sterilization compatibility presents another critical consideration, as many hydrogel formulations contain temperature-sensitive components that may degrade during conventional sterilization processes. Radiation sterilization methods have shown promise for preserving hydrogel integrity while ensuring microbial safety, though careful validation is required to prevent formation of toxic byproducts or alterations in mechanical properties that could compromise hemostatic efficacy.
Regulatory Approval Pathways for Hemostatic Biomaterials
The regulatory landscape for hemostatic biomaterials, particularly injectable hydrogels, involves complex approval pathways that vary significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies most hemostatic agents as Class III medical devices or combination products, requiring premarket approval (PMA) with substantial clinical evidence demonstrating safety and efficacy. Injectable hydrogel hemostatic agents typically follow the PMA pathway due to their implantable nature and critical intended use in bleeding control.
The European Union, under the Medical Device Regulation (MDR), categorizes these products as Class III devices, necessitating conformity assessment through a notified body and comprehensive clinical evaluation. The CE marking process requires manufacturers to implement a quality management system and conduct post-market surveillance, with specific requirements for novel biomaterials like injectable hydrogels.
In Asia, regulatory frameworks show considerable variation. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake designation for innovative medical technologies, potentially expediting approval for novel hemostatic hydrogels. China's National Medical Products Administration (NMPA) has recently reformed its approval process, introducing special review pathways for innovative medical devices, though requirements remain stringent for implantable materials.
For injectable hydrogel hemostatic agents specifically, regulatory bodies focus on several critical aspects: biocompatibility testing according to ISO 10993 standards, hemostatic efficacy validation through standardized bleeding models, degradation profile and tissue integration assessment, and sterility assurance. The FDA's guidance on hemostatic devices emphasizes the importance of demonstrating both immediate hemostatic efficacy and long-term safety outcomes.
Manufacturers pursuing multi-market approval face challenges in harmonizing their development strategies to meet divergent regulatory requirements. The International Medical Device Regulators Forum (IMDRF) has made progress in standardizing certain aspects of medical device regulation, but significant differences persist in clinical evidence requirements and risk classification methodologies for hemostatic biomaterials.
Emerging regulatory considerations for injectable hydrogels include the evaluation of their interaction with other hemostatic modalities, their performance in patients with coagulopathies, and their long-term tissue effects. Regulatory agencies are increasingly requesting real-world evidence and patient-reported outcomes to supplement traditional clinical endpoints for these advanced hemostatic technologies.
The European Union, under the Medical Device Regulation (MDR), categorizes these products as Class III devices, necessitating conformity assessment through a notified body and comprehensive clinical evaluation. The CE marking process requires manufacturers to implement a quality management system and conduct post-market surveillance, with specific requirements for novel biomaterials like injectable hydrogels.
In Asia, regulatory frameworks show considerable variation. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake designation for innovative medical technologies, potentially expediting approval for novel hemostatic hydrogels. China's National Medical Products Administration (NMPA) has recently reformed its approval process, introducing special review pathways for innovative medical devices, though requirements remain stringent for implantable materials.
For injectable hydrogel hemostatic agents specifically, regulatory bodies focus on several critical aspects: biocompatibility testing according to ISO 10993 standards, hemostatic efficacy validation through standardized bleeding models, degradation profile and tissue integration assessment, and sterility assurance. The FDA's guidance on hemostatic devices emphasizes the importance of demonstrating both immediate hemostatic efficacy and long-term safety outcomes.
Manufacturers pursuing multi-market approval face challenges in harmonizing their development strategies to meet divergent regulatory requirements. The International Medical Device Regulators Forum (IMDRF) has made progress in standardizing certain aspects of medical device regulation, but significant differences persist in clinical evidence requirements and risk classification methodologies for hemostatic biomaterials.
Emerging regulatory considerations for injectable hydrogels include the evaluation of their interaction with other hemostatic modalities, their performance in patients with coagulopathies, and their long-term tissue effects. Regulatory agencies are increasingly requesting real-world evidence and patient-reported outcomes to supplement traditional clinical endpoints for these advanced hemostatic technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!