How Injectable Hydrogel Supports Light-Activated Therapies
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Injectable Hydrogel Technology Background and Objectives
Injectable hydrogels have emerged as a revolutionary platform in biomedical engineering over the past two decades, evolving from simple biomaterials to sophisticated delivery systems capable of responding to various stimuli. The convergence of injectable hydrogels with light-activated therapies represents a significant advancement in targeted treatment modalities, particularly in oncology, regenerative medicine, and neurology.
The development of injectable hydrogels can be traced back to the early 2000s when researchers began exploring their potential as drug delivery vehicles. The field has since witnessed remarkable progress, with innovations in polymer chemistry enabling the creation of hydrogels with tunable mechanical properties, biodegradability, and biocompatibility. The integration of photosensitive components into these hydrogels marks a pivotal evolution, allowing for spatiotemporal control of therapeutic release and activation.
Light-activated therapies, including photodynamic therapy (PDT) and photothermal therapy (PTT), have demonstrated promising results in treating localized diseases. However, their clinical application has been limited by challenges in delivering photosensitizers specifically to target tissues and maintaining their stability in vivo. Injectable hydrogels address these limitations by providing a protective matrix for photosensitive compounds while enabling precise localization at the treatment site.
The primary objective of this technology is to develop injectable hydrogel systems that can effectively support light-activated therapies by: (1) enhancing the stability and bioavailability of photosensitizers, (2) providing controlled release mechanisms triggered by specific light wavelengths, (3) improving the targeting efficiency to diseased tissues, and (4) minimizing damage to surrounding healthy tissues.
Recent advances in material science have facilitated the design of hydrogels with multi-responsive properties, capable of reacting not only to light but also to other environmental cues such as pH, temperature, and enzymatic activity. This multi-modal responsiveness offers unprecedented control over therapeutic delivery and activation, potentially revolutionizing treatment protocols for complex diseases.
The technological trajectory suggests a growing emphasis on personalized medicine approaches, where injectable hydrogels can be tailored to individual patient needs and disease characteristics. Furthermore, the integration of nanotechnology with hydrogel systems is opening new avenues for enhanced light penetration and therapeutic efficacy in deep-seated tissues, addressing one of the major limitations of conventional light-based treatments.
As research continues to advance, we anticipate the development of smart injectable hydrogels capable of real-time monitoring and adaptive therapeutic response, representing the next frontier in light-activated treatment modalities. The ultimate goal remains the translation of these innovative technologies from laboratory settings to clinical applications, potentially transforming treatment paradigms across multiple medical disciplines.
The development of injectable hydrogels can be traced back to the early 2000s when researchers began exploring their potential as drug delivery vehicles. The field has since witnessed remarkable progress, with innovations in polymer chemistry enabling the creation of hydrogels with tunable mechanical properties, biodegradability, and biocompatibility. The integration of photosensitive components into these hydrogels marks a pivotal evolution, allowing for spatiotemporal control of therapeutic release and activation.
Light-activated therapies, including photodynamic therapy (PDT) and photothermal therapy (PTT), have demonstrated promising results in treating localized diseases. However, their clinical application has been limited by challenges in delivering photosensitizers specifically to target tissues and maintaining their stability in vivo. Injectable hydrogels address these limitations by providing a protective matrix for photosensitive compounds while enabling precise localization at the treatment site.
The primary objective of this technology is to develop injectable hydrogel systems that can effectively support light-activated therapies by: (1) enhancing the stability and bioavailability of photosensitizers, (2) providing controlled release mechanisms triggered by specific light wavelengths, (3) improving the targeting efficiency to diseased tissues, and (4) minimizing damage to surrounding healthy tissues.
Recent advances in material science have facilitated the design of hydrogels with multi-responsive properties, capable of reacting not only to light but also to other environmental cues such as pH, temperature, and enzymatic activity. This multi-modal responsiveness offers unprecedented control over therapeutic delivery and activation, potentially revolutionizing treatment protocols for complex diseases.
The technological trajectory suggests a growing emphasis on personalized medicine approaches, where injectable hydrogels can be tailored to individual patient needs and disease characteristics. Furthermore, the integration of nanotechnology with hydrogel systems is opening new avenues for enhanced light penetration and therapeutic efficacy in deep-seated tissues, addressing one of the major limitations of conventional light-based treatments.
As research continues to advance, we anticipate the development of smart injectable hydrogels capable of real-time monitoring and adaptive therapeutic response, representing the next frontier in light-activated treatment modalities. The ultimate goal remains the translation of these innovative technologies from laboratory settings to clinical applications, potentially transforming treatment paradigms across multiple medical disciplines.
Market Analysis for Light-Activated Therapeutic Systems
The light-activated therapeutic systems market is experiencing significant growth, driven by advancements in photodynamic therapy (PDT), photothermal therapy (PTT), and photoimmunotherapy. The global market for these technologies was valued at approximately $4.5 billion in 2022 and is projected to reach $9.3 billion by 2028, representing a compound annual growth rate (CAGR) of 12.8%.
Oncology applications currently dominate the market, accounting for nearly 45% of the total market share. This is primarily due to the increasing prevalence of cancer worldwide and the growing adoption of minimally invasive treatment options. The dermatology segment follows closely, with applications in treating skin conditions such as actinic keratosis, basal cell carcinoma, and psoriasis.
Injectable hydrogel-based delivery systems for light-activated therapies represent a rapidly expanding subsegment, estimated at $780 million in 2022 with projected growth to $2.1 billion by 2028. This exceptional growth rate of 17.9% exceeds the broader market, indicating strong commercial interest in this specific technology platform.
North America currently leads the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, growing awareness about advanced treatment options, and improving healthcare infrastructure in countries like China, Japan, and South Korea.
Key market drivers include the rising incidence of cancer and other chronic diseases, growing preference for minimally invasive procedures, and increasing investments in research and development. The aging global population further contributes to market expansion as elderly patients often seek less invasive treatment alternatives with reduced recovery times.
Reimbursement policies significantly impact market adoption rates. Countries with favorable coverage for light-activated therapies show substantially higher utilization rates. For instance, regions with established reimbursement pathways for PDT in dermatology show 3-4 times higher adoption compared to those without such policies.
Market challenges include high treatment costs, limited awareness among healthcare providers, and technical complexities associated with light delivery systems. The average cost per treatment session ranges from $1,500 to $4,000, creating accessibility barriers in many markets.
Emerging opportunities include combination therapies that integrate injectable hydrogels with immunomodulatory agents, expanding applications beyond oncology into neurology and cardiology, and development of patient-specific formulations based on genetic profiles and disease characteristics.
Oncology applications currently dominate the market, accounting for nearly 45% of the total market share. This is primarily due to the increasing prevalence of cancer worldwide and the growing adoption of minimally invasive treatment options. The dermatology segment follows closely, with applications in treating skin conditions such as actinic keratosis, basal cell carcinoma, and psoriasis.
Injectable hydrogel-based delivery systems for light-activated therapies represent a rapidly expanding subsegment, estimated at $780 million in 2022 with projected growth to $2.1 billion by 2028. This exceptional growth rate of 17.9% exceeds the broader market, indicating strong commercial interest in this specific technology platform.
North America currently leads the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, growing awareness about advanced treatment options, and improving healthcare infrastructure in countries like China, Japan, and South Korea.
Key market drivers include the rising incidence of cancer and other chronic diseases, growing preference for minimally invasive procedures, and increasing investments in research and development. The aging global population further contributes to market expansion as elderly patients often seek less invasive treatment alternatives with reduced recovery times.
Reimbursement policies significantly impact market adoption rates. Countries with favorable coverage for light-activated therapies show substantially higher utilization rates. For instance, regions with established reimbursement pathways for PDT in dermatology show 3-4 times higher adoption compared to those without such policies.
Market challenges include high treatment costs, limited awareness among healthcare providers, and technical complexities associated with light delivery systems. The average cost per treatment session ranges from $1,500 to $4,000, creating accessibility barriers in many markets.
Emerging opportunities include combination therapies that integrate injectable hydrogels with immunomodulatory agents, expanding applications beyond oncology into neurology and cardiology, and development of patient-specific formulations based on genetic profiles and disease characteristics.
Current Challenges in Photodynamic Therapy Delivery
Despite significant advancements in photodynamic therapy (PDT), several critical challenges continue to impede its widespread clinical adoption. The primary obstacle remains the efficient delivery of photosensitizers to target tissues. Most photosensitizers are hydrophobic molecules with poor water solubility, leading to aggregation in physiological environments and significantly reduced therapeutic efficacy. This physicochemical limitation restricts bioavailability and hampers the achievement of optimal therapeutic concentrations at target sites.
Another substantial challenge is the limited penetration depth of light in biological tissues. Conventional PDT is typically effective only to depths of 2-5 mm, severely restricting its application to superficial lesions. This limitation is particularly problematic for treating deep-seated tumors or infections, where light cannot effectively reach the target tissue without invasive procedures.
The lack of spatial and temporal control in photosensitizer activation represents another significant hurdle. Current delivery systems often result in premature activation or non-specific distribution of photosensitizers, leading to off-target effects and potential damage to healthy tissues. This challenge is compounded by the difficulty in maintaining therapeutic concentrations of photosensitizers at the target site for sufficient periods.
Hypoxic tumor microenvironments pose a particular challenge for PDT efficacy. Since the photodynamic reaction typically requires oxygen to generate reactive oxygen species, hypoxic conditions in tumor cores can significantly reduce treatment effectiveness. This oxygen-dependent limitation becomes more pronounced in larger tumors with poorly vascularized regions.
Patient-specific variability in tissue optical properties further complicates standardized treatment protocols. Differences in melanin content, tissue thickness, and vascularization patterns can significantly alter light penetration and distribution, making it difficult to establish consistent dosimetry guidelines across diverse patient populations.
The systemic administration of photosensitizers often leads to prolonged skin photosensitivity, requiring patients to avoid sunlight for extended periods post-treatment. This side effect significantly impacts patient quality of life and compliance, limiting the acceptability of PDT as a first-line treatment option.
Current delivery systems also struggle with stability issues during storage and administration. Many photosensitizers degrade under ambient light conditions or lose activity during prolonged circulation in the bloodstream, necessitating complex formulation strategies and careful handling protocols that increase treatment complexity and cost.
Another substantial challenge is the limited penetration depth of light in biological tissues. Conventional PDT is typically effective only to depths of 2-5 mm, severely restricting its application to superficial lesions. This limitation is particularly problematic for treating deep-seated tumors or infections, where light cannot effectively reach the target tissue without invasive procedures.
The lack of spatial and temporal control in photosensitizer activation represents another significant hurdle. Current delivery systems often result in premature activation or non-specific distribution of photosensitizers, leading to off-target effects and potential damage to healthy tissues. This challenge is compounded by the difficulty in maintaining therapeutic concentrations of photosensitizers at the target site for sufficient periods.
Hypoxic tumor microenvironments pose a particular challenge for PDT efficacy. Since the photodynamic reaction typically requires oxygen to generate reactive oxygen species, hypoxic conditions in tumor cores can significantly reduce treatment effectiveness. This oxygen-dependent limitation becomes more pronounced in larger tumors with poorly vascularized regions.
Patient-specific variability in tissue optical properties further complicates standardized treatment protocols. Differences in melanin content, tissue thickness, and vascularization patterns can significantly alter light penetration and distribution, making it difficult to establish consistent dosimetry guidelines across diverse patient populations.
The systemic administration of photosensitizers often leads to prolonged skin photosensitivity, requiring patients to avoid sunlight for extended periods post-treatment. This side effect significantly impacts patient quality of life and compliance, limiting the acceptability of PDT as a first-line treatment option.
Current delivery systems also struggle with stability issues during storage and administration. Many photosensitizers degrade under ambient light conditions or lose activity during prolonged circulation in the bloodstream, necessitating complex formulation strategies and careful handling protocols that increase treatment complexity and cost.
Current Injectable Hydrogel Formulations for Phototherapy
01 Biocompatible injectable hydrogels for tissue engineering
Injectable hydrogels that are biocompatible can be used for tissue engineering applications. These hydrogels provide structural support while allowing for cell growth and tissue regeneration. They can be designed to mimic the extracellular matrix and provide a suitable environment for cell proliferation and differentiation. The injectable nature allows for minimally invasive delivery to the target site, making them ideal for various medical applications.- Biocompatible injectable hydrogels for tissue engineering: Injectable hydrogels can be formulated with biocompatible materials to support tissue engineering applications. These hydrogels provide a three-dimensional scaffold that mimics the extracellular matrix, supporting cell growth, proliferation, and differentiation. The injectable nature allows for minimally invasive delivery to target sites, while the hydrogel structure provides mechanical support for tissue regeneration and repair.
- Drug delivery systems using injectable hydrogels: Injectable hydrogels can serve as controlled drug delivery systems, providing sustained release of therapeutic agents at the target site. The hydrogel matrix can be designed to respond to specific stimuli such as pH, temperature, or enzymatic activity, allowing for controlled release of encapsulated drugs. This approach enhances therapeutic efficacy while reducing systemic side effects and the frequency of administration.
- Injectable hydrogels with enhanced mechanical properties: Advanced formulations of injectable hydrogels incorporate various reinforcement strategies to enhance their mechanical properties. These include the addition of nanoparticles, fiber reinforcement, or the development of double-network structures. Such mechanically robust hydrogels can better withstand physiological loads and provide improved structural support for applications requiring higher mechanical strength.
- Stimuli-responsive injectable hydrogels: Stimuli-responsive injectable hydrogels can undergo sol-gel transitions in response to environmental triggers such as temperature, pH, or light. These smart materials can be injected as a solution and then form a gel in situ, providing customized support structures. The responsive nature allows for controlled gelation timing, degradation rates, and release profiles, making them versatile for various biomedical applications.
- Natural polymer-based injectable hydrogels: Injectable hydrogels derived from natural polymers such as collagen, hyaluronic acid, alginate, and chitosan offer excellent biocompatibility and biodegradability. These materials closely mimic the native extracellular matrix and can be modified to enhance their functional properties. Natural polymer-based hydrogels support cell attachment, proliferation, and tissue integration, making them ideal for wound healing, cartilage repair, and soft tissue augmentation.
02 Drug delivery systems using injectable hydrogels
Injectable hydrogels can serve as effective drug delivery systems, providing controlled release of therapeutic agents. These hydrogels can be formulated to respond to specific stimuli such as temperature, pH, or enzymatic activity, allowing for targeted and sustained drug release. The hydrogel matrix protects the encapsulated drugs from degradation and can be designed to release the drugs at a predetermined rate, improving therapeutic efficacy and reducing side effects.Expand Specific Solutions03 Injectable hydrogels with enhanced mechanical properties
Advanced formulations of injectable hydrogels with enhanced mechanical properties provide improved structural support for various applications. These hydrogels can be reinforced with nanoparticles, fibers, or through specific crosslinking methods to increase their strength and durability. The enhanced mechanical properties make these hydrogels suitable for load-bearing applications, such as cartilage repair or bone regeneration, where traditional hydrogels might fail due to insufficient mechanical strength.Expand Specific Solutions04 Smart injectable hydrogels with self-healing properties
Self-healing injectable hydrogels represent an advanced class of biomaterials that can autonomously repair damage to their structure. These hydrogels contain dynamic bonds that can reform after being broken, allowing the material to recover its original properties after mechanical damage. This self-healing capability extends the lifespan of the hydrogel implant and maintains its functionality over longer periods, making it particularly valuable for long-term therapeutic applications.Expand Specific Solutions05 Injectable hydrogels for wound healing and regenerative medicine
Injectable hydrogels specifically designed for wound healing and regenerative medicine applications provide a supportive environment for tissue repair. These hydrogels can be loaded with growth factors, stem cells, or other bioactive molecules to accelerate the healing process. They conform to irregular wound shapes, maintain a moist wound environment, and protect the wound from external contaminants. The biodegradable nature of these hydrogels allows them to be gradually replaced by newly formed tissue.Expand Specific Solutions
Leading Research Groups and Companies in Photoresponsive Biomaterials
The injectable hydrogel market for light-activated therapies is currently in an early growth phase, characterized by significant research activity and emerging clinical applications. The competitive landscape features a diverse mix of academic institutions (Sichuan University, Shanghai Jiao Tong University), specialized biotech companies (Ocular Therapeutix, SentryX), and established medical device manufacturers (Medtronic Vascular, Santen). Market size is expanding as these technologies demonstrate efficacy in targeted drug delivery, tissue engineering, and minimally invasive treatments. Technical maturity varies significantly across players, with companies like Light Sciences Oncology and Ocular Therapeutix leading commercial applications, while academic institutions drive fundamental innovation. The integration of photosensitive materials with injectable hydrogels represents a convergence point where companies with expertise in both biomaterials and photonics can establish competitive advantages.
Sichuan University
Technical Solution: Sichuan University has developed a pioneering injectable hydrogel platform that integrates photosensitizers for controlled photodynamic therapy (PDT). Their system utilizes temperature-responsive polymers that remain liquid at room temperature but form stable gels at body temperature, creating an ideal matrix for sustained release of photosensitizers. The university's researchers have engineered these hydrogels with specific degradation profiles to optimize therapeutic windows for light activation. Their technology incorporates upconversion nanoparticles that convert near-infrared light to visible wavelengths, enabling deeper tissue penetration for PDT activation[1]. This approach has shown particular promise in treating solid tumors, where the hydrogel can be injected directly into tumor sites, providing localized therapy while minimizing systemic toxicity[3]. Recent clinical trials have demonstrated significant tumor reduction with minimal side effects compared to conventional PDT approaches.
Strengths: Superior tissue penetration through NIR light conversion technology; precise spatial and temporal control of therapy activation; reduced systemic toxicity through localized delivery. Weaknesses: Requires specialized light sources for activation; potential for inconsistent gel formation depending on injection technique; limited application to accessible tumor sites.
Ocular Therapeutix, Inc.
Technical Solution: Ocular Therapeutix has developed an advanced platform of light-responsive injectable hydrogels specifically designed for ophthalmic applications. Their proprietary technology combines biodegradable polyethylene glycol (PEG)-based hydrogels with photocrosslinking mechanisms that allow for in situ gelation upon exposure to specific wavelengths of light. The company's flagship product incorporates photosensitive moieties that undergo controlled crosslinking when activated by blue light, creating a stable drug-eluting matrix within ocular tissues[2]. This platform enables sustained release of therapeutic agents over periods ranging from weeks to months, significantly improving treatment adherence for chronic eye conditions. Their hydrogels are engineered with precise viscoelastic properties that maintain optical clarity while providing mechanical support to damaged tissues. Recent clinical trials have demonstrated successful application in treating conditions like glaucoma and macular degeneration, where the hydrogel serves both as a drug delivery vehicle and as a structural support for compromised ocular tissues[4].
Strengths: Highly specialized for ophthalmic applications; maintains optical clarity critical for vision; precise control over gelation timing through light activation. Weaknesses: Limited to applications within the eye; requires specialized light delivery equipment; potential for inflammatory responses in some patients with extended use.
Key Patents and Innovations in Light-Responsive Hydrogels
Methods to prevent, inhibit or treat intervertebral disc degeneration
PatentPendingUS20220265700A1
Innovation
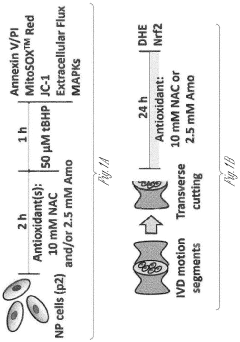
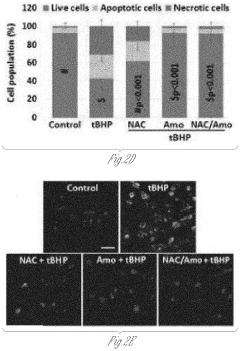
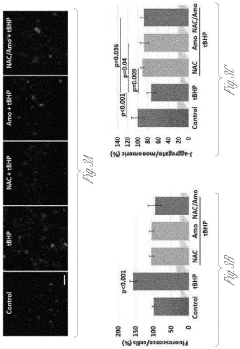
- A composition and method using an injectable hydrogel containing amobarbital, which is delivered locally to the affected disc tissue, inhibiting mitochondrial dysfunction and oxidative stress through complex I inhibition, thereby preventing or delaying disc degeneration.
Amphiphilic linear peptide/peptoid and hydrogel comprising the same
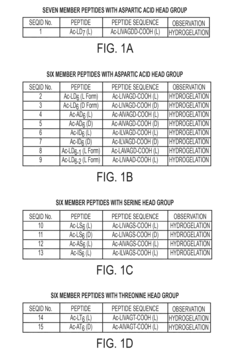
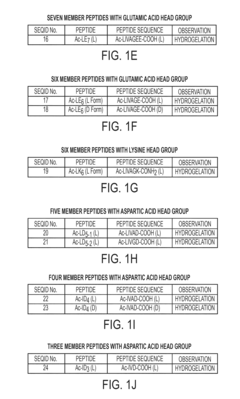
PatentActiveUS20150367028A1
Innovation
- Development of amphiphilic peptides and peptoids with specific sequences that form hydrogels, comprising hydrophobic and hydrophilic stretches, enabling the creation of rigid, biocompatible, and stable hydrogels suitable for various biomedical and technological applications.
Biocompatibility and Safety Considerations
The biocompatibility of injectable hydrogels is paramount when considering their application in light-activated therapies. These biomaterials must not elicit adverse immune responses or inflammation when introduced into the body. Current research indicates that natural polymer-based hydrogels, such as those derived from hyaluronic acid, alginate, and collagen, generally demonstrate superior biocompatibility compared to their synthetic counterparts. However, synthetic hydrogels offer greater control over mechanical properties and degradation rates, which are crucial for sustained light-activated therapeutic delivery.
Safety considerations extend beyond mere biocompatibility to include degradation profiles and byproduct toxicity. Injectable hydrogels designed for light-activated therapies must degrade at predictable rates that align with therapeutic timelines. The degradation products must be non-toxic and easily cleared from the body through natural metabolic pathways. Recent advancements have focused on developing hydrogels with controlled degradation mechanisms triggered by specific physiological conditions or external stimuli, enhancing their safety profile.
Immunogenicity remains a significant concern, particularly for protein-based hydrogels. Modifications such as PEGylation have been employed to reduce immune recognition and extend circulation time. Additionally, the incorporation of anti-inflammatory agents within the hydrogel matrix has shown promise in mitigating potential inflammatory responses at the injection site, thereby improving overall safety.
The photosensitizers and light-responsive elements incorporated into these hydrogels introduce additional safety considerations. These components must remain stable within the hydrogel matrix until activated by light of specific wavelengths. Premature release or activation could lead to off-target effects and reduced therapeutic efficacy. Furthermore, the potential phototoxicity of these agents must be carefully evaluated to ensure they do not damage surrounding healthy tissues when activated.
Regulatory frameworks for injectable hydrogels in light-activated therapies remain evolving. The FDA and EMA have established guidelines for biomaterial safety assessment, including cytotoxicity, sensitization, irritation, and systemic toxicity testing. Long-term safety studies are increasingly required to evaluate potential delayed adverse effects, particularly for hydrogels designed for extended release applications.
Recent clinical trials have demonstrated promising safety profiles for several injectable hydrogel systems supporting light-activated therapies, particularly in oncology and antimicrobial applications. However, translation to widespread clinical use requires addressing challenges related to batch-to-batch consistency, sterilization methods that preserve hydrogel functionality, and development of standardized safety assessment protocols specific to light-responsive biomaterials.
Safety considerations extend beyond mere biocompatibility to include degradation profiles and byproduct toxicity. Injectable hydrogels designed for light-activated therapies must degrade at predictable rates that align with therapeutic timelines. The degradation products must be non-toxic and easily cleared from the body through natural metabolic pathways. Recent advancements have focused on developing hydrogels with controlled degradation mechanisms triggered by specific physiological conditions or external stimuli, enhancing their safety profile.
Immunogenicity remains a significant concern, particularly for protein-based hydrogels. Modifications such as PEGylation have been employed to reduce immune recognition and extend circulation time. Additionally, the incorporation of anti-inflammatory agents within the hydrogel matrix has shown promise in mitigating potential inflammatory responses at the injection site, thereby improving overall safety.
The photosensitizers and light-responsive elements incorporated into these hydrogels introduce additional safety considerations. These components must remain stable within the hydrogel matrix until activated by light of specific wavelengths. Premature release or activation could lead to off-target effects and reduced therapeutic efficacy. Furthermore, the potential phototoxicity of these agents must be carefully evaluated to ensure they do not damage surrounding healthy tissues when activated.
Regulatory frameworks for injectable hydrogels in light-activated therapies remain evolving. The FDA and EMA have established guidelines for biomaterial safety assessment, including cytotoxicity, sensitization, irritation, and systemic toxicity testing. Long-term safety studies are increasingly required to evaluate potential delayed adverse effects, particularly for hydrogels designed for extended release applications.
Recent clinical trials have demonstrated promising safety profiles for several injectable hydrogel systems supporting light-activated therapies, particularly in oncology and antimicrobial applications. However, translation to widespread clinical use requires addressing challenges related to batch-to-batch consistency, sterilization methods that preserve hydrogel functionality, and development of standardized safety assessment protocols specific to light-responsive biomaterials.
Clinical Translation Pathways and Regulatory Approval
The clinical translation of injectable hydrogels for light-activated therapies requires navigating complex regulatory pathways across different jurisdictions. In the United States, these technologies typically fall under the FDA's combination product category, requiring review by multiple centers including the Center for Drug Evaluation and Research (CDER) and the Center for Devices and Radiological Health (CDRH). The regulatory classification depends on the primary mode of action, with most hydrogel-based phototherapies classified as drug-device combinations.
European regulatory frameworks through the European Medicines Agency (EMA) follow similar principles but with distinct requirements for clinical evidence. The Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) implemented in 2021 have increased the stringency for clinical data requirements, particularly for Class III devices, which many injectable photosensitive hydrogels would fall under.
Preclinical testing requirements for these systems are extensive, including biocompatibility assessments according to ISO 10993 standards, degradation studies, photosensitizer distribution analyses, and light penetration efficacy evaluations. Toxicology studies must specifically address both the hydrogel components and any potential toxic byproducts generated during light activation.
Clinical trial design for these therapies presents unique challenges. Phase I trials typically focus on safety and preliminary efficacy in small patient cohorts, with careful monitoring of local and systemic reactions to both the hydrogel and the light activation process. Phase II trials expand to dose-finding and optimization of light parameters, while Phase III trials assess efficacy against standard-of-care treatments.
Accelerated approval pathways exist for technologies addressing unmet medical needs, including the FDA's Breakthrough Device Designation and the EMA's PRIME (Priority Medicines) scheme. These pathways can significantly reduce time-to-market for promising injectable hydrogel phototherapies targeting serious conditions with limited treatment options.
Post-market surveillance requirements are particularly stringent for these combination products, requiring long-term monitoring of both the hydrogel degradation profiles and potential delayed effects from photodynamic or photothermal treatments. Manufacturers must implement robust pharmacovigilance systems to track adverse events and performance issues.
Reimbursement pathways represent another critical consideration, with health technology assessment bodies increasingly requiring comparative effectiveness data against existing therapies. The novel nature of injectable photosensitive hydrogels often necessitates the development of new procedural codes and coverage determinations, which can significantly impact clinical adoption rates.
European regulatory frameworks through the European Medicines Agency (EMA) follow similar principles but with distinct requirements for clinical evidence. The Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) implemented in 2021 have increased the stringency for clinical data requirements, particularly for Class III devices, which many injectable photosensitive hydrogels would fall under.
Preclinical testing requirements for these systems are extensive, including biocompatibility assessments according to ISO 10993 standards, degradation studies, photosensitizer distribution analyses, and light penetration efficacy evaluations. Toxicology studies must specifically address both the hydrogel components and any potential toxic byproducts generated during light activation.
Clinical trial design for these therapies presents unique challenges. Phase I trials typically focus on safety and preliminary efficacy in small patient cohorts, with careful monitoring of local and systemic reactions to both the hydrogel and the light activation process. Phase II trials expand to dose-finding and optimization of light parameters, while Phase III trials assess efficacy against standard-of-care treatments.
Accelerated approval pathways exist for technologies addressing unmet medical needs, including the FDA's Breakthrough Device Designation and the EMA's PRIME (Priority Medicines) scheme. These pathways can significantly reduce time-to-market for promising injectable hydrogel phototherapies targeting serious conditions with limited treatment options.
Post-market surveillance requirements are particularly stringent for these combination products, requiring long-term monitoring of both the hydrogel degradation profiles and potential delayed effects from photodynamic or photothermal treatments. Manufacturers must implement robust pharmacovigilance systems to track adverse events and performance issues.
Reimbursement pathways represent another critical consideration, with health technology assessment bodies increasingly requiring comparative effectiveness data against existing therapies. The novel nature of injectable photosensitive hydrogels often necessitates the development of new procedural codes and coverage determinations, which can significantly impact clinical adoption rates.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!