Study of Injectable Hydrogel's Potential in Synthetic Biology
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Injectable Hydrogel Background and Objectives
Injectable hydrogels represent a revolutionary class of biomaterials that have gained significant attention in the scientific community over the past two decades. These materials combine the properties of hydrogels—three-dimensional networks of hydrophilic polymers capable of absorbing large amounts of water—with the ability to be delivered in a minimally invasive manner through injection. The evolution of injectable hydrogels has progressed from simple natural polymers to sophisticated synthetic and hybrid systems with tunable properties.
The field has witnessed remarkable growth since the early 2000s, with significant milestones including the development of stimuli-responsive hydrogels, self-healing systems, and bioactive formulations. Recent advances in polymer chemistry and materials science have enabled precise control over gelation kinetics, mechanical properties, and degradation profiles, making injectable hydrogels increasingly versatile platforms for various applications.
In the context of synthetic biology, injectable hydrogels present unique opportunities as they can serve as three-dimensional matrices for engineered biological systems. The intersection of these two fields represents a frontier with immense potential for innovation in healthcare, environmental remediation, and biomanufacturing. Synthetic biology principles can be applied to design hydrogels with programmable functionalities, while hydrogels can provide suitable microenvironments for synthetic biological circuits and engineered cells.
The primary objectives of exploring injectable hydrogels in synthetic biology include developing programmable biomaterials that can respond to biological signals, creating artificial cellular niches with defined biochemical and mechanical properties, and establishing delivery systems for engineered biological components. These objectives align with the broader goals of synthetic biology to design and construct novel biological parts, devices, and systems.
Furthermore, this technological convergence aims to address critical challenges in regenerative medicine, targeted drug delivery, biosensing, and the development of artificial organs. By combining the spatial control offered by hydrogels with the molecular precision of synthetic biology, researchers seek to create systems that can dynamically interact with biological environments in predetermined ways.
The ultimate goal is to develop injectable hydrogel platforms that can house synthetic biological circuits capable of performing complex functions such as sensing pathological conditions, producing therapeutic molecules on demand, or facilitating tissue regeneration through controlled release of growth factors and morphogens. This would represent a significant advancement in our ability to interface with and modulate biological systems for therapeutic and diagnostic purposes.
The field has witnessed remarkable growth since the early 2000s, with significant milestones including the development of stimuli-responsive hydrogels, self-healing systems, and bioactive formulations. Recent advances in polymer chemistry and materials science have enabled precise control over gelation kinetics, mechanical properties, and degradation profiles, making injectable hydrogels increasingly versatile platforms for various applications.
In the context of synthetic biology, injectable hydrogels present unique opportunities as they can serve as three-dimensional matrices for engineered biological systems. The intersection of these two fields represents a frontier with immense potential for innovation in healthcare, environmental remediation, and biomanufacturing. Synthetic biology principles can be applied to design hydrogels with programmable functionalities, while hydrogels can provide suitable microenvironments for synthetic biological circuits and engineered cells.
The primary objectives of exploring injectable hydrogels in synthetic biology include developing programmable biomaterials that can respond to biological signals, creating artificial cellular niches with defined biochemical and mechanical properties, and establishing delivery systems for engineered biological components. These objectives align with the broader goals of synthetic biology to design and construct novel biological parts, devices, and systems.
Furthermore, this technological convergence aims to address critical challenges in regenerative medicine, targeted drug delivery, biosensing, and the development of artificial organs. By combining the spatial control offered by hydrogels with the molecular precision of synthetic biology, researchers seek to create systems that can dynamically interact with biological environments in predetermined ways.
The ultimate goal is to develop injectable hydrogel platforms that can house synthetic biological circuits capable of performing complex functions such as sensing pathological conditions, producing therapeutic molecules on demand, or facilitating tissue regeneration through controlled release of growth factors and morphogens. This would represent a significant advancement in our ability to interface with and modulate biological systems for therapeutic and diagnostic purposes.
Market Analysis for Synthetic Biology Applications
The synthetic biology market has experienced remarkable growth in recent years, with a global market value reaching $9.5 billion in 2021 and projected to grow at a CAGR of 24.1% through 2030. This expansion is driven by increasing investments in research and development, growing demand for sustainable solutions, and advancements in genetic engineering technologies. Injectable hydrogels represent a significant opportunity within this expanding market.
Healthcare applications currently dominate the synthetic biology market landscape, accounting for approximately 35% of market share. Within this segment, injectable hydrogels are gaining traction for drug delivery systems, tissue engineering, and regenerative medicine applications. The pharmaceutical industry has shown particular interest in hydrogel technologies for controlled release formulations, with several products already in clinical trials.
Agricultural applications represent the second-largest market segment, where hydrogel technologies are being explored for improved crop yields, sustainable farming practices, and environmental remediation. The ability of injectable hydrogels to deliver beneficial microorganisms, nutrients, or plant growth regulators directly to soil systems presents significant commercial potential.
Industrial biotechnology applications are rapidly emerging, with companies developing hydrogel-based bioreactors and enzyme immobilization systems. These technologies enable more efficient biomanufacturing processes, reducing production costs and environmental impact. Market analysis indicates that this segment may experience the fastest growth rate over the next decade.
Consumer products incorporating synthetic biology elements, including cosmetics and food additives, represent a smaller but rapidly growing market segment. Injectable hydrogels are being investigated for applications in functional foods, cosmeceuticals, and other consumer-facing products, though regulatory hurdles remain significant in these areas.
Regional analysis reveals North America as the dominant market for synthetic biology applications, accounting for approximately 40% of global market share. However, Asia-Pacific regions, particularly China, South Korea, and Singapore, are experiencing the fastest growth rates due to increasing government investments and favorable regulatory environments.
Market barriers include high development costs, regulatory uncertainties, and public perception challenges. The average time-to-market for synthetic biology products incorporating injectable hydrogels ranges from 3-7 years, depending on the application and regulatory pathway. Despite these challenges, venture capital funding in this space has increased by 215% since 2018, indicating strong investor confidence in future market potential.
Customer segmentation analysis reveals that academic research institutions currently represent the largest customer base for injectable hydrogel technologies in synthetic biology, followed by pharmaceutical companies and agricultural technology firms. As technologies mature, this balance is expected to shift toward commercial applications.
Healthcare applications currently dominate the synthetic biology market landscape, accounting for approximately 35% of market share. Within this segment, injectable hydrogels are gaining traction for drug delivery systems, tissue engineering, and regenerative medicine applications. The pharmaceutical industry has shown particular interest in hydrogel technologies for controlled release formulations, with several products already in clinical trials.
Agricultural applications represent the second-largest market segment, where hydrogel technologies are being explored for improved crop yields, sustainable farming practices, and environmental remediation. The ability of injectable hydrogels to deliver beneficial microorganisms, nutrients, or plant growth regulators directly to soil systems presents significant commercial potential.
Industrial biotechnology applications are rapidly emerging, with companies developing hydrogel-based bioreactors and enzyme immobilization systems. These technologies enable more efficient biomanufacturing processes, reducing production costs and environmental impact. Market analysis indicates that this segment may experience the fastest growth rate over the next decade.
Consumer products incorporating synthetic biology elements, including cosmetics and food additives, represent a smaller but rapidly growing market segment. Injectable hydrogels are being investigated for applications in functional foods, cosmeceuticals, and other consumer-facing products, though regulatory hurdles remain significant in these areas.
Regional analysis reveals North America as the dominant market for synthetic biology applications, accounting for approximately 40% of global market share. However, Asia-Pacific regions, particularly China, South Korea, and Singapore, are experiencing the fastest growth rates due to increasing government investments and favorable regulatory environments.
Market barriers include high development costs, regulatory uncertainties, and public perception challenges. The average time-to-market for synthetic biology products incorporating injectable hydrogels ranges from 3-7 years, depending on the application and regulatory pathway. Despite these challenges, venture capital funding in this space has increased by 215% since 2018, indicating strong investor confidence in future market potential.
Customer segmentation analysis reveals that academic research institutions currently represent the largest customer base for injectable hydrogel technologies in synthetic biology, followed by pharmaceutical companies and agricultural technology firms. As technologies mature, this balance is expected to shift toward commercial applications.
Current Challenges in Injectable Hydrogel Technology
Despite significant advancements in injectable hydrogel technology for synthetic biology applications, several critical challenges continue to impede their widespread implementation. The primary obstacle remains achieving precise control over mechanical properties while maintaining biocompatibility. Current hydrogel systems often exhibit inconsistent gelation kinetics, leading to unpredictable structural integrity and mechanical performance in vivo. This variability significantly impacts their functionality as scaffolds for engineered biological systems.
Degradation rate control presents another substantial challenge. Injectable hydrogels must maintain structural integrity long enough to fulfill their intended function while eventually degrading at an appropriate rate. The complex biological environment introduces variables that can accelerate or retard degradation through enzymatic activity, pH fluctuations, and mechanical stresses, making predictable performance difficult to achieve.
The integration of synthetic biological components within hydrogel matrices faces significant hurdles related to maintaining cellular viability and functionality. Encapsulated engineered cells often experience reduced metabolic activity and compromised genetic circuit performance due to diffusion limitations of nutrients, signaling molecules, and waste products. The three-dimensional architecture of hydrogels can create microenvironments with oxygen and nutrient gradients that adversely affect cellular behavior.
Scalable manufacturing represents a considerable technical barrier. Current production methods for injectable hydrogels with consistent properties suffer from batch-to-batch variations, limiting their translation from laboratory to clinical or industrial applications. The complexity increases substantially when incorporating biological components, as maintaining sterility and bioactivity throughout the manufacturing process requires sophisticated protocols.
Immunogenicity and foreign body responses remain persistent concerns. Even hydrogels designed to be biocompatible can trigger inflammatory responses when combined with synthetic biological components, potentially compromising both the hydrogel integrity and the function of the engineered biological system. The interface between synthetic materials and biological systems creates unique challenges for predicting host responses.
Regulatory hurdles compound these technical challenges. The combination of injectable biomaterials with synthetic biological components creates novel entities that do not fit neatly into existing regulatory frameworks. The lack of standardized testing protocols and safety benchmarks for these hybrid technologies slows their progression through development pipelines.
Achieving spatiotemporal control over the release of bioactive molecules from hydrogels presents another significant challenge. Current systems often exhibit burst release profiles rather than the sustained, controlled release necessary for many synthetic biology applications. Engineering hydrogels with programmable release kinetics that respond to specific biological triggers remains an active area of research with considerable technical obstacles.
Degradation rate control presents another substantial challenge. Injectable hydrogels must maintain structural integrity long enough to fulfill their intended function while eventually degrading at an appropriate rate. The complex biological environment introduces variables that can accelerate or retard degradation through enzymatic activity, pH fluctuations, and mechanical stresses, making predictable performance difficult to achieve.
The integration of synthetic biological components within hydrogel matrices faces significant hurdles related to maintaining cellular viability and functionality. Encapsulated engineered cells often experience reduced metabolic activity and compromised genetic circuit performance due to diffusion limitations of nutrients, signaling molecules, and waste products. The three-dimensional architecture of hydrogels can create microenvironments with oxygen and nutrient gradients that adversely affect cellular behavior.
Scalable manufacturing represents a considerable technical barrier. Current production methods for injectable hydrogels with consistent properties suffer from batch-to-batch variations, limiting their translation from laboratory to clinical or industrial applications. The complexity increases substantially when incorporating biological components, as maintaining sterility and bioactivity throughout the manufacturing process requires sophisticated protocols.
Immunogenicity and foreign body responses remain persistent concerns. Even hydrogels designed to be biocompatible can trigger inflammatory responses when combined with synthetic biological components, potentially compromising both the hydrogel integrity and the function of the engineered biological system. The interface between synthetic materials and biological systems creates unique challenges for predicting host responses.
Regulatory hurdles compound these technical challenges. The combination of injectable biomaterials with synthetic biological components creates novel entities that do not fit neatly into existing regulatory frameworks. The lack of standardized testing protocols and safety benchmarks for these hybrid technologies slows their progression through development pipelines.
Achieving spatiotemporal control over the release of bioactive molecules from hydrogels presents another significant challenge. Current systems often exhibit burst release profiles rather than the sustained, controlled release necessary for many synthetic biology applications. Engineering hydrogels with programmable release kinetics that respond to specific biological triggers remains an active area of research with considerable technical obstacles.
Current Injectable Hydrogel Formulations and Methods
01 Composition and formulation of injectable hydrogels
Injectable hydrogels can be formulated using various polymers and cross-linking agents to create biocompatible matrices suitable for medical applications. These formulations typically include natural polymers (like hyaluronic acid, collagen, or alginate) or synthetic polymers (such as polyethylene glycol) that can be cross-linked in situ after injection. The composition can be tailored to control properties such as gelation time, mechanical strength, degradation rate, and biocompatibility, making them versatile for different medical applications.- Composition of injectable hydrogels for drug delivery: Injectable hydrogels can be formulated with specific polymers and active ingredients to create controlled drug delivery systems. These formulations allow for sustained release of therapeutic agents at the target site. The hydrogel matrix protects the encapsulated drugs while maintaining their bioactivity and controlling their release kinetics, making them particularly useful for delivering sensitive biomolecules or small molecule drugs that require localized administration.
- Biodegradable injectable hydrogels for tissue engineering: Biodegradable injectable hydrogels provide temporary scaffolds for tissue regeneration and repair. These hydrogels can be designed with varying degradation rates to match the tissue healing process. The biodegradable nature allows for gradual replacement by native tissue while providing structural support during the healing process. These materials often incorporate bioactive components that promote cell adhesion, proliferation, and differentiation to enhance tissue regeneration.
- Stimuli-responsive injectable hydrogels: Stimuli-responsive injectable hydrogels can undergo sol-gel transitions in response to external triggers such as temperature, pH, or light. These smart materials can be injected as liquids and form solid gels in situ upon exposure to physiological conditions. This property enables minimally invasive administration while providing precise spatial control of gel formation. Applications include targeted drug delivery, tissue engineering, and wound healing where in situ gelation is advantageous.
- Injectable hydrogels with enhanced mechanical properties: Advanced formulations of injectable hydrogels incorporate reinforcing components to enhance their mechanical strength and stability. These reinforced hydrogels can withstand physiological stresses while maintaining their structure and function. Techniques include incorporation of nanoparticles, fiber reinforcement, double-network structures, or chemical crosslinking strategies. The improved mechanical properties make these hydrogels suitable for load-bearing applications such as cartilage repair or intervertebral disc replacement.
- Injectable hydrogels for cell encapsulation and delivery: Injectable hydrogels can serve as carriers for living cells, providing a protective microenvironment that supports cell viability and function. These cell-laden hydrogels can be precisely delivered to target tissues where the encapsulated cells can secrete therapeutic factors or directly participate in tissue regeneration. The hydrogel composition can be tailored to provide appropriate biochemical and mechanical cues to direct cell behavior, including differentiation, migration, and matrix production.
02 Drug delivery applications of injectable hydrogels
Injectable hydrogels serve as effective drug delivery systems that can provide controlled release of therapeutic agents. These systems can encapsulate various drugs, proteins, growth factors, or other bioactive molecules and release them at controlled rates. The hydrogel matrix protects the encapsulated drugs from degradation while maintaining their bioactivity. By modifying the hydrogel composition, the release kinetics can be tailored to achieve sustained or triggered drug delivery, improving therapeutic efficacy and reducing side effects.Expand Specific Solutions03 Tissue engineering and regenerative medicine applications
Injectable hydrogels provide three-dimensional scaffolds for tissue engineering and regenerative medicine. These hydrogels can support cell adhesion, proliferation, and differentiation, making them ideal for tissue regeneration. They can be designed to mimic the extracellular matrix of specific tissues and can incorporate growth factors or stem cells to enhance tissue formation. The minimally invasive nature of injectable hydrogels allows them to fill irregular-shaped defects and conform to the surrounding tissue architecture, promoting better integration with host tissues.Expand Specific Solutions04 Stimuli-responsive injectable hydrogels
Stimuli-responsive injectable hydrogels can undergo physical or chemical changes in response to external stimuli such as temperature, pH, light, or electrical signals. These smart hydrogels can transition from a flowable solution to a solid gel state upon exposure to specific physiological conditions, enabling precise control over their properties and behavior in vivo. This responsiveness can be utilized for targeted drug delivery, controlled tissue regeneration, or as biosensors. The ability to respond to environmental cues makes these hydrogels particularly valuable for personalized medicine applications.Expand Specific Solutions05 Injectable hydrogels for wound healing and hemostasis
Injectable hydrogels can be formulated specifically for wound healing and hemostatic applications. These hydrogels can form a protective barrier over wounds, maintain a moist environment conducive to healing, and deliver therapeutic agents directly to the wound site. Some formulations incorporate antimicrobial components to prevent infection or growth factors to accelerate tissue regeneration. Hemostatic hydrogels can rapidly form a physical barrier to stop bleeding and may contain coagulation-promoting agents. Their injectable nature allows for easy application to irregularly shaped wounds or difficult-to-access areas.Expand Specific Solutions
Leading Companies and Research Institutions
The injectable hydrogel market in synthetic biology is currently in an early growth phase, characterized by significant research activity but limited commercial applications. The market size is projected to expand substantially as hydrogel technologies mature, with potential applications spanning regenerative medicine, drug delivery, and contraception. Technical maturity varies across applications, with academic institutions (University of Delaware, Johns Hopkins University, Duke University) leading fundamental research while specialized companies like Contraline are advancing specific applications. Established corporations (IBM, Boston Scientific) are beginning to invest in the space, indicating growing commercial interest. The field represents a convergence of materials science and synthetic biology, with cross-disciplinary collaboration between research institutions and industry partners driving innovation in this emerging biotechnology sector.
Contraline, Inc.
Technical Solution: Contraline has developed a proprietary injectable hydrogel technology called ADAM™, which represents a significant advancement in the application of hydrogels for synthetic biology. While initially focused on male contraception, their technology platform demonstrates broader potential for synthetic biology applications. Their hydrogel system features a unique combination of natural and synthetic polymers that create a biocompatible matrix capable of controlled interaction with biological systems[1]. The company's innovation lies in their precision injection technology that allows for targeted placement of the hydrogel in specific anatomical locations with minimal invasiveness. Contraline's hydrogels are engineered with tunable porosity that can selectively filter biological molecules based on size and charge, creating semi-permeable membranes that can compartmentalize synthetic biological systems while allowing nutrient exchange[2]. Their formulation includes proprietary crosslinking chemistry that provides long-term stability in physiological environments while maintaining flexibility and tissue-like mechanical properties. Recent developments have expanded their platform to include hydrogels with embedded biosensing capabilities that can monitor local biological conditions and potentially trigger programmed responses from synthetic biological components[3].
Strengths: Exceptional longevity and stability in vivo; minimally invasive delivery system with high precision; proven biocompatibility in human clinical trials. Weaknesses: Currently optimized for specific anatomical applications; may require significant adaptation for broader synthetic biology applications; relatively new technology with limited long-term data in diverse biological contexts.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has developed advanced injectable hydrogels for synthetic biology applications, focusing on biomimetic matrices that support cell growth and tissue engineering. Their proprietary technology utilizes self-assembling peptide hydrogels that can be injected as liquids and form stable 3D structures in situ upon exposure to physiological conditions[1]. These hydrogels incorporate bioactive molecules and growth factors that can be released in a controlled manner to guide cellular behavior and tissue development. The university's research team has demonstrated successful encapsulation of various cell types, including stem cells and engineered bacteria, within these hydrogels to create functional synthetic biological systems[2]. Their recent innovations include stimuli-responsive hydrogels that can change properties in response to specific biological signals, enabling dynamic control over synthetic biological circuits and engineered cellular functions within the hydrogel matrix[3].
Strengths: Superior biocompatibility and biodegradability profiles; precise control over mechanical properties to match native tissues; advanced functionalization capabilities for cell-specific interactions. Weaknesses: Relatively high production costs; potential challenges in scaling up manufacturing; some formulations may have limited stability during long-term storage.
Key Patents and Scientific Breakthroughs
Novel hydrogels and uses thereof
PatentInactiveUS20110052692A1
Innovation
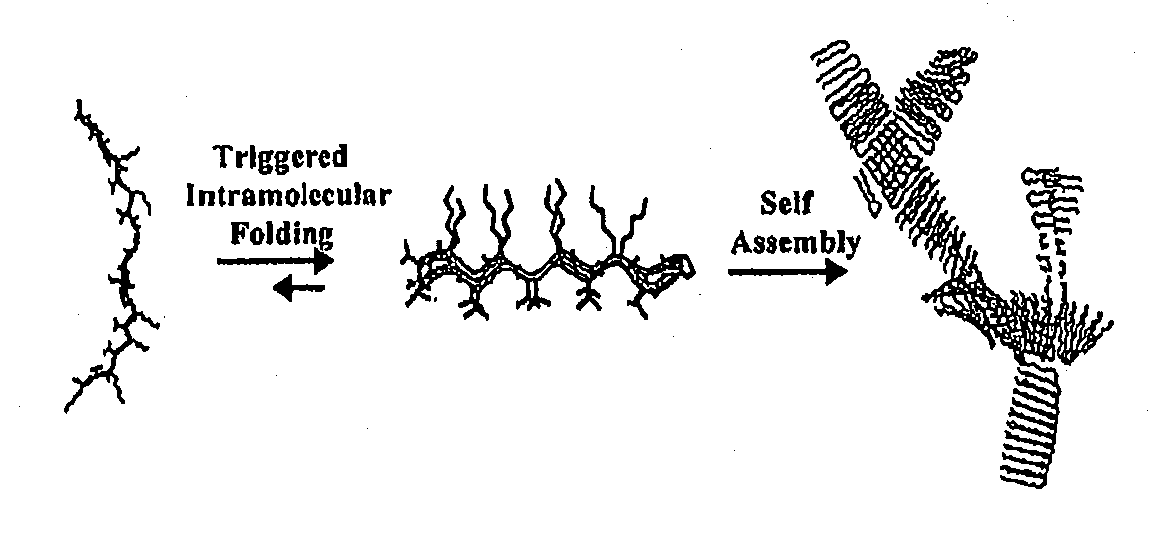
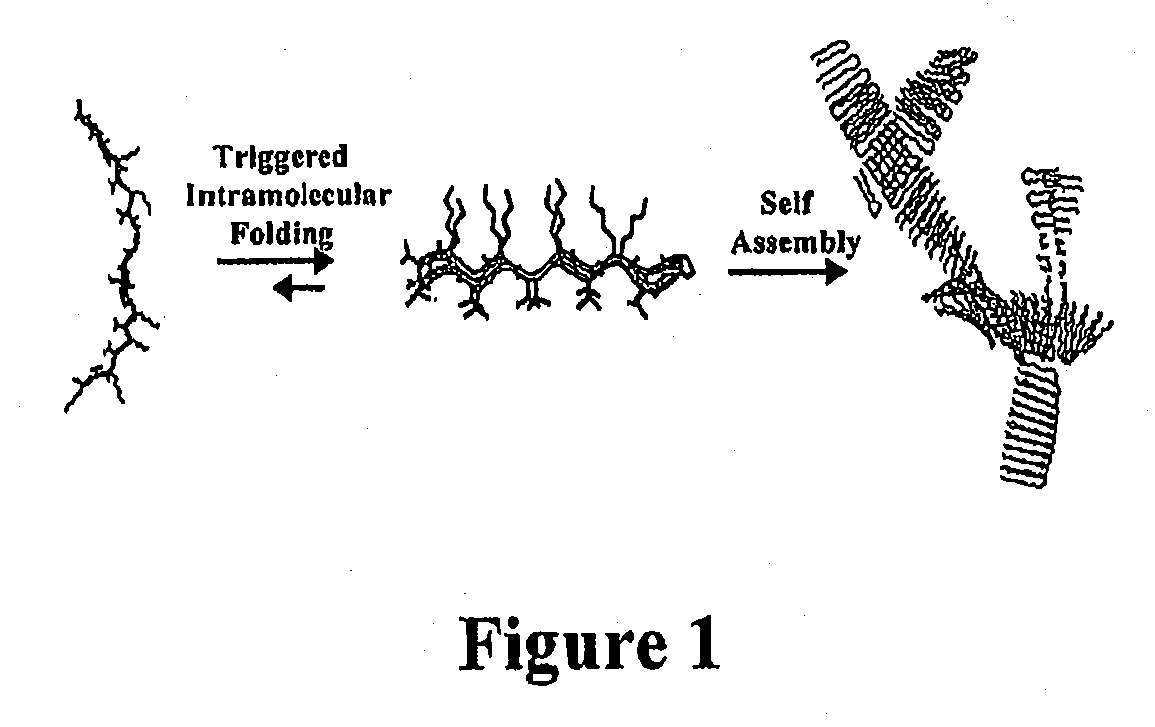
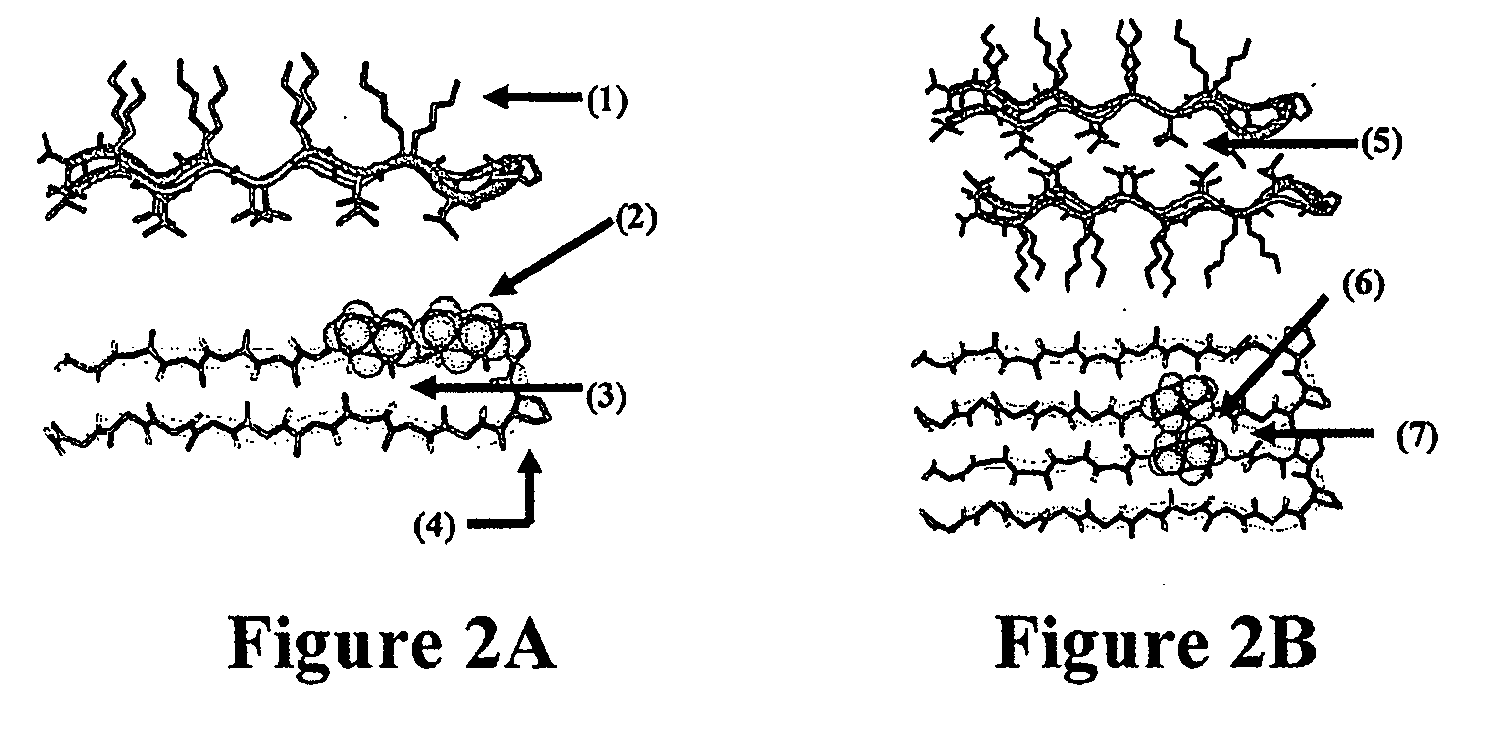
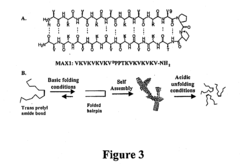
- Development of novel peptide-based hydrogels that undergo self-assembly in response to environmental stimuli, such as pH, ionic strength, and temperature, forming a rigid, porous structure without the need for exogenous crosslinking agents, allowing for controlled hydrogelation and reversibility.
Biocompatibility and Safety Considerations
The biocompatibility of injectable hydrogels represents a critical consideration for their application in synthetic biology. These materials must interact harmoniously with biological systems without triggering adverse immune responses or toxicity. Current research indicates that natural polymer-based hydrogels, such as those derived from hyaluronic acid, alginate, and collagen, generally exhibit superior biocompatibility compared to their synthetic counterparts. However, synthetic hydrogels offer greater tunability and consistency in performance, creating an ongoing challenge in balancing biocompatibility with functional requirements.
Safety evaluations for injectable hydrogels must address both short-term and long-term biological responses. Immediate concerns include potential inflammatory reactions at the injection site, while long-term considerations focus on degradation products and their metabolic pathways. Recent studies have demonstrated that hydrogel composition significantly influences macrophage polarization, with M2 phenotype promotion generally associated with improved tissue integration and reduced fibrotic encapsulation.
Regulatory frameworks for injectable hydrogels in synthetic biology applications remain complex and evolving. The FDA and EMA have established guidelines for biomaterial safety assessment, but the unique nature of cell-material interactions in synthetic biology contexts often necessitates case-specific evaluation protocols. The development of standardized testing methodologies represents an ongoing challenge for the field, particularly for novel applications where historical safety data is limited.
Sterilization methods present another critical consideration, as they must effectively eliminate microbial contamination without compromising the structural integrity or functional properties of the hydrogel. Common approaches include filtration for thermosensitive formulations, gamma irradiation for more robust compositions, and ethylene oxide treatment, each with distinct advantages and limitations depending on the specific hydrogel chemistry.
Recent advances in immunomodulatory hydrogels demonstrate promising approaches to enhancing biocompatibility. By incorporating anti-inflammatory agents or designing materials that actively suppress pro-inflammatory signaling pathways, researchers have developed hydrogels capable of creating more favorable microenvironments for engineered biological systems. Additionally, the incorporation of extracellular matrix components or biomimetic peptides has shown potential for improving cell-material interactions and promoting desired cellular behaviors.
The degradation kinetics of injectable hydrogels must be carefully controlled to match the intended application timeline in synthetic biology. Premature degradation may compromise functional performance, while persistent materials may interfere with natural biological processes. Enzymatically degradable linkages and hydrolytically sensitive bonds offer tunable approaches to controlling material persistence in biological environments.
Safety evaluations for injectable hydrogels must address both short-term and long-term biological responses. Immediate concerns include potential inflammatory reactions at the injection site, while long-term considerations focus on degradation products and their metabolic pathways. Recent studies have demonstrated that hydrogel composition significantly influences macrophage polarization, with M2 phenotype promotion generally associated with improved tissue integration and reduced fibrotic encapsulation.
Regulatory frameworks for injectable hydrogels in synthetic biology applications remain complex and evolving. The FDA and EMA have established guidelines for biomaterial safety assessment, but the unique nature of cell-material interactions in synthetic biology contexts often necessitates case-specific evaluation protocols. The development of standardized testing methodologies represents an ongoing challenge for the field, particularly for novel applications where historical safety data is limited.
Sterilization methods present another critical consideration, as they must effectively eliminate microbial contamination without compromising the structural integrity or functional properties of the hydrogel. Common approaches include filtration for thermosensitive formulations, gamma irradiation for more robust compositions, and ethylene oxide treatment, each with distinct advantages and limitations depending on the specific hydrogel chemistry.
Recent advances in immunomodulatory hydrogels demonstrate promising approaches to enhancing biocompatibility. By incorporating anti-inflammatory agents or designing materials that actively suppress pro-inflammatory signaling pathways, researchers have developed hydrogels capable of creating more favorable microenvironments for engineered biological systems. Additionally, the incorporation of extracellular matrix components or biomimetic peptides has shown potential for improving cell-material interactions and promoting desired cellular behaviors.
The degradation kinetics of injectable hydrogels must be carefully controlled to match the intended application timeline in synthetic biology. Premature degradation may compromise functional performance, while persistent materials may interfere with natural biological processes. Enzymatically degradable linkages and hydrolytically sensitive bonds offer tunable approaches to controlling material persistence in biological environments.
Scalability and Manufacturing Challenges
The scalability of injectable hydrogels represents a significant challenge in transitioning from laboratory-scale production to commercial manufacturing for synthetic biology applications. Current production methods often rely on batch processes that are difficult to scale without compromising the structural integrity and functional properties of the hydrogels. The heterogeneity in crosslinking density and network formation becomes more pronounced at larger scales, leading to inconsistent mechanical properties and bioactive molecule distribution throughout the hydrogel matrix.
Manufacturing challenges extend beyond mere scale-up issues to include sterilization processes that can potentially denature embedded bioactive components or alter the hydrogel's physical properties. Traditional sterilization methods such as autoclaving may compromise the structural integrity of temperature-sensitive hydrogels, while gamma irradiation can induce unwanted crosslinking or degradation of polymer chains. Alternative approaches like filtration are limited to specific components and cannot be applied to the final hydrogel product.
Quality control presents another significant hurdle, as current analytical methods struggle to provide real-time monitoring of critical parameters during the manufacturing process. The viscoelastic nature of hydrogels makes conventional quality assessment techniques insufficient for ensuring batch-to-batch consistency. Additionally, the shelf-life stability of injectable hydrogels containing biological components remains problematic, with potential degradation of both the polymer network and the encapsulated bioactive molecules over time.
Regulatory considerations further complicate the manufacturing landscape, particularly for hydrogels intended for clinical applications in synthetic biology. The complex composition of these materials, often combining synthetic polymers with biological components, creates regulatory ambiguity that can extend development timelines and increase costs. Manufacturing facilities must meet stringent requirements for both pharmaceutical and biological product production, necessitating substantial capital investment.
Cost-effective production represents perhaps the most pressing challenge for widespread adoption of injectable hydrogels in synthetic biology. Current manufacturing approaches involve expensive raw materials, complex processing steps, and significant quality control overhead. The economics become particularly unfavorable when considering personalized applications that require small batch production with high quality standards. Innovations in continuous manufacturing processes, automated quality control systems, and standardized production protocols are essential to overcome these limitations and realize the full potential of injectable hydrogels in synthetic biology applications.
Manufacturing challenges extend beyond mere scale-up issues to include sterilization processes that can potentially denature embedded bioactive components or alter the hydrogel's physical properties. Traditional sterilization methods such as autoclaving may compromise the structural integrity of temperature-sensitive hydrogels, while gamma irradiation can induce unwanted crosslinking or degradation of polymer chains. Alternative approaches like filtration are limited to specific components and cannot be applied to the final hydrogel product.
Quality control presents another significant hurdle, as current analytical methods struggle to provide real-time monitoring of critical parameters during the manufacturing process. The viscoelastic nature of hydrogels makes conventional quality assessment techniques insufficient for ensuring batch-to-batch consistency. Additionally, the shelf-life stability of injectable hydrogels containing biological components remains problematic, with potential degradation of both the polymer network and the encapsulated bioactive molecules over time.
Regulatory considerations further complicate the manufacturing landscape, particularly for hydrogels intended for clinical applications in synthetic biology. The complex composition of these materials, often combining synthetic polymers with biological components, creates regulatory ambiguity that can extend development timelines and increase costs. Manufacturing facilities must meet stringent requirements for both pharmaceutical and biological product production, necessitating substantial capital investment.
Cost-effective production represents perhaps the most pressing challenge for widespread adoption of injectable hydrogels in synthetic biology. Current manufacturing approaches involve expensive raw materials, complex processing steps, and significant quality control overhead. The economics become particularly unfavorable when considering personalized applications that require small batch production with high quality standards. Innovations in continuous manufacturing processes, automated quality control systems, and standardized production protocols are essential to overcome these limitations and realize the full potential of injectable hydrogels in synthetic biology applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!