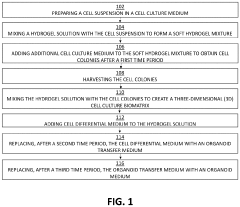
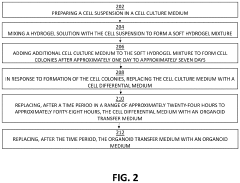
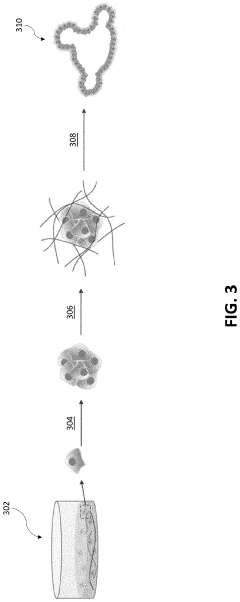
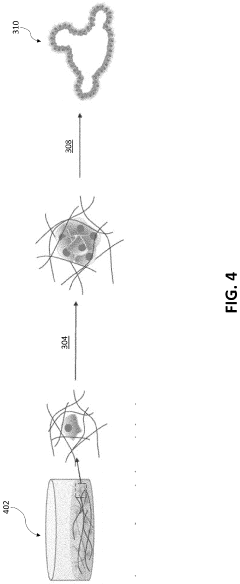
Injectable Hydrogel as a Medium in Organoid Cultivation Techniques
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Injectable Hydrogel Development and Objectives
Injectable hydrogels have emerged as a revolutionary biomaterial platform in the field of organoid cultivation, representing a significant advancement from traditional two-dimensional cell culture methods. The development of these hydrogels traces back to the early 2000s when researchers began exploring three-dimensional matrices that could better mimic the extracellular environment. Over the past decade, injectable hydrogels have evolved from simple polymeric networks to sophisticated biomimetic materials with tunable properties specifically designed for organoid applications.
The technological evolution of injectable hydrogels has been driven by interdisciplinary collaboration between material scientists, bioengineers, and cell biologists. Early iterations focused primarily on biocompatibility and basic mechanical support, while contemporary formulations incorporate bioactive components, degradation kinetics control, and stimuli-responsive elements. This progression reflects the growing understanding of the complex microenvironmental requirements for successful organoid cultivation.
Current technological trends in this field include the development of hydrogels with spatiotemporal control over mechanical properties, integration of tissue-specific extracellular matrix components, and incorporation of growth factor delivery systems. The convergence of advanced manufacturing techniques such as 3D bioprinting with injectable hydrogel technology has further expanded the design possibilities and precision of these cultivation matrices.
The primary technical objectives for injectable hydrogels in organoid cultivation include achieving physiologically relevant stiffness gradients, optimizing biodegradation rates to match organoid development timelines, and enhancing nutrient diffusion while maintaining structural integrity. Additionally, there is a focused effort to develop formulations that enable non-invasive monitoring of organoid development through optical transparency or integration with imaging modalities.
Another critical goal is the standardization of injectable hydrogel compositions to improve reproducibility in organoid research, addressing one of the major challenges in the field. Researchers are working toward creating defined synthetic alternatives to naturally derived materials like Matrigel, which suffer from batch-to-batch variability and undefined composition.
Looking forward, the field aims to develop injectable hydrogels capable of directing organoid differentiation through controlled presentation of biochemical and biophysical cues. The ultimate objective is to create "programmable" hydrogel systems that can be customized for specific organoid types and developmental stages, potentially revolutionizing personalized medicine applications and drug discovery platforms.
The technological evolution of injectable hydrogels has been driven by interdisciplinary collaboration between material scientists, bioengineers, and cell biologists. Early iterations focused primarily on biocompatibility and basic mechanical support, while contemporary formulations incorporate bioactive components, degradation kinetics control, and stimuli-responsive elements. This progression reflects the growing understanding of the complex microenvironmental requirements for successful organoid cultivation.
Current technological trends in this field include the development of hydrogels with spatiotemporal control over mechanical properties, integration of tissue-specific extracellular matrix components, and incorporation of growth factor delivery systems. The convergence of advanced manufacturing techniques such as 3D bioprinting with injectable hydrogel technology has further expanded the design possibilities and precision of these cultivation matrices.
The primary technical objectives for injectable hydrogels in organoid cultivation include achieving physiologically relevant stiffness gradients, optimizing biodegradation rates to match organoid development timelines, and enhancing nutrient diffusion while maintaining structural integrity. Additionally, there is a focused effort to develop formulations that enable non-invasive monitoring of organoid development through optical transparency or integration with imaging modalities.
Another critical goal is the standardization of injectable hydrogel compositions to improve reproducibility in organoid research, addressing one of the major challenges in the field. Researchers are working toward creating defined synthetic alternatives to naturally derived materials like Matrigel, which suffer from batch-to-batch variability and undefined composition.
Looking forward, the field aims to develop injectable hydrogels capable of directing organoid differentiation through controlled presentation of biochemical and biophysical cues. The ultimate objective is to create "programmable" hydrogel systems that can be customized for specific organoid types and developmental stages, potentially revolutionizing personalized medicine applications and drug discovery platforms.
Market Analysis for Organoid Cultivation Technologies
The global organoid cultivation market is experiencing robust growth, with the injectable hydrogel segment emerging as a particularly dynamic component. Current market valuations place the overall organoid technology sector at approximately 1.5 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 22.5% through 2030, potentially reaching 6.2 billion USD by the end of the decade.
Injectable hydrogels as cultivation media represent about 18% of this market currently, with their share expected to expand to 25-30% by 2028 due to their superior performance characteristics compared to traditional Matrigel-based approaches. This growth is primarily driven by increasing R&D investments in regenerative medicine, personalized therapeutics, and drug discovery applications.
North America dominates the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (22%), with the latter showing the fastest growth trajectory. The pharmaceutical and biotechnology sectors account for the largest end-user segment (65%), followed by academic and research institutions (25%).
Key market drivers include the rising prevalence of chronic diseases necessitating novel treatment approaches, growing demand for personalized medicine, and increasing applications in toxicology testing as alternatives to animal models. The injectable hydrogel segment specifically benefits from advantages in providing more physiologically relevant microenvironments, improved nutrient diffusion, and enhanced structural support for 3D organoid development.
Significant market restraints include high costs associated with specialized hydrogel formulations, technical challenges in achieving consistent organoid development, and regulatory uncertainties surrounding clinical applications of organoid technologies. The average cost per research-grade injectable hydrogel kit ranges from 800-1,200 USD, representing a substantial expense for research institutions.
Customer segmentation reveals pharmaceutical companies primarily value injectable hydrogels for drug screening and toxicity testing, while academic institutions focus on basic research applications. Healthcare providers are increasingly interested in patient-derived organoid applications using specialized hydrogels for personalized medicine approaches.
The competitive landscape features established players like Corning, BD Biosciences, and Thermo Fisher Scientific alongside specialized startups such as Cellendes, QGel, and Advanced BioMatrix focusing exclusively on advanced hydrogel technologies for organoid cultivation. Recent market consolidation through strategic acquisitions indicates growing recognition of injectable hydrogels as critical components in the organoid technology value chain.
Injectable hydrogels as cultivation media represent about 18% of this market currently, with their share expected to expand to 25-30% by 2028 due to their superior performance characteristics compared to traditional Matrigel-based approaches. This growth is primarily driven by increasing R&D investments in regenerative medicine, personalized therapeutics, and drug discovery applications.
North America dominates the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (22%), with the latter showing the fastest growth trajectory. The pharmaceutical and biotechnology sectors account for the largest end-user segment (65%), followed by academic and research institutions (25%).
Key market drivers include the rising prevalence of chronic diseases necessitating novel treatment approaches, growing demand for personalized medicine, and increasing applications in toxicology testing as alternatives to animal models. The injectable hydrogel segment specifically benefits from advantages in providing more physiologically relevant microenvironments, improved nutrient diffusion, and enhanced structural support for 3D organoid development.
Significant market restraints include high costs associated with specialized hydrogel formulations, technical challenges in achieving consistent organoid development, and regulatory uncertainties surrounding clinical applications of organoid technologies. The average cost per research-grade injectable hydrogel kit ranges from 800-1,200 USD, representing a substantial expense for research institutions.
Customer segmentation reveals pharmaceutical companies primarily value injectable hydrogels for drug screening and toxicity testing, while academic institutions focus on basic research applications. Healthcare providers are increasingly interested in patient-derived organoid applications using specialized hydrogels for personalized medicine approaches.
The competitive landscape features established players like Corning, BD Biosciences, and Thermo Fisher Scientific alongside specialized startups such as Cellendes, QGel, and Advanced BioMatrix focusing exclusively on advanced hydrogel technologies for organoid cultivation. Recent market consolidation through strategic acquisitions indicates growing recognition of injectable hydrogels as critical components in the organoid technology value chain.
Current Challenges in Hydrogel-Based Organoid Systems
Despite significant advancements in hydrogel-based organoid cultivation techniques, several critical challenges persist that limit their widespread application and clinical translation. The primary obstacle remains the inconsistency in organoid development within hydrogel matrices. Even under seemingly identical conditions, organoids often exhibit considerable variability in size, morphology, and functionality, hampering experimental reproducibility and standardization for clinical applications.
The mechanical properties of injectable hydrogels present another significant challenge. While stiffness and viscoelasticity critically influence cell behavior and organoid formation, achieving precise control over these parameters remains difficult. The dynamic nature of developing organoids requires hydrogels that can adapt their mechanical properties over time, a feature not fully realized in current systems. Additionally, the degradation kinetics of hydrogels often fail to synchronize with organoid growth rates, leading to suboptimal development.
Diffusion limitations within hydrogel matrices constitute a substantial barrier to organoid maturation. As organoids grow, the transport of nutrients, oxygen, and waste products becomes increasingly restricted, particularly in the core regions. This diffusion constraint frequently results in necrotic centers in larger organoids, limiting their size and functional maturity. Current hydrogel formulations struggle to provide adequate vascularization support, essential for developing physiologically relevant organoid systems.
The biocompatibility of synthetic components in injectable hydrogels raises concerns regarding long-term culture and potential clinical applications. Some crosslinking agents and additives used to enhance mechanical properties may exhibit cytotoxicity or trigger inflammatory responses, compromising organoid viability and functionality. Furthermore, batch-to-batch variations in natural hydrogel components like Matrigel introduce additional inconsistencies in experimental outcomes.
Scalability represents another significant hurdle in hydrogel-based organoid cultivation. Current protocols often yield limited quantities of organoids, insufficient for high-throughput drug screening or regenerative medicine applications. The labor-intensive nature of organoid culture, coupled with the high costs of specialized hydrogel materials, further restricts their accessibility and widespread adoption in research and clinical settings.
Integration challenges between hydrogels and analytical platforms impede comprehensive characterization of organoids. Many imaging and molecular analysis techniques are not fully compatible with three-dimensional hydrogel cultures, necessitating organoid extraction or fixation that disrupts their native state. This limitation hinders real-time monitoring of organoid development and function, essential for understanding dynamic biological processes.
The mechanical properties of injectable hydrogels present another significant challenge. While stiffness and viscoelasticity critically influence cell behavior and organoid formation, achieving precise control over these parameters remains difficult. The dynamic nature of developing organoids requires hydrogels that can adapt their mechanical properties over time, a feature not fully realized in current systems. Additionally, the degradation kinetics of hydrogels often fail to synchronize with organoid growth rates, leading to suboptimal development.
Diffusion limitations within hydrogel matrices constitute a substantial barrier to organoid maturation. As organoids grow, the transport of nutrients, oxygen, and waste products becomes increasingly restricted, particularly in the core regions. This diffusion constraint frequently results in necrotic centers in larger organoids, limiting their size and functional maturity. Current hydrogel formulations struggle to provide adequate vascularization support, essential for developing physiologically relevant organoid systems.
The biocompatibility of synthetic components in injectable hydrogels raises concerns regarding long-term culture and potential clinical applications. Some crosslinking agents and additives used to enhance mechanical properties may exhibit cytotoxicity or trigger inflammatory responses, compromising organoid viability and functionality. Furthermore, batch-to-batch variations in natural hydrogel components like Matrigel introduce additional inconsistencies in experimental outcomes.
Scalability represents another significant hurdle in hydrogel-based organoid cultivation. Current protocols often yield limited quantities of organoids, insufficient for high-throughput drug screening or regenerative medicine applications. The labor-intensive nature of organoid culture, coupled with the high costs of specialized hydrogel materials, further restricts their accessibility and widespread adoption in research and clinical settings.
Integration challenges between hydrogels and analytical platforms impede comprehensive characterization of organoids. Many imaging and molecular analysis techniques are not fully compatible with three-dimensional hydrogel cultures, necessitating organoid extraction or fixation that disrupts their native state. This limitation hinders real-time monitoring of organoid development and function, essential for understanding dynamic biological processes.
Current Hydrogel Formulations for Organoid Support
01 Composition of injectable hydrogels for drug delivery
Injectable hydrogels can be formulated as drug delivery systems that provide controlled release of therapeutic agents. These hydrogels are designed to encapsulate various drugs and release them at a controlled rate once injected into the body. The composition typically includes biocompatible polymers that form a three-dimensional network capable of holding water and drugs. These systems can be tailored to respond to specific physiological conditions, such as pH or temperature, to trigger drug release at the target site.- Composition of injectable hydrogels for tissue engineering: Injectable hydrogels can be formulated with various polymers and biomaterials to create a suitable medium for tissue engineering applications. These hydrogels provide a three-dimensional scaffold that supports cell growth, proliferation, and differentiation. The composition typically includes natural or synthetic polymers, crosslinking agents, and bioactive molecules that promote tissue regeneration. The hydrogel matrix mimics the extracellular environment, allowing for cell attachment and migration while maintaining structural integrity after injection.
- Drug delivery systems using injectable hydrogels: Injectable hydrogels serve as effective drug delivery systems that provide controlled release of therapeutic agents. These hydrogels can encapsulate various drugs, proteins, or growth factors and release them at a controlled rate over time. The release kinetics can be tailored by modifying the hydrogel composition, crosslinking density, and degradation rate. This approach enhances therapeutic efficacy by maintaining drug concentrations within the therapeutic window while reducing systemic side effects. The injectable nature allows for minimally invasive administration directly to the target site.
- Stimuli-responsive injectable hydrogels: Stimuli-responsive injectable hydrogels can undergo sol-gel transitions in response to external stimuli such as temperature, pH, or light. These smart materials remain in a liquid state during injection and then rapidly form a gel once inside the body in response to physiological conditions. This property enables precise placement of the hydrogel at the target site through minimally invasive procedures. The responsive nature of these hydrogels also allows for controlled degradation and release of encapsulated substances, making them versatile for various biomedical applications.
- Injectable hydrogels for cell encapsulation and delivery: Injectable hydrogels provide an excellent medium for cell encapsulation and delivery in regenerative medicine applications. These hydrogels create a protective microenvironment that shields cells from mechanical stress during injection while supporting their viability and function. The three-dimensional network allows for nutrient diffusion, waste removal, and cell-cell interactions. By encapsulating stem cells, progenitor cells, or differentiated cells within injectable hydrogels, targeted delivery to specific tissues can be achieved, enhancing therapeutic outcomes in tissue repair and regeneration.
- Crosslinking mechanisms for injectable hydrogels: Various crosslinking mechanisms can be employed to form stable injectable hydrogels, including chemical, physical, and enzymatic methods. Chemical crosslinking involves the formation of covalent bonds between polymer chains, providing mechanical stability to the hydrogel. Physical crosslinking relies on non-covalent interactions such as hydrogen bonding, ionic interactions, or hydrophobic associations. Enzymatic crosslinking utilizes specific enzymes to catalyze the formation of crosslinks. The choice of crosslinking mechanism affects the gelation time, mechanical properties, biodegradability, and biocompatibility of the resulting hydrogel, which can be tailored for specific biomedical applications.
02 Injectable hydrogels for tissue engineering and regeneration
Injectable hydrogels serve as scaffolds for tissue engineering and regenerative medicine applications. These biomaterials provide a three-dimensional environment that supports cell growth, proliferation, and differentiation. The hydrogels can be designed to mimic the extracellular matrix of natural tissues, promoting tissue regeneration and repair. They can be loaded with growth factors, stem cells, or other bioactive molecules to enhance their regenerative properties. The injectable nature allows for minimally invasive delivery to the target tissue site.Expand Specific Solutions03 Stimuli-responsive injectable hydrogel systems
Stimuli-responsive injectable hydrogels can undergo sol-gel transitions in response to external stimuli such as temperature, pH, light, or electrical signals. These smart materials remain in a liquid state during injection and then rapidly form a gel once inside the body in response to physiological conditions. This property makes them particularly useful for various biomedical applications, including controlled drug release, tissue engineering, and wound healing. The responsive nature allows for precise control over the material properties and functionality after injection.Expand Specific Solutions04 Injectable hydrogels with enhanced mechanical properties
Advanced injectable hydrogels are designed with improved mechanical properties to better match the characteristics of target tissues. These hydrogels incorporate various reinforcement strategies, such as double-network structures, nanocomposite formulations, or chemical crosslinking methods, to enhance their strength, elasticity, and durability. The improved mechanical properties ensure that the hydrogels maintain their structural integrity under physiological conditions and mechanical loading, making them suitable for applications in load-bearing tissues or as structural supports in the body.Expand Specific Solutions05 Biodegradable injectable hydrogels for medical applications
Biodegradable injectable hydrogels are designed to gradually break down in the body after fulfilling their intended function. These hydrogels are composed of naturally derived polymers, synthetic biodegradable polymers, or their combinations. The degradation rate can be tailored by adjusting the chemical composition and crosslinking density. Biodegradable injectable hydrogels are particularly valuable for temporary applications where permanent implantation is not desired, such as wound healing, drug delivery, or as temporary scaffolds for tissue regeneration.Expand Specific Solutions
Leading Companies and Research Institutions in Organoid Field
Injectable hydrogel technology for organoid cultivation is currently in a growth phase, with the market expanding rapidly due to increasing applications in regenerative medicine, drug discovery, and personalized medicine. The global market size for this technology is projected to reach significant value as research institutions and biotech companies invest heavily in 3D cell culture technologies. Technologically, the field is advancing from early-stage development toward maturity, with companies like Thewell Bioscience leading with their VitroGel® platform that mimics natural ECM environments. Other key players include Cellendes, Onyel Biotech specializing in microfluidic integration, and Beijing Cornerstone Life Technology developing novel hydrogel formulations. Research institutions such as EPFL, Columbia University, and the Agency for Science, Technology & Research are driving innovation through collaborative approaches, pushing the boundaries of organoid cultivation techniques.
Molecular Devices (Austria) GmbH
Technical Solution: Molecular Devices has developed the MatriGrid™ system, an advanced synthetic hydrogel platform specifically engineered for high-throughput organoid cultivation and screening. Their technology utilizes a proprietary blend of PEG-based polymers with bioactive peptide modifications that promote cell adhesion and organization. The injectable hydrogel features rapid gelation kinetics (under 5 minutes) triggered by mild photocrosslinking, allowing for precise spatial control of the matrix formation. The MatriGrid™ system incorporates degradable crosslinkers that enable dynamic remodeling of the matrix as organoids develop. A key innovation is their gradient-forming technology that creates biomimetic stiffness variations (ranging from 0.5-50 kPa) within a single hydrogel construct, allowing for the study of mechanobiological effects on organoid development. The system is compatible with their automated imaging platforms, enabling real-time monitoring of organoid growth and morphological changes. Molecular Devices has validated their technology with various organoid types, including intestinal, pancreatic, and brain organoids, demonstrating enhanced reproducibility and functional maturation compared to conventional methods[4].
Strengths: Excellent optical clarity for high-resolution imaging; compatible with automated screening platforms; highly reproducible with minimal batch-to-batch variation; supports long-term culture stability. Weaknesses: Requires specialized equipment for optimal photocrosslinking; higher initial investment cost; limited customization options for certain specialized applications.
Beijing Cornerstone Life Technology Co., Ltd
Technical Solution: Beijing Cornerstone Life Technology has developed an innovative injectable hydrogel system called BioMatrix™ specifically designed for organoid cultivation. Their technology utilizes a combination of natural and synthetic polymers to create a biomimetic extracellular matrix environment. The hydrogel incorporates hyaluronic acid and modified gelatin components with thermoresponsive properties, allowing for liquid-state handling at room temperature and rapid gelation at physiological temperature (37°C). This enables minimally invasive delivery and in situ formation. The company's proprietary crosslinking technology allows for precise control over mechanical properties (stiffness ranging from 0.2-20 kPa) and degradation rates (adjustable from days to months). BioMatrix™ hydrogels feature integrated growth factor reservoirs that provide sustained release of bioactive molecules critical for organoid development. The system has been validated for various organoid types including intestinal, liver, and pancreatic organoids, demonstrating enhanced viability and functionality compared to conventional Matrigel cultures[3].
Strengths: Excellent batch-to-batch consistency; tunable mechanical and degradation properties; compatible with various bioprinting applications; cost-effective compared to animal-derived matrices. Weaknesses: Limited long-term stability in certain applications; requires optimization for specific organoid types; relatively new technology with fewer independent validation studies.
Key Patents and Breakthroughs in Injectable Hydrogels
Hydrogel for stem cell and organoid culture
PatentPendingUS20200239824A1
Innovation
- The use of soft polysaccharide hydrogels with shear-thinning and self-healing properties, capable of conversion to hard polysaccharide hydrogels, which are suitable for injection and exhibit a homogenous matrix structure, allowing for controlled experimental conditions and in vivo applications, incorporating functional ligands and bioactive molecules for enhanced cell culture and organoid formation.
Injectable hydrogel composition, method for the preparation and uses thereof
PatentInactiveEP2813213A1
Innovation
- An injectable hydrogel composition comprising an aqueous dispersion of inorganic nanoparticles linked to a block copolymer with a lower critical solution temperature (LCST), allowing for thermo-induced gelation without chemical reactions, enabling low viscosity before injection and osmotic activity post-injection.
Regulatory Pathway for Translational Organoid Technologies
The regulatory landscape for translational organoid technologies utilizing injectable hydrogels presents significant complexity due to the novel nature of these combined technologies. Regulatory bodies such as the FDA in the United States and the EMA in Europe have established frameworks that these technologies must navigate, typically classified as combination products involving both biological and device components.
For injectable hydrogel-based organoid systems, developers must consider multiple regulatory pathways. The primary determination centers on the product's primary mode of action (PMOA), which dictates whether it falls under biologics (CBER), drugs (CDER), or medical devices (CDRH) jurisdiction within the FDA structure. Most organoid technologies incorporating injectable hydrogels are reviewed through the Investigational New Drug (IND) pathway, with subsequent Biologics License Application (BLA) or New Drug Application (NDA) submissions.
Preclinical testing requirements are particularly stringent for these technologies, requiring comprehensive biocompatibility assessments of the hydrogel components, degradation studies, and evaluation of potential immunogenicity. The FDA's guidance on human cell and tissue products (HCT/Ps) under 21 CFR Part 1271 provides additional regulatory considerations when organoids contain human-derived materials.
Quality control standards present unique challenges, as developers must establish robust characterization methods for both the hydrogel properties and the resulting organoid structures. This includes developing validated assays for functional assessment, structural integrity, and cellular composition that can satisfy regulatory scrutiny.
Clinical translation pathways typically begin with Phase I safety studies, though the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation offers accelerated pathways for qualifying technologies that address serious conditions. Similarly, the EMA provides the Priority Medicines (PRIME) scheme for expedited development of promising therapies.
Post-market surveillance requirements are particularly important for these technologies due to their novelty and potential long-term effects. Regulatory bodies typically mandate comprehensive follow-up studies to monitor safety and efficacy over extended periods, with particular attention to potential tumorigenicity concerns associated with organoid technologies.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are gradually addressing the regulatory disparities across major markets, though significant regional differences in the approval processes for these advanced therapeutic products remain.
For injectable hydrogel-based organoid systems, developers must consider multiple regulatory pathways. The primary determination centers on the product's primary mode of action (PMOA), which dictates whether it falls under biologics (CBER), drugs (CDER), or medical devices (CDRH) jurisdiction within the FDA structure. Most organoid technologies incorporating injectable hydrogels are reviewed through the Investigational New Drug (IND) pathway, with subsequent Biologics License Application (BLA) or New Drug Application (NDA) submissions.
Preclinical testing requirements are particularly stringent for these technologies, requiring comprehensive biocompatibility assessments of the hydrogel components, degradation studies, and evaluation of potential immunogenicity. The FDA's guidance on human cell and tissue products (HCT/Ps) under 21 CFR Part 1271 provides additional regulatory considerations when organoids contain human-derived materials.
Quality control standards present unique challenges, as developers must establish robust characterization methods for both the hydrogel properties and the resulting organoid structures. This includes developing validated assays for functional assessment, structural integrity, and cellular composition that can satisfy regulatory scrutiny.
Clinical translation pathways typically begin with Phase I safety studies, though the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation offers accelerated pathways for qualifying technologies that address serious conditions. Similarly, the EMA provides the Priority Medicines (PRIME) scheme for expedited development of promising therapies.
Post-market surveillance requirements are particularly important for these technologies due to their novelty and potential long-term effects. Regulatory bodies typically mandate comprehensive follow-up studies to monitor safety and efficacy over extended periods, with particular attention to potential tumorigenicity concerns associated with organoid technologies.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are gradually addressing the regulatory disparities across major markets, though significant regional differences in the approval processes for these advanced therapeutic products remain.
Scalability and Commercialization Challenges
The scaling of injectable hydrogel-based organoid cultivation from laboratory to commercial applications faces significant challenges that must be addressed for widespread adoption. Current production methods for high-quality hydrogels suitable for organoid culture remain largely artisanal, with batch-to-batch variations that impede standardization. This inconsistency presents a major hurdle for pharmaceutical companies and clinical applications requiring reproducible results across large sample sizes.
Manufacturing scale-up introduces additional complexities related to maintaining hydrogel homogeneity and biocompatibility. As production volumes increase, ensuring uniform distribution of bioactive components and consistent mechanical properties becomes increasingly difficult. The delicate balance of stiffness, porosity, and degradation kinetics that supports optimal organoid development can be disrupted during large-scale manufacturing processes.
Cost factors present another substantial barrier to commercialization. High-grade materials required for injectable hydrogels, particularly those incorporating specialized ECM components or growth factors, command premium prices that escalate with production scale. Current estimates suggest that material costs alone for clinical-grade hydrogel systems range from $200-500 per milliliter, making widespread application economically challenging without significant process optimization.
Regulatory pathways for hydrogel-based organoid cultivation systems remain complex and poorly defined. The combination of biomaterials, potentially patient-derived cells, and growth factors creates a regulatory classification challenge. Companies pursuing commercialization must navigate different regulatory frameworks across global markets, with some jurisdictions treating these systems as advanced therapy medicinal products (ATMPs) requiring extensive clinical validation.
Storage stability and shelf-life limitations further complicate commercial viability. Many hydrogel formulations exhibit limited stability under standard storage conditions, with degradation of bioactive components and alterations in mechanical properties occurring over relatively short timeframes. Current preservation methods, including lyophilization and cryopreservation, often compromise the functional properties of hydrogels upon reconstitution.
Supply chain management introduces additional complexity, particularly for hydrogels incorporating patient-specific or rare biological components. Establishing robust procurement channels for consistent raw materials while maintaining cold chain logistics presents significant operational challenges that increase costs and complexity.
Despite these challenges, several companies are making progress toward commercial-scale production. Strategic approaches include developing synthetic hydrogel alternatives with improved stability profiles, establishing automated manufacturing processes to reduce variability, and creating modular systems that separate stable components from bioactive elements until point of use.
Manufacturing scale-up introduces additional complexities related to maintaining hydrogel homogeneity and biocompatibility. As production volumes increase, ensuring uniform distribution of bioactive components and consistent mechanical properties becomes increasingly difficult. The delicate balance of stiffness, porosity, and degradation kinetics that supports optimal organoid development can be disrupted during large-scale manufacturing processes.
Cost factors present another substantial barrier to commercialization. High-grade materials required for injectable hydrogels, particularly those incorporating specialized ECM components or growth factors, command premium prices that escalate with production scale. Current estimates suggest that material costs alone for clinical-grade hydrogel systems range from $200-500 per milliliter, making widespread application economically challenging without significant process optimization.
Regulatory pathways for hydrogel-based organoid cultivation systems remain complex and poorly defined. The combination of biomaterials, potentially patient-derived cells, and growth factors creates a regulatory classification challenge. Companies pursuing commercialization must navigate different regulatory frameworks across global markets, with some jurisdictions treating these systems as advanced therapy medicinal products (ATMPs) requiring extensive clinical validation.
Storage stability and shelf-life limitations further complicate commercial viability. Many hydrogel formulations exhibit limited stability under standard storage conditions, with degradation of bioactive components and alterations in mechanical properties occurring over relatively short timeframes. Current preservation methods, including lyophilization and cryopreservation, often compromise the functional properties of hydrogels upon reconstitution.
Supply chain management introduces additional complexity, particularly for hydrogels incorporating patient-specific or rare biological components. Establishing robust procurement channels for consistent raw materials while maintaining cold chain logistics presents significant operational challenges that increase costs and complexity.
Despite these challenges, several companies are making progress toward commercial-scale production. Strategic approaches include developing synthetic hydrogel alternatives with improved stability profiles, establishing automated manufacturing processes to reduce variability, and creating modular systems that separate stable components from bioactive elements until point of use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!